Abstract
We previously demonstrated that subantimicrobial-dose-doxycycline (SDD) treatment of post-menopausal osteopenic women significantly reduced periodontal disease progression, and biomarkers of collagen destruction and bone resorption locally in periodontal pockets, in a double-blind placebo-controlled clinical trial. We now hypothesize that SDD may also improve biomarkers of bone loss systemically in the same women, consistent with previous studies on tetracyclines (e.g., doxycycline) in organ culture and animal models of bone-deficiency disease. 128 post-menopausal osteopenic women with chronic periodontitis randomly received SDD or placebo tablets daily for 2 years adjunctive to periodontal maintenance therapy every 3-4 months. Blood was collected at baseline and at one- and two-year appointments, and sera were analyzed for bone resorption and bone formation/turnover biomarkers. In subsets of the study population, adjunctive SDD significantly reduced serum biomarkers of bone resorption (biomarkers of bone formation were unaffected), consistent with reduced risk of future systemic bone loss in these post-menopausal women not yet on anti-osteoporotic drugs.
Keywords: subantimicrobial-dose-doxycycline, serum biomarkers, post-menopause, osteopenia, periodontitis
Introduction
Loss of alveolar bone is a signature event in the diagnosis and pathogenesis of periodontitis. Therefore, numerous research groups have attempted to reduce this bone loss using pharmaceutical agents, some novel, others long recognized to be effective in managing bone-deficiency diseases such as osteoporosis. However, only a few systemically administered drugs have demonstrated the potential to inhibit both locally (periodontitis) and systemically (osteoporosis) induced bone loss. To date, these medications have included orally administered agents such as bisphosphonates (Tenenbaum et al., 2002), non-steroidal anti-inflammatory drugs (Williams et al., 1989), tetracyclines, e.g., subantimicrobial-dose-doxycycline (SDD) and the chemically modified non-bacteriostatic tetracyclines (Golub et al., 1998; Payne et al., 2007), and, more recently, the resolvins (Van Dyke, 2008) and intermittent injections of parathyroid hormone (Barros et al., 2003).
Regarding tetracyclines, our group recently described the beneficial long-term effects of SDD in post-menopausal women exhibiting both local and mild systemic bone loss (periodontitis and osteopenia, respectively). In brief, the two-year SDD regimen significantly reduced the progression of periodontal attachment loss (Reinhardt et al., 2007) and, in subgroups of the study population, decreased alveolar bone loss (Payne et al., 2007) in a double-blind placebo-controlled trial. Consistent with these clinical observations, SDD also reduced biomarkers of collagen degradation and bone resorption locally in gingival crevicular fluid (GCF) of these women (Golub et al., 2008). We now hypothesize that this treatment may also improve biomarkers of bone remodeling systemically in the circulation of these post-menopausal osteopenic women with periodontitis.
Materials & Methods
The details of this trial— including methods for clinical (e.g., probing depth, clinical attachment levels), dental, and medical radiographic, microbiological, and GCF measurements of local “biomarkers” of collagenolysis and bone resorption—were described by us recently (Payne et al., 2007; Reinhardt et al., 2007; Walker et al., 2007; Golub et al., 2008). The study protocol was approved by the Institutional Review Boards of both study centers, the University of Nebraska Medical Center and Stony Brook University, and all participants provided written informed consent.
Briefly, the study was a double-blind placebo-controlled clinical trial, with each of 128 post-menopausal osteopenic women with periodontitis randomized to receive SDD (64 women) or placebo (n = 64) tablets systemically (oral route) every 12 hrs over 2 yrs. Both groups also received daily calcium (1200 mg) and vitamin D (400 IU) supplements for 2 yrs, and periodontal maintenance therapy every 3-4 mos, at no cost to the subjects. The clinical measurements of periodontal disease severity were taken every 6 mos, while dental radiographs and scans of the lumbar spine and femoral neck (dual-energy x-ray absorptiometry) and microbiologic, GCF, and blood samples were collected at the baseline and at one- and two-year appointments. Note that none of the enrolled women was diagnosed with osteoporosis, and none was taking medications for this disease (e.g., bisphosphonates).
Regarding blood samples, the sera were separated, then frozen (-80°C) until analyzed for bone-remodeling biomarkers and serum doxycycline levels, as follows:
Bone-specific alkaline phosphatase, a biomarker of osteoblast activity and bone formation (Pedrazzoni et al., 1996), was measured by EIA (Quidel Corp., San Diego, CA, USA) with a monoclonal antibody to this enzyme. A 20-µL quantity of serum was used for this and for osteocalcin analysis (see below). Recovery values for this and the other bone metabolism markers were essentially 100%.
Osteocalcin is considered a biomarker of bone turnover, not just bone formation, even though it is produced only by osteoblasts (Looker et al., 2000). Serum samples from each woman were analyzed with an ELISA (Nordic Bioscience Diagnostics, Herlev, Denmark) kit with monoclonal antibodies recognizing both intact and N-terminal mid-fragments of human osteocalcin.
ICTP, a pyridinoline-crosslink-containing degradation fragment of the C-terminal telopeptide region of type I collagen, indicative of bone resorption, was measured by radio-immunoassay with 125I-labeled antibody against 14- to 43-kDa fragments of bone collagen digested by bacterial collagenase (Immunodiagnostic Systems, Fountain Hills, AZ, USA), as we described previously (Golub et al., 1997, 2008).
CTX, a deoxypyridinoline-containing degradation fragment of the C-terminal telopeptide region of type I collagen, generated by breakdown mediated by cathepsin-K and matrix metalloproteinases, was measured by ELISA (Nordic Bioscience Diagnostics, Herlev, Denmark).
Serum samples from the placebo- and SDD-treated participants, collected at the baseline and one- and two-year appointments, were analyzed for doxycycline concentrations by high-performance liquid chromatography, as we previously described (Liu et al., 2001).
Statistical Analyses
As described previously (Payne et al., 2007; Reinhardt et al., 2007), we used generalized estimating equations methodology to estimate the treatment effect on follow-up serum biomarker levels after adjustment for baseline serum biomarker levels and other baseline confounding factors (Liang and Zeger, 1986). A natural log transformation was used for the CTX measure. We used a similar modeling approach to compare CTX measures between women with detectable and undetectable levels of serum doxycycline. The association between ICTP and CTX at each time-point, following a natural log transformation of the measures, was estimated with a Pearson correlation coefficient. The primary analysis followed an intent-to-treat paradigm. Pre-specified subgroup analyses—defined by baseline smoking status, time since onset of menopause, adherence to study medications, and significant concomitant medication use—were performed by tests of interactions. As we described previously (Payne et al., 2007), sample size was justified based on the primary study aim and endpoint, to compare radiographic evidence of alveolar bone density changes from baseline between the SDD and placebo groups.
Results
Based on intent-to-treat analyses, a two-year regimen of SDD produced no significant changes, compared with placebo therapy, in the serum levels of bone-specific alkaline phosphatase (p = 0.3) and osteocalcin (p = 0.5) (Table 1), which are biomarkers of bone formation and bone turnover, respectively. The serum biomarkers of bone resorption, ICTP and CTX, were positively correlated at all 3 appointments (baseline, one- and two-year, r = 0.34, 0.34, 0.26, respectively; p ≤ 0.006). However, based on intent-to-treat analyses, SDD therapy did not produce statistically significant effects on these biomarkers, ICTP (p = 0.1) and CTX (p = 0.5), relative to placebo (Table 2).
Table 1.
The Effect of a Two-year Regimen of SDD on Serum Bone Formation and Bone Turnover Biomarkers, Bone-specific Alkaline Phosphatase and Osteocalcin [Data are presented as the median, mean, and standard deviation (SD) values.]
| Bone-specific Alkaline Phosphatase (U/L) |
Osteocalcin (ng/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group Time-point | Number of Subjects | Median | Mean | SD | p-value* | Median | Mean | SD | p-value* |
| Placebo | 0.3 | 0.5 | |||||||
| Baseline | 64 | 28.8 | 30.8 | 9.8 | 31.9 | 34.0 | 14.0 | ||
| 1-year | 61 | 28.9 | 30.0 | 9.8 | 27.1 | 28.9 | 11.3 | ||
| 2-year | 62 | 27.3 | 29.0 | 8.9 | 28.2 | 28.8 | 11.4 | ||
| SDD | |||||||||
| Baseline | 64 | 28.6 | 30.3 | 10.1 | 35.1 | 35.9 | 14.0 | ||
| 1-year | 55 | 27.0 | 28.7 | 8.9 | 31.6 | 31.2 | 12.1 | ||
| 2-year | 51 | 26.6 | 27.3 | 7.0 | 31.1 | 29.6 | 9.9 | ||
The p-value corresponds to the comparison between placebo and SDD over the one-year and two-year visits after adjustment for baseline levels.
Table 2.
The Effect of a Two-year Regimen of SDD on Serum Concentrations of the Bone Resorption Biomarkers, ICTP and CTX [Data are presented as the median, mean, and standard deviation (SD) values.]
| Pyridinoline-crosslink Fragment of Type I Collagen (ICTP; ng/mL) |
Deoxypyridinoline-crosslink Fragment of Type I Collagen (CTX; ng/mL × 10) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group Time-point | Number of Subjects | Median | Mean | SD | p-value* | Median | Mean | SD | p-value* |
| Placebo | 0.1 | 0.5 | |||||||
| Baseline | 64 | 3.4 | 3.5 | 1.2 | 2.3 | 2.7 | 2.1 | ||
| 1-year | 61 | 3.4 | 3.6 | 1.0 | 2.0 | 2.2 | 1.9 | ||
| 2-year | 62 | 3.4 | 3.6 | 1.2 | 2.2 | 2.6 | 2.2 | ||
| SDD | |||||||||
| Baseline | 64 | 3.5 | 3.5 | 1.0 | 2.4 | 2.8 | 2.0 | ||
| 1-year | 55 | 3.2 | 3.4 | 1.0 | 1.9 | 2.3 | 1.7 | ||
| 2-year | 51 | 3.3 | 3.4 | 1.0 | 1.8 | 2.2 | 1.6 | ||
The p-value corresponds to the comparison between placebo and SDD over the one-year and two-year visits after adjustment for baseline levels.
However, SDD therapy did produce statistically significant effects relative to placebo among subsets of the post-menopausal women. Those women treated for 2 yrs with SDD, who were post-menopausal ≤ 5 yrs at the baseline visit (n = 23 placebo and n = 25 SDD), showed a highly significant reduction in serum ICTP (p = 0.0003) and a marginal reduction in CTX (p = 0.06) (Figs. 1, 2). A similar pattern was seen in the subset of post-menopausal women not on significant concomitant medications (n = 39 placebo and n = 34 SDD at the two-year time-point). When the two-year data were compared with baseline, the SDD-treated group showed a significant reduction in serum ICTP (p = 0.05) and a marginal reduction in CTX (p = 0.06). Moreover, when we investigated the association between serum CTX and the presence (or absence) of doxycycline in the serum samples (which addresses whether the individual was in the placebo or SDD group, and study medication adherence), those with HPLC-detectable doxycycline (> 0.1 µg/mL; median serum concentration 0.59 µg/mL) showed a statistically significant reduction in CTX (p = 0.02) compared with those with no detectable serum doxycycline levels (data not shown).
Figure 1.
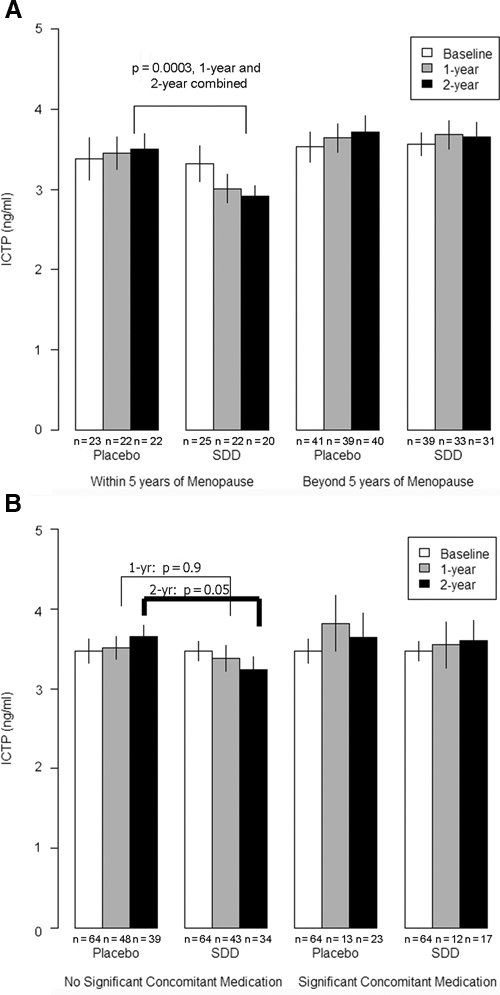
The effect of SDD relative to placebo on ICTP levels in serum in (A) subgroups of women within 5 yrs of menopause at baseline (no significant effect of SDD in women beyond 5 yrs of menopause, p = 0.8) and (B) women who did not receive significant concomitant medications (no significant SDD effect in women using significant concomitant medications, p = 0.5), where the baseline reference group represents all study participants, free of significant concomitant medication use (an eligibility criterion). Each bar represents the mean value, and whiskers are drawn to ± 1 standard error of the mean; subgroup sample size is shown below each bar value. SDD, subantimicrobial-dose doxycycline. ICTP, pyridinoline-crosslink degradation fragment of type I collagen.
Figure 2.
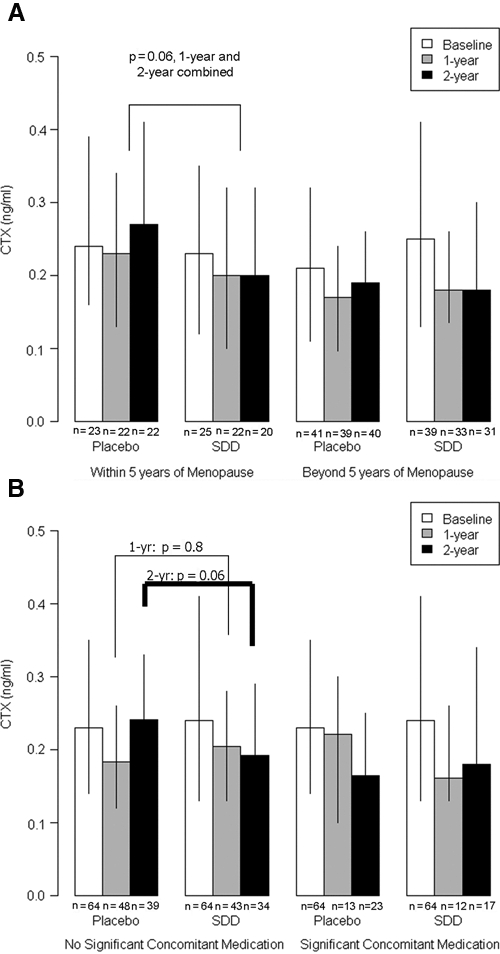
The effect of SDD relative to placebo on CTX levels in serum in (A) subgroups of women within 5 yrs of menopause (no significant SDD effect in women beyond 5 yrs of menopause, p = 0.4) and (B) women who did not receive significant concomitant medications (no significant SDD effect among women using significant concomitant medications, p = 0.8), where the baseline reference group represents all study participants, free of significant concomitant medication use (an eligibility criterion). Each bar represents the median value, and whiskers are drawn to the 25th and 75th percentiles; subgroup sample size is shown below each bar value. SDD, subantimicrobial-dose doxycycline. CTX, deoxypyridinoline-crosslink degradation fragments of type I collagen.
Discussion
Over the past several decades, numerous clinical trials have demonstrated that SDD adjunctive to mechanical debridement significantly reduces the severity of chronic periodontitis (Golub et al., 1998; Giannobile, 2008; Preshaw, 2008). Moreover, the clinical improvements were associated with significant reductions in biomarkers of inflammation and collagen destruction and bone destruction locally in periodontal pockets. These GCF analytes included the matrix metalloproteinases, notably, MMP-8 (collagenase-2), MMP-13 (collagenase-3), and MMP-9 (gelatinase-B), and other neutral proteinases, e.g., plasminogen activator, pro-inflammatory cytokines, EMMPRIN (extracellular MMP inducer, a membrane-bound glycoprotein), and ICTP (Golub et al., 1998; Emingil et al., 2006, 2008; Giannobile, 2008; Preshaw, 2008). SDD also demonstrated efficacy in other periodontal disease categories, including persons with generalized severe periodontitis (Novak et al., 2002), institutionalized geriatric individuals (Mohammad et al., 2005), smokers (Preshaw et al., 2005), persons with diabetes (Ryan et al., 2006), and cardiovascular patients (Tüter et al., 2007).
Recently, we reported that post-menopausal women, who exhibited local as well as mild systemic bone loss (periodontitis and osteopenia, respectively), and who were not treated with bone-sparing agents such as bisphosphonates, also responded favorably to SDD therapy (Payne et al., 2007; Reinhardt et al., 2007) in this two-year clinical trial. These women showed significant reductions in clinical (based on intent-to-treat analyses) and radiological (based on subgroup analyses) measures of periodontal disease severity and progression, with similar adverse events relative to the placebo group. These post-menopausal women also exhibited reductions in biomarkers of inflammation, collagen destruction, and bone resorption locally in the GCF (Golub et al., 2008). However, two studies (see below) also demonstrated a beneficial effect of SDD on systemic inflammatory biomarkers of disease. In this regard, Brown et al. (2004) reported that a six-month regimen of SDD (in the absence of scaling and root planing; SRP) significantly reduced systemic inflammation biomarkers (e.g., plasma C-reactive protein) in individuals with severe cardiovascular disease (CVD), and Tüter et al. (2007) found that a short-term regimen (1½ mos) of SDD, in combination with SRP, reduced clinical measures of periodontitis and improved some systemic CVD biomarkers, including serum levels of HDL cholesterol and APO-A lipoprotein.
Thus, the current study is the first to describe the effects of SDD on biomarkers of systemic bone turnover. In this regard, the two-year regimen of SDD did not produce a significant change in serum levels of either bone-specific alkaline phosphatase, a diagnostic marker of bone formation, or osteocalcin, a secretion product of osteoblasts thought to reflect bone turnover. Recently, it was suggested that the lack of change in GCF osteocalcin levels, in another study on persons with periodontitis (Golub et al. 1997), actually reflected an increased rate of bone formation (balanced by a decrease in bone resorption), consistent with animal models of bone-deficiency disease (ovariectomized aged rat/osteoporosis; diabetes-induced osteopenia) in which tetracyclines enhanced osteoblast activity and bone formation (Bain et al., 1997; Williams et al., 1999). However, in the current clinical trial, the two-year regimen of SDD did not significantly affect either bone formation marker in the serum. Therefore, perhaps tetracyclines increase osteoblast activity and bone formation only in the short term, and this beneficial effect of the drugs may be lost over a prolonged period of treatment, such as 2 yrs.
In contrast to the data on bone formation biomarkers, SDD administration appeared to produce long-term beneficial effects on 2 systemic bone resorption markers in subgroups of these post-menopausal women. Reminiscent of previous observations locally in the GCF of these (and other) women (Golub et al., 1997, 2008), the SDD treatment in the current study did produce a highly statistically significant reduction in ICTP systemically in the serum of women within 5 yrs of menopause, a time-period associated with high-turnover bone loss (Heaney et al., 1978). However, in those women administered SDD beyond this time-period, which is generally associated with low-turnover bone loss (Heaney et al., 1978; Cochran, 2008), the effect of the medication on this bone resorption biomarker was lost. These data were consistent with a similar pattern of change with a second, more current, serum diagnostic biomarker of bone resorption, CTX, and with the observation that the 2 measurements of systemic bone resorption were positively correlated with each other over the two-year clinical trial. Additional evidence of the systemic bone-sparing potential of long-term SDD therapy in these osteopenic women is the observation that the detection of doxycycline in the serum, at a median concentration of 0.59 µg/mL (a level of this drug which, in previous studies, demonstrated efficacy and safety in the management of chronic periodontitis), was associated with a significant reduction of serum CTX. These beneficial effects on systemic bone resorption biomarkers are also consistent with earlier basic studies demonstrating that tetracyclines can inhibit bone resorption: (a) in organ culture, regardless of whether the osteoclastic/bone resorption activity was induced by parathyroid hormone, endotoxin, or prostaglandin E2 (Golub et al., 1984); and (b) in animal models of bone-deficiency disease, including the ovariectomized aged rat model of osteoporosis, and a rat model of type I diabetes-induced osteopenia (Golub et al., 1998; Williams et al., 1999).
Future studies are required to identify more clearly the mechanisms by which SDD (and other tetracyclines) inhibit local and systemic bone loss. Likely candidates include the ability of non-antibiotic properties of these compounds to suppress the expression of pro-inflammatory cytokines including IL-1β and IL-6, dampening osteoclast chemotaxis (Kirkwood et al., 1999). These compounds also may inhibit the expression, by osteoblasts and T- and B-lymphocytes, of RANKL, reducing its availability “to bind RANK on osteoclast precursors tipping the balance” (Cochran, 2008; Giannobile, 2008) against osteoclast differentiation and bone resorption. The initial mechanism proposed was the ability of tetracyclines to block collagenase and other MMPs produced by osteoblasts and pre-osteoclasts (Golub et al., 1998). Of course, the desired ultimate outcome of both periodontal and osteoporosis therapies is to reduce tooth loss and skeletal fractures, respectively. Evidence now suggests that long-term administration of non-antimicrobial tetracyclines may achieve both goals (Zernicke et al., 1997; Cunha-Cruz et al., 2008).
In conclusion, just as the two-year SDD regimen was found to reduce periodontal disease progression (and reduced collagenolytic and bone resorption biomarkers in periodontal pockets) in post-menopausal women (Payne et al., 2007; Reinhardt et al., 2007; Golub et al., 2008), we now propose (based on the reduction of bone resorption markers systemically seen in the current study) that longer term (e.g., 3-5 yrs) host-modulating therapy also may reduce the risk of progression of mild systemic bone loss (osteopenia) into the more serious bone-deficiency disease, osteoporosis. This hypothesis derives support from our earlier studies in a standard animal model of post-menopausal osteoporosis, the ovariectomized aged rat. Treating these rats with a chemically modified (non-antimicrobial) doxycycline, CMT-8: (a) reduced the severity of both systemic osteoporosis (tibia) and local alveolar bone loss; and (b) normalized the pathologically excessive collagenase activity in the gingiva (Golub et al., 1999). Moreover, additional data from our study on post-menopausal osteopenic women (Payne et al., unpublished observations) showed that elevated serum CTX levels (which were reduced by SDD therapy in subgroups) were associated with lumbar spine bone mineral density loss over 2 yrs.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCR/NIH. SDD and placebo tablets were provided by CollaGenex Pharmaceuticals, Inc. (Newtown, PA). LMG is (1) an inventor on SDD patents and these were fully assigned to Stony Brook University, and (2) a consultant to Galderma R & D and Oral Science.
Footnotes
The project was supported by Grant Number R01DE012872 from the NIDCR (JBP, PI and LMG, Co-PI). TS grants were supported by the Academy of Finland and Helsinki University Central Hospital Research Foundation.
References
- Bain S, Ramamurthy NS, Impeduglia T, Scolman S, Golub LM, Rubin C, et al. (1997). Tetracycline prevents cancellous bone loss and maintains near-normal rates of bone formation in streptozotocin diabetic rats. Bone 21:147-153 [DOI] [PubMed] [Google Scholar]
- Barros SP, Silva MA, Somerman MJ, Nociti FH., Jr (2003). Parathyroid hormone protects against periodontitis-associated bone loss. J Dent Res 82:791-795 [DOI] [PubMed] [Google Scholar]
- Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. (2004). Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS): pilot trial. Arterioscler Thromb Vasc Biol 24:733-738 [DOI] [PubMed] [Google Scholar]
- Cochran DL. (2008). Inflammation and bone loss in periodontal disease. J Periodontol 79(8 Suppl):1569S-1576S [DOI] [PubMed] [Google Scholar]
- Cunha-Cruz J, Hujoel PP, Maupome G, Saver B. (2008). Systemic antibiotics and tooth loss in periodontal disease. J Dent Res 87:871-876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emingil G, Gurkan A, Atilla G, Berdeli A, Cinarcik S. (2006). Adjunctive low-dose doxycycline therapy effect on clinical parameters and gingival crevicular fluid tissue plasminogen activator levels in chronic periodontitis. Inflamm Res 55:550-558 [DOI] [PubMed] [Google Scholar]
- Emingil G, Atilla G, Sorsa T, Tervahartiala T. (2008). The effect of adjunctive subantimicrobial dose doxycycline therapy on GCF EMMPRIN levels in chronic periodontitis. J Periodontol 79:469-476 [DOI] [PubMed] [Google Scholar]
- Giannobile WV. (2008). Host-response therapeutics for periodontal diseases. J Periodontol 79(8 Suppl):1592S-1600S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub LM, Ramamurthy N, McNamara TF, Gomes B, Wolff M, Casino A, et al. (1984). Tetracyclines inhibit tissue collagenase activity: a new mechanism in the treatment of periodontal disease. J Periodontal Res 19:651-655 [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, et al. (1997). A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res 46:310-319 [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. (1998). Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res 12:12-26 [DOI] [PubMed] [Google Scholar]
- Golub LM, Ramamurthy NS, Llavaneras A, Ryan ME, Lee HM, Liu Y, et al. (1999). A chemically modified nonantimicrobial tetracycline (CMT-8) inhibits gingival matrix metalloproteinases, periodontal breakdown, and extra-oral bone loss in a ovariectomized rats. Ann NY Acad Sci 878:290-310 [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Stoner JA, Sorsa T, Reinhardt RA, Wolff MS, et al. (2008). Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol 79:1409-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney RP, Recker RR, Saville PD. (1978). Menopausal changes in bone remodeling. J Lab Clin Med 92:964-970 [PubMed] [Google Scholar]
- Kirkwood KL, Golub LM, Bradford PG. (1999). Non-antimicrobial and antimicrobial tetracyclines inhibit IL-6 expression in murine osteoblasts. Ann NY Acad Sci 878:667-670 [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. (1986). Longitudinal data analysis using generalized linear models. Biometrika 73:13-22 [Google Scholar]
- Liu Y, Ramamurthy N, Marecek J, Lee HM, Chen JL, Ryan ME, et al. (2001). The lipophilicity, pharmacokinetics, and cellular uptake of different chemically-modified tetracyclines (CMTS). Curr Med Chem 8:243-252 [DOI] [PubMed] [Google Scholar]
- Looker AC, Bauer DC, Chesnut CH, 3rd, Gundberg CM, Hochberg MC, Klee G, et al. (2000). Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int 11:467-480 [DOI] [PubMed] [Google Scholar]
- Mohammad AR, Preshaw PM, Bradshaw MH, Hefti AF, Powala CV, Romanowicz M. (2005). Adjunctive subantimicrobial dose doxycycline in the management of institutionalized geriatric patients with chronic periodontitis. Gerodontology 22:37-43 [DOI] [PubMed] [Google Scholar]
- Novak MJ, Johns LP, Miller RC, Bradshaw MH. (2002). Adjunctive benefits of subantimicrobial dose doxycycline in the management of severe generalized chronic periodontitis. J Periodontol 73:762-769 [DOI] [PubMed] [Google Scholar]
- Payne JB, Stoner JA, Nummikoski PV, Reinhardt RA, Goren AD, Wolff MS, et al. (2007). Subantimicrobial dose doxycycline effects on alveolar bone loss in postmenopausal women. J Clin Periodontol 34:776-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzoni M, Alfano FS, Girasole G, Giuliani N, Fantuzzi M, Gatti C, et al. (1996). Clinical observations with a new specific assay for bone alkaline phosphatase: a cross-sectional study in osteoporotic and pagetic subjects and a longitudinal evaluation of the esponse to ovariectomy, estrogens, and bisphosphonates. Calcif Tissue Int 59:334-338 [DOI] [PubMed] [Google Scholar]
- Preshaw PM. (2008). Host response modulation in periodontics. Periodontol 2000 48:92-110 [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Hefti AF, Bradshaw MH. (2005). Adjunctive sub-antimicrobial dose doxycycline in smokers and non-smokers with chronic periodontitis. J Clin Periodontol 32:610-616 [DOI] [PubMed] [Google Scholar]
- Reinhardt RA, Stoner JA, Golub LM, Wolff MS, Lee HM, Meinberg TA, et al. (2007). Efficacy of subantimicrobial dose doxycycline in postmenopausal women: clinical outcomes. J Clin Periodontol 34:768-775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M, Carnu O, Tenzler R. (2006). The impact of periodontitis on metabolic control and risk for diabetic complications. Grand Rounds Oral- Sys Med 2:24-34a [Google Scholar]
- Tenenbaum HC, Shelemay A, Girard B, Zohar R, Fritz PC. (2002). Bisphosphonates and periodontics: potential applications for regulation of bone mass in the periodontium and other therapeutic/diagnostic uses. J Periodontol 73:813-822 [DOI] [PubMed] [Google Scholar]
- Tüter G, Kurtis B, Serdar M, Aykan T, Okyay K, Yücel A, et al. (2007). Effects of scaling and root planing and sub-antimicrobial dose doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. J Clin Periodontol 34:673-681 [DOI] [PubMed] [Google Scholar]
- Van Dyke T. (2008). The management of inflammation in periodontal disease. J Periodontol 79(8 Suppl):1601S-1608S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C, Puumala S, Golub LM, Stoner JA, Reinhardt RA, Lee HM, et al. (2007). Subantimicrobial dose doxycycline effects on osteopenic bone loss: microbiologic results. J Periodontol 78:1590-1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RC, Jeffcoat MK, Howell TH, Rolla A, Stubbs D, Teoh KW, et al. (1989). Altering the progression of human alveolar bone loss with the nonsteroidal anti-inflammatory drug flurbiprofen. J Periodontol 60:485-490 [DOI] [PubMed] [Google Scholar]
- Williams S, Barnes J, Wakisaka A, Ogasa H, Liang CT. (1999). Treatment of osteoporosis with MMP inhibitors. Ann NY Acad Sci 878:191-200 [DOI] [PubMed] [Google Scholar]
- Zernicke RF, Wohl GR, Greenwald RA, Moak SA, Leng W, Golub LM. (1997). Administration of systemic matrix-metalloproteinase inhibitors maintains bone mechanical integrity in adjuvant arthritis. J Rheumatol 24:1324-1331 [PubMed] [Google Scholar]


