Abstract
Cytochrome P450 content and activities are increased in the kidneys of spontaneously hypertensive rats (SHR) as compared with those of normotensive, Wistar-Kyoto (WKY), control rats during the period of rapid elevation of blood pressure. We studied the effect of heme arginate, a potent inducer of heme oxygenase (EC 1.14.99.3), on microsomal cytochrome P450 levels and activities and blood pressure in SHR at 7 wk of age. Administration of heme arginate (15 mg/kg body weight for 4 d) resulted in a marked decrease in blood pressure from 156.3 +/- 4.7 to 129.8 +/- 4.5 mm Hg (P less than 0.001), whereas blood pressure in SHR receiving the vehicle control was not affected. The blood pressure of age-matched WKY was not affected by heme arginate. Heme oxygenase activity increased in both hepatic and renal microsomes of SHR and WKY by two- to four-fold after treatment with heme arginate. Maximal increase of heme oxygenase mRNA occurred 5-7 h after the last injection of heme arginate and returned to control levels after 24 h. The increase in heme oxygenase activity was associated with a parallel decrease in cytochrome P450 content and in the activity of cytochrome P450 omega/omega-1 arachidonate hydroxylases in kidneys of SHR. It is postulated that heme arginate treatment resulted in induction of heme oxygenase which consequently led to a diminution of cytochrome P450, especially the arachidonate omega/omega-1 hydroxylases leading to a marked decrease in 19-hydroxyeicosatetraenoic acid (HETE) and 20-HETE. The effect of heme arginate on blood pressure may be mediated via these biochemical events inasmuch as both 19-HETE and 20-HETE produced by the kidney may promote hypertension by causing vasoconstriction and sodium retention.
Full text
PDF

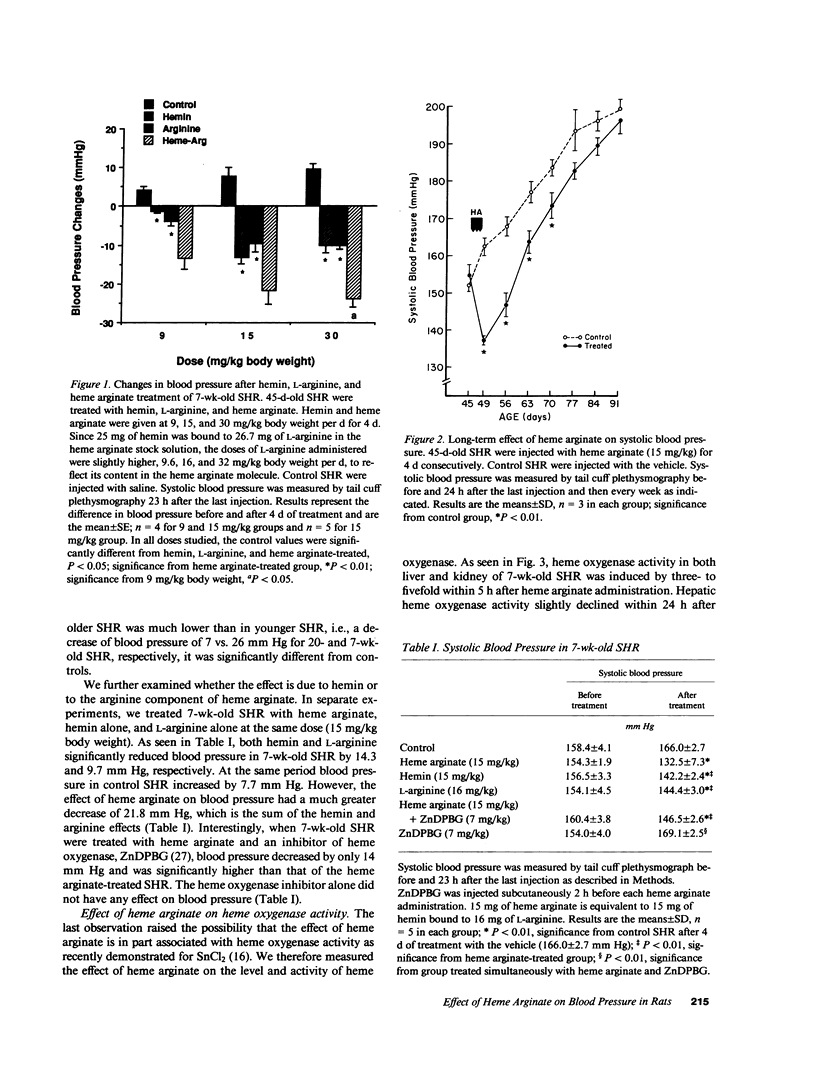
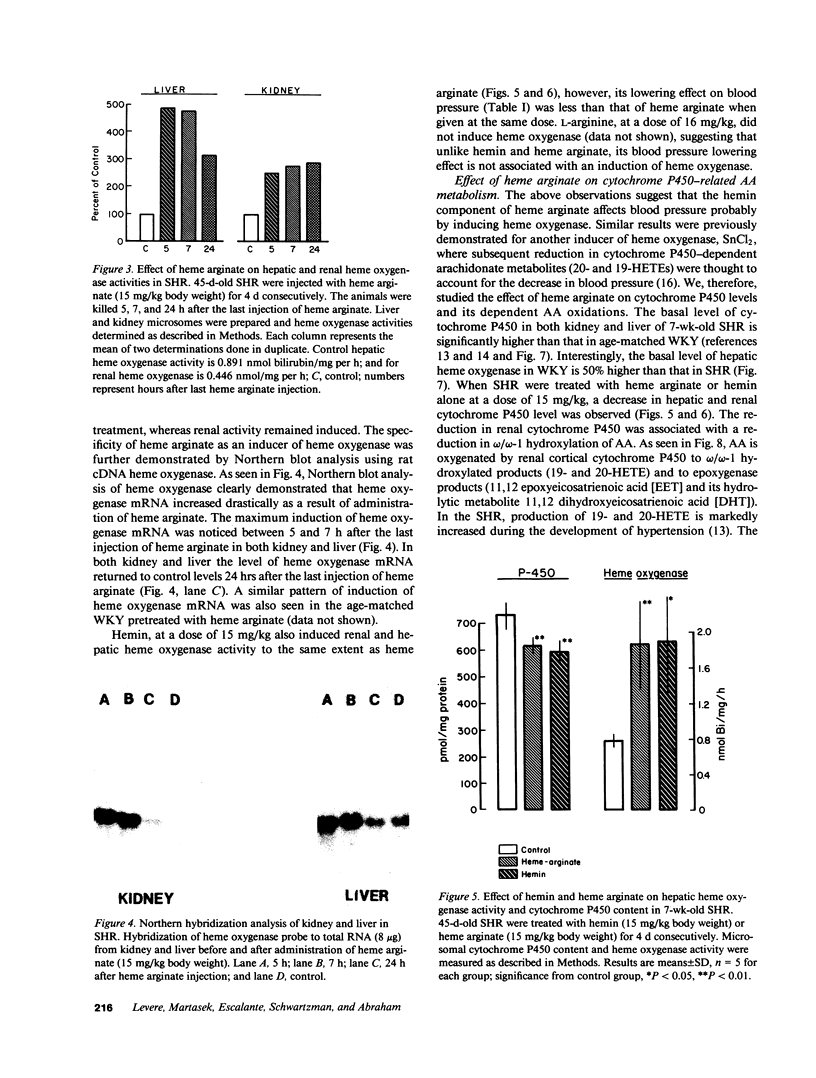
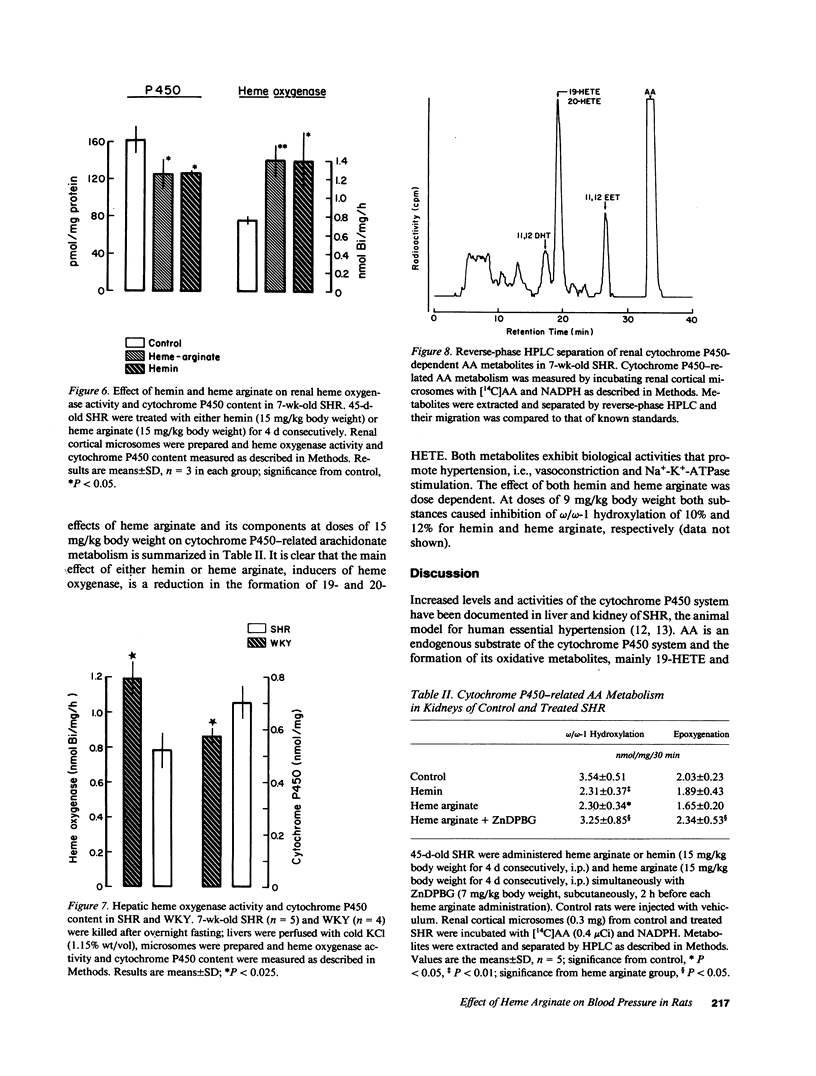


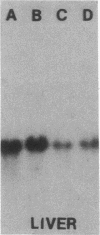
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Lin J. H., Mitrione S. M., Schwartzman M. L., Levere R. D., Shibahara S. Expression of heme oxygenase gene in rat and human liver. Biochem Biophys Res Commun. 1988 Jan 29;150(2):717–722. doi: 10.1016/0006-291x(88)90450-0. [DOI] [PubMed] [Google Scholar]
- Abraham N. G., Lin J. H., Schwartzman M. L., Levere R. D., Shibahara S. The physiological significance of heme oxygenase. Int J Biochem. 1988;20(6):543–558. doi: 10.1016/0020-711x(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Abraham N. G., Lutton J. D., Freedman M. L., Levere R. D. Benzene modulation of liver cell structure and heme-cytochrome P-450 metabolism. Am J Med Sci. 1986 Aug;292(2):81–86. doi: 10.1097/00000441-198608000-00003. [DOI] [PubMed] [Google Scholar]
- Aisaka K., Gross S. S., Griffith O. W., Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun. 1989 Apr 28;160(2):881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- Beierwaltes W. H., Arendshorst W. J., Klemmer P. J. Electrolyte and water balance in young spontaneously hypertensive rats. Hypertension. 1982 Nov-Dec;4(6):908–915. doi: 10.1161/01.hyp.4.6.908. [DOI] [PubMed] [Google Scholar]
- Dilley J. R., Stier C. T., Jr, Arendshorst W. J. Abnormalities in glomerular function in rats developing spontaneous hypertension. Am J Physiol. 1984 Jan;246(1 Pt 2):F12–F20. doi: 10.1152/ajprenal.1984.246.1.F12. [DOI] [PubMed] [Google Scholar]
- Escalante B., Falck J. R., Yadagiri P., Sun L. M., Laniado-Schwartzman M. 19(S)-hydroxyeicosatetraenoic acid is a potent stimulator of renal Na+-K+-ATPase. Biochem Biophys Res Commun. 1988 May 16;152(3):1269–1274. doi: 10.1016/s0006-291x(88)80422-4. [DOI] [PubMed] [Google Scholar]
- Escalante B., Sessa W. C., Falck J. R., Yadagiri P., Schwartzman M. L. Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. J Pharmacol Exp Ther. 1989 Jan;248(1):229–232. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ibraham N. G., Friedland M. L., Levere R. D. Heme metabolism in erythroid and hepatic cells. Prog Hematol. 1983;13:75–130. [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Kappas A., Drummond G. S. Control of heme metabolism with synthetic metalloporphyrins. J Clin Invest. 1986 Feb;77(2):335–339. doi: 10.1172/JCI112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe K., Watanabe T. X., Shiono K., Sokabe H. Influence on blood pressure of renal isografts between spontaneously hypertensive and normotensive rats, utilizing the F1 hybrids. Jpn Heart J. 1978 Nov;19(6):886–894. doi: 10.1536/ihj.19.886. [DOI] [PubMed] [Google Scholar]
- Kordac V., Kozáková M., Martásek P. Changes of myocardial functions in acute hepatic porphyrias. Role of heme arginate administration. Ann Med. 1989 Aug;21(4):273–276. doi: 10.3109/07853898909149205. [DOI] [PubMed] [Google Scholar]
- Kordac V., Martásek P. Haem arginate in acute hepatic porphyrias. Br Med J (Clin Res Ed) 1986 Oct 25;293(6554):1098–1098. doi: 10.1136/bmj.293.6554.1098-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martasek P., Solangi K., Goodman A. I., Levere R. D., Chernick R. J., Abraham N. G. Properties of human kidney heme oxygenase: inhibition by synthetic heme analogues and metalloporphyrins. Biochem Biophys Res Commun. 1988 Dec 15;157(2):480–487. doi: 10.1016/s0006-291x(88)80274-2. [DOI] [PubMed] [Google Scholar]
- Merrick B. A., Davies M. H., Cook D. E., Holcslaw T. L., Schnell R. C. Alterations in hepatic microsomal drug metabolism and cytochrome P-450 proteins in spontaneously hypertensive rats. Pharmacology. 1985;30(3):129–135. doi: 10.1159/000138061. [DOI] [PubMed] [Google Scholar]
- Mustajoki P., Tenhunen R., Tokola O., Gothoni G. Haem arginate in the treatment of acute hepatic porphyrias. Br Med J (Clin Res Ed) 1986 Aug 30;293(6546):538–539. doi: 10.1136/bmj.293.6546.538-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO K., AOKI K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963 Mar;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdoti D., Abraham N. G., McGiff J. C., Schwartzman M. L. Renal cytochrome P-450-dependent metabolism of arachidonic acid in spontaneously hypertensive rats. Biochem Pharmacol. 1988 Feb 1;37(3):521–527. doi: 10.1016/0006-2952(88)90223-7. [DOI] [PubMed] [Google Scholar]
- Sacerdoti D., Escalante B., Abraham N. G., McGiff J. C., Levere R. D., Schwartzman M. L. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989 Jan 20;243(4889):388–390. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- Schwartzman M. L., Abraham N. G., Carroll M. A., Levere R. D., McGiff J. C. Regulation of arachidonic acid metabolism by cytochrome P-450 in rabbit kidney. Biochem J. 1986 Aug 15;238(1):283–290. doi: 10.1042/bj2380283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Müller R. M., Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987 Sep 25;262(27):12889–12892. [PubMed] [Google Scholar]
- Shibahara S., Müller R., Taguchi H., Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionatto C. S., Anderson K. E., Drummond G. S., Kappas A. Studies on the mechanism of Sn-protoporphyrin suppression of hyperbilirubinemia. Inhibition of heme oxidation and bilirubin production. J Clin Invest. 1985 Feb;75(2):513–521. doi: 10.1172/JCI111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970 Mar;75(3):410–421. [PubMed] [Google Scholar]