Abstract
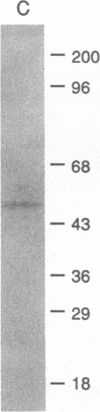
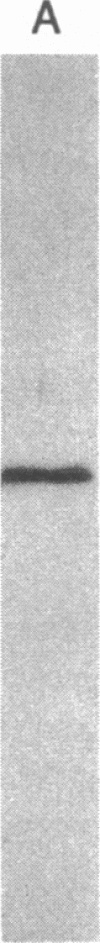
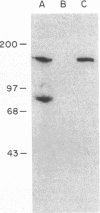
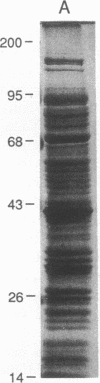
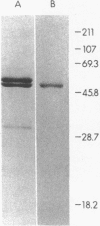
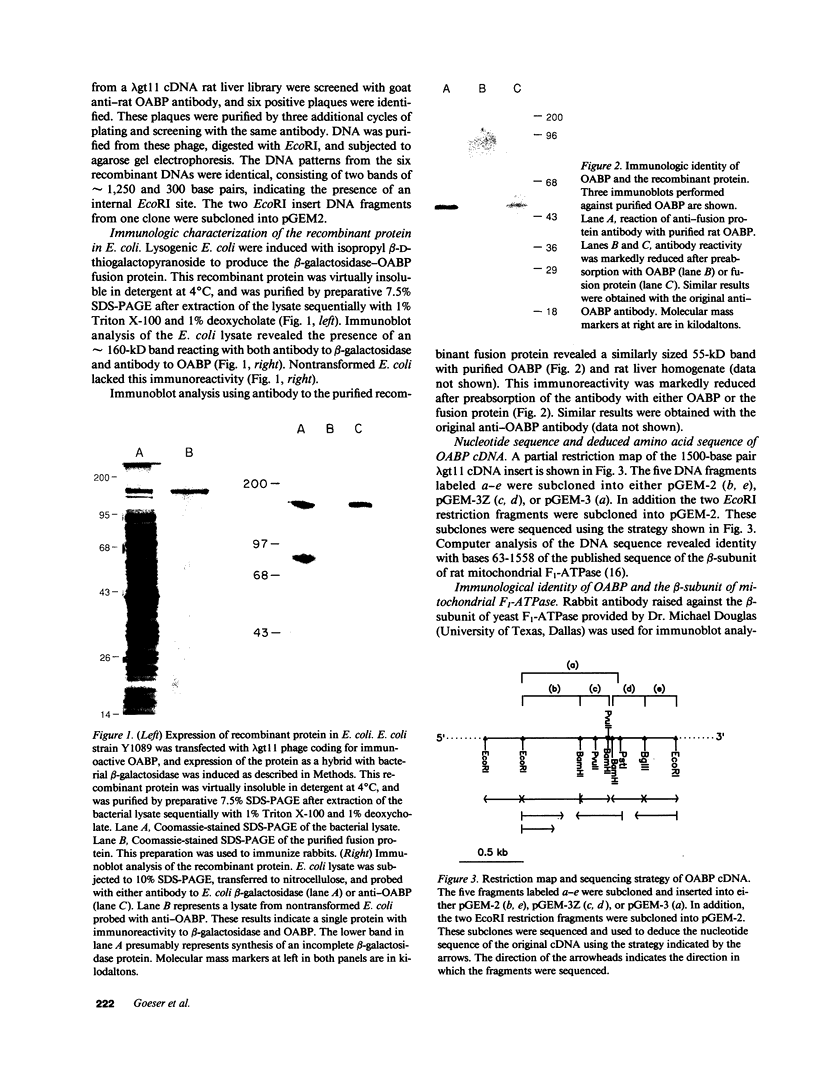
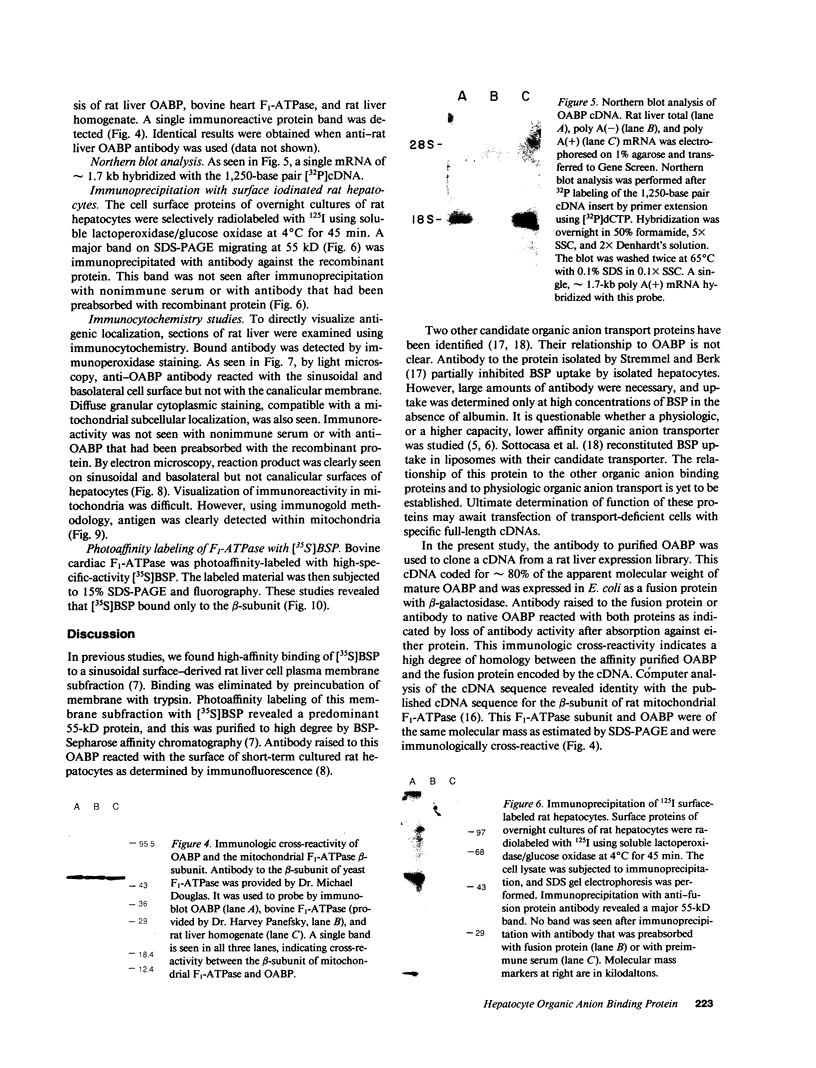
A 55-kD organic anion binding protein (OABP) was identified previously in liver cell plasma membrane sinusoidal subfractions. Although this protein was localized to the surface of hepatocytes by immunofluorescence, immunoblot analysis revealed reactivity toward both plasma membrane and mitochondrial fractions. To clarify these findings, an immunoreactive clone from a rat liver cDNA expression library was isolated, the 1,500-base pair cDNA insert was sequenced, and the corresponding beta-galactosidase fusion protein was expressed and purified. The resulting sequence corresponded to that of the rat mitochondrial F1-adenosine triphosphatase (F1-ATPase) beta-subunit. This protein and OABP are of similar size and are mutually immunologically cross-reactive. That the antigen was present on the cell surface as well as in mitochondria was suggested from studies of immunoprecipitation after cell-surface iodination, and light- and electron-microscopic immunocytochemistry. Photoaffinity labeling of bovine F1-ATPase with high-specific-activity [35S]sulfobromophthalein revealed binding only to the beta-subunit. Hepatocyte uptake of bilirubin and sulfobromophthalein requires cellular ATP and mitochondria also transport these organic anions, which at high doses inhibit respiration. The presence of an organic anion binding site on the F1-ATPase beta-subunit suggests that it may play a role in these processes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burr R., Schwenk M., Pfaff E. Interaction of bromosulfophthalein with mitochondrial membranes--inhibition of respiration. Biochem Pharmacol. 1977 Mar 15;26(6):461–466. doi: 10.1016/0006-2952(77)90317-3. [DOI] [PubMed] [Google Scholar]
- Burr R., Schwenk M., Pfaff E. Interaction of bromosulfophthalein with mitochondrial membranes--uptake of bromosulfophthalein and effect on ANS-fluorescence. Biochem Pharmacol. 1977 Mar 15;26(6):457–460. doi: 10.1016/0006-2952(77)90316-1. [DOI] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Eghbali M., Giambrone M. A., Eghbali M., Zern M. A. Differential effects of gamma-interferon on collagen and fibronectin gene expression. J Biol Chem. 1987 Sep 25;262(27):13348–13351. [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORESKY C. A. INITIAL DISTRIBUTION AND RATE OF UPTAKE OF SULFOBROMOPHTHALEIN IN THE LIVER. Am J Physiol. 1964 Jul;207:13–26. doi: 10.1152/ajplegacy.1964.207.1.13. [DOI] [PubMed] [Google Scholar]
- Garboczi D. N., Fox A. H., Gerring S. L., Pedersen P. L. Beta subunit of rat liver mitochondrial ATP synthase: cDNA cloning, amino acid sequence, expression in Escherichia coli, and structural relationship to adenylate kinase. Biochemistry. 1988 Jan 26;27(2):553–560. doi: 10.1021/bi00402a008. [DOI] [PubMed] [Google Scholar]
- Ghadiminejad I., Baum H. Evidence for the cell-surface localization of antigens cross-reacting with the "mitochondrial antibodies" of primary biliary cirrhosis. Hepatology. 1987 Jul-Aug;7(4):743–749. doi: 10.1002/hep.1840070421. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kurisu H., Nilprabhassorn P., Wolkoff A. W. Preparation of [35S]sulfobromophthalein of high specific activity. Anal Biochem. 1989 May 15;179(1):72–74. doi: 10.1016/0003-2697(89)90202-9. [DOI] [PubMed] [Google Scholar]
- Lan J. A., Chervu L. R., Johansen K. L., Wolkoff A. W. Uptake of technetium 99m hepatobiliary imaging agents by cultured rat hepatocytes. Gastroenterology. 1988 Dec;95(6):1625–1631. doi: 10.1016/s0016-5085(88)80087-8. [DOI] [PubMed] [Google Scholar]
- Laperche Y., Oudea P. Inhibition by sulfobromophthalein of mitochondrial translocation of anions and adenine nucleotides: effects upon liver adenosine triphosphate and possible correlation with inhibition of bile flow in the rat. J Pharmacol Exp Ther. 1976 Apr;197(1):235–244. [PubMed] [Google Scholar]
- Mihara K., Omura T., Harano T., Brenner S., Fleischer S., Rajagopalan K. V., Blobel G. Rat liver L-glutamate dehydrogenase, malate dehydrogenase, D-beta-hydroxybutyrate dehydrogenase, and sulfite oxidase are each synthesized as larger precursors by cytoplasmic free polysomes. J Biol Chem. 1982 Apr 10;257(7):3355–3358. [PubMed] [Google Scholar]
- Novikoff P. M., La Russo N. F., Novikoff A. B., Stockert R. J., Yam A., Le Sage G. D. Immunocytochemical localization of lysosomal beta-galactosidase in rat liver. J Cell Biol. 1983 Nov;97(5 Pt 1):1559–1565. doi: 10.1083/jcb.97.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Kagawa Y. Human F1-ATPase: molecular cloning of cDNA for the beta subunit. J Biochem. 1986 Jan;99(1):135–141. doi: 10.1093/oxfordjournals.jbchem.a135452. [DOI] [PubMed] [Google Scholar]
- Paietta E., Stockert R. J., Morell A. G., Diehl V., Wiernik P. H. Unique antigen of cultured Hodgkin's cells. A putative sialyltransferase. J Clin Invest. 1986 Aug;78(2):349–354. doi: 10.1172/JCI112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt B. F., Waggoner J. G., Berk P. D. Hepatic organic anion uptake in the rat. J Clin Invest. 1975 Nov;56(5):1280–1292. doi: 10.1172/JCI108204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Baldini G., Sandri G., Lunazzi G., Tiribelli C. Reconstitution in vitro of sulfobromophthalein transport by bilitranslocase. Biochim Biophys Acta. 1982 Feb 23;685(2):123–128. doi: 10.1016/0005-2736(82)90088-8. [DOI] [PubMed] [Google Scholar]
- Stollman Y. R., Gärtner U., Theilmann L., Ohmi N., Wolkoff A. W. Hepatic bilirubin uptake in the isolated perfused rat liver is not facilitated by albumin binding. J Clin Invest. 1983 Aug;72(2):718–723. doi: 10.1172/JCI111021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Berk P. D. Hepatocellular uptake of sulfobromophthalein and bilirubin is selectively inhibited by an antibody to the liver plasma membrane sulfobromophthalein/bilirubin binding protein. J Clin Invest. 1986 Sep;78(3):822–826. doi: 10.1172/JCI112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Fearnley I. M., Gay N. J., Gibson B. W., Northrop F. D., Powell S. J., Runswick M. J., Saraste M., Tybulewicz V. L. Primary structure and subunit stoichiometry of F1-ATPase from bovine mitochondria. J Mol Biol. 1985 Aug 20;184(4):677–701. doi: 10.1016/0022-2836(85)90313-4. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Chung C. T. Identification, purification, and partial characterization of an organic anion binding protein from rat liver cell plasma membrane. J Clin Invest. 1980 May;65(5):1152–1161. doi: 10.1172/JCI109770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Klausner R. D., Ashwell G., Harford J. Intracellular segregation of asialoglycoproteins and their receptor: a prelysosomal event subsequent to dissociation of the ligand-receptor complex. J Cell Biol. 1984 Feb;98(2):375–381. doi: 10.1083/jcb.98.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Samuelson A. C., Johansen K. L., Nakata R., Withers D. M., Sosiak A. Influence of Cl- on organic anion transport in short-term cultured rat hepatocytes and isolated perfused rat liver. J Clin Invest. 1987 Apr;79(4):1259–1268. doi: 10.1172/JCI112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Sosiak A., Greenblatt H. C., Van Renswoude J., Stockert R. J. Immunological studies of an organic anion-binding protein isolated from rat liver cell plasma membrane. J Clin Invest. 1985 Aug;76(2):454–459. doi: 10.1172/JCI111993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W. The role of an albumin receptor in hepatic organic anion uptake: the controversy continues. Hepatology. 1987 Jul-Aug;7(4):777–779. doi: 10.1002/hep.1840070427. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]