Abstract
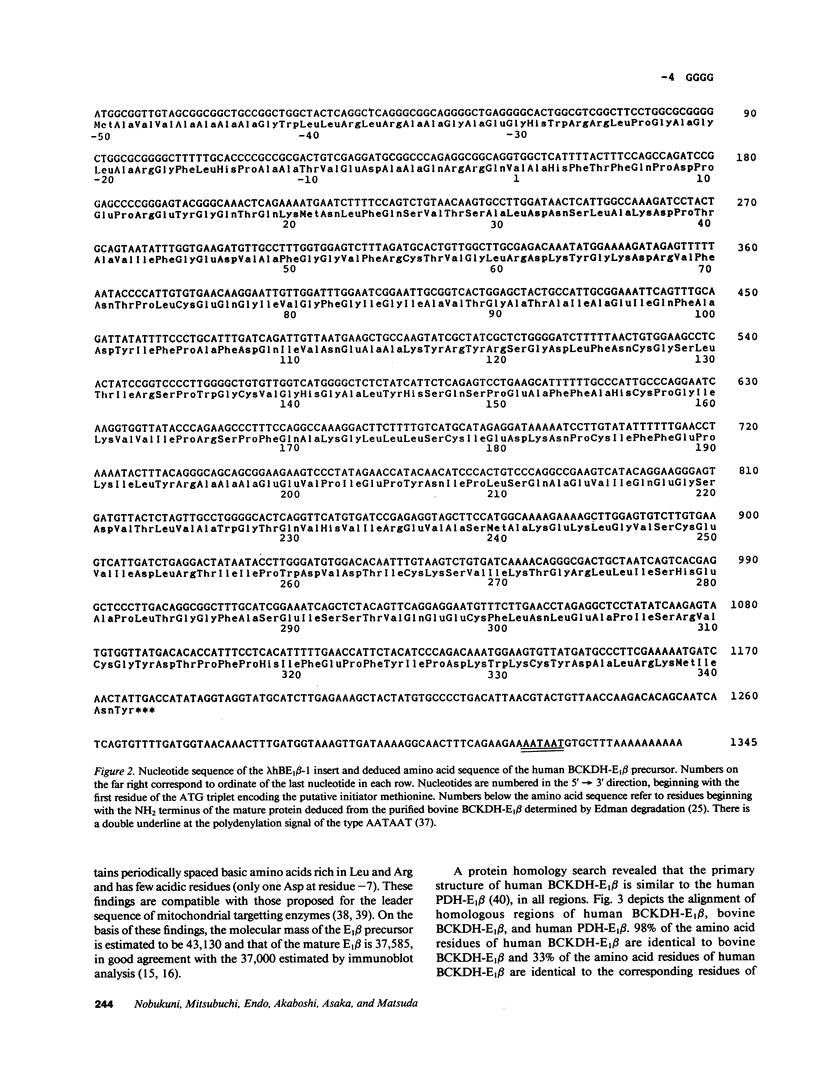
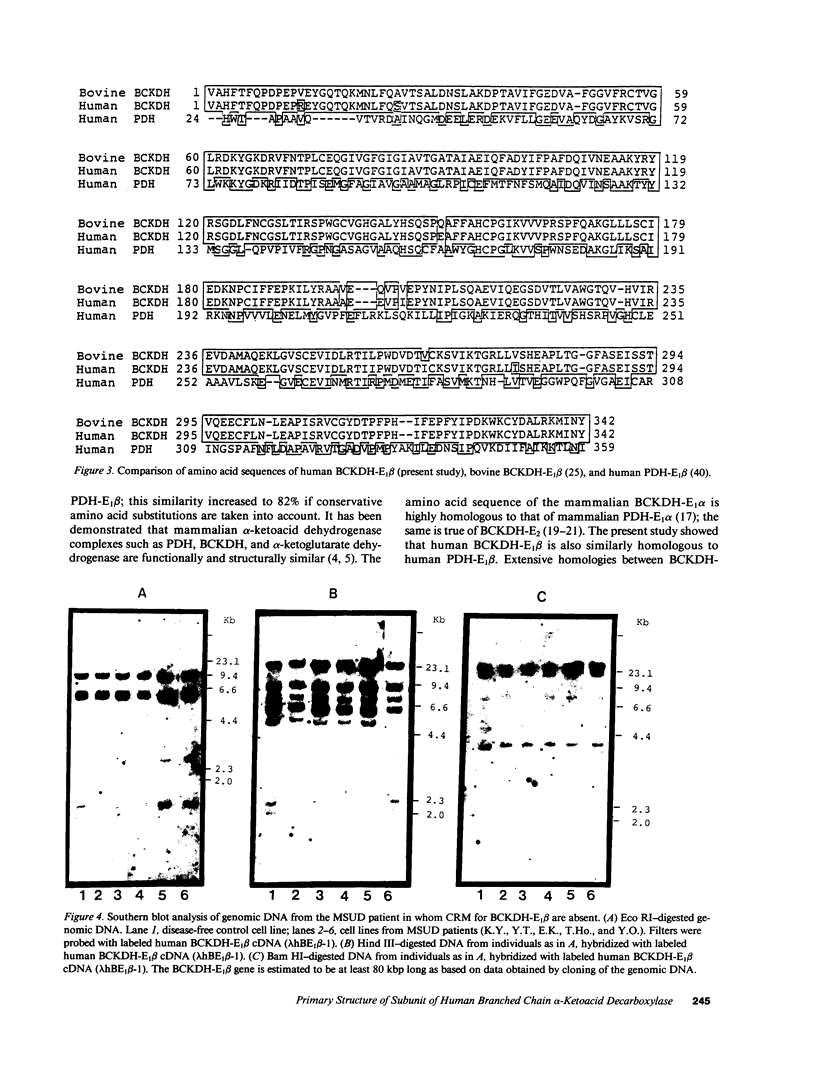
A defect in the E1 beta subunit of the branched chain alpha-ketoacid dehydrogenase (BCKDH) complex is one cause of maple syrup urine disease (MSUD). In an attempt to elucidate the molecular basis of MSUD, we isolated and characterized a 1.35 kbp cDNA clone encoding the entire precursor of the E1 beta subunit of BCKDH complex from a human placental cDNA library. Nucleotide sequence analysis revealed that the isolated cDNA clone (lambda hBE1 beta-1) contained a 5'-untranslated sequence of four nucleotides, the translated sequence of 1,176 nucleotides and the 3'-untranslated sequence of 169 nucleotides. Comparison of the amino acid sequence predicted from the nucleotide sequence of the cDNA insert of the clone with the NH2-terminal amino acid sequence of the purified mature bovine BCKDH-E1 beta subunit showed that the cDNA insert encodes for a 342-amino acid subunit with a Mr = 37,585. The subunit is synthesized as the precursor with a leader sequence of 50 amino acids and is processed at the NH2 terminus. A search for protein homology revealed that the primary structure of human BCKDH-E1 beta was similar to the bovine BCKDH-E1 beta and to the E1 beta subunit of human pyruvate dehydrogenase complex, in all regions. The structures and functions of mammalian alpha-ketoacid dehydrogenase complexes are apparently highly conserved. Genomic DNA from lymphoblastoid cell lines derived from normal and five MSUD patients, in whom E1 beta was not detected by immunoblot analysis, gave the same restriction maps on Southern blot analysis. The gene has at least 80 kbp.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Cook K. G., Bradford A. P., Yeaman S. J., Aitken A., Fearnley I. M., Walker J. E. Regulation of bovine kidney branched-chain 2-oxoacid dehydrogenase complex by reversible phosphorylation. Eur J Biochem. 1984 Dec 17;145(3):587–591. doi: 10.1111/j.1432-1033.1984.tb08597.x. [DOI] [PubMed] [Google Scholar]
- Cook K. G., Bradford A. P., Yeaman S. J. Resolution and reconstitution of bovine kidney branched-chain 2-oxo acid dehydrogenase complex. Biochem J. 1985 Feb 1;225(3):731–735. doi: 10.1042/bj2250731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. G., Lawson R., Yeaman S. J. Multi-site phosphorylation of branched-chain 2-oxoacid dehydrogenase complex within mitochondria isolated from rat liver, kidney and heart. FEBS Lett. 1983 Nov 28;164(1):85–88. doi: 10.1016/0014-5793(83)80024-6. [DOI] [PubMed] [Google Scholar]
- Damuni Z., Merryfield M. L., Humphreys J. S., Reed L. J. Purification and properties of branched-chain alpha-keto acid dehydrogenase phosphatase from bovine kidney. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4335–4338. doi: 10.1073/pnas.81.14.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Armstrong N., Heffelfinger S. C., Sewell E. T., Priest J. H., Elsas L. J. Absence of branched chain acyl-transferase as a cause of maple syrup urine disease. J Clin Invest. 1985 Mar;75(3):858–860. doi: 10.1172/JCI111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Besharse J. C., Elsas L. J., 2nd Purification and characterization of branched chain alpha-ketoacid dehydrogenase from bovine liver mitochondria. J Biol Chem. 1979 Jun 25;254(12):5522–5526. [PubMed] [Google Scholar]
- Danner D. J., Litwer S., Herring W. J., Pruckler J. Construction and nucleotide sequence of a cDNA encoding the full-length preprotein for human branched chain acyltransferase. J Biol Chem. 1989 May 5;264(13):7742–7746. [PubMed] [Google Scholar]
- Fatania H. R., Patston P. A., Randle P. J. Dephosphorylation and reactivation of phosphorylated purified ox-kidney branched-chain dehydrogenase complex by co-purified phosphatase. FEBS Lett. 1983 Jul 25;158(2):234–238. doi: 10.1016/0014-5793(83)80585-7. [DOI] [PubMed] [Google Scholar]
- Fisher C. W., Chuang J. L., Griffin T. A., Lau K. S., Cox R. P., Chuang D. T. Molecular phenotypes in cultured maple syrup urine disease cells. Complete E1 alpha cDNA sequence and mRNA and subunit contents of the human branched chain alpha-keto acid dehydrogenase complex. J Biol Chem. 1989 Feb 25;264(6):3448–3453. [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Danner D. J. Identification of specific subunits of highly purified bovine liver branched-chain ketoacid dehydrogenase. Biochemistry. 1983 Nov 22;22(24):5519–5522. doi: 10.1021/bi00293a011. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hummel K. B., Litwer S., Bradford A. P., Aitken A., Danner D. J., Yeaman S. J. Nucleotide sequence of a cDNA for branched chain acyltransferase with analysis of the deduced protein structure. J Biol Chem. 1988 May 5;263(13):6165–6168. [PubMed] [Google Scholar]
- Indo Y., Akaboshi I., Nobukuni Y., Endo F., Matsuda I. Maple syrup urine disease: a possible biochemical basis for the clinical heterogeneity. Hum Genet. 1988 Sep;80(1):6–10. doi: 10.1007/BF00451447. [DOI] [PubMed] [Google Scholar]
- Indo Y., Kitano A., Endo F., Akaboshi I., Matsuda I. Altered kinetic properties of the branched-chain alpha-keto acid dehydrogenase complex due to mutation of the beta-subunit of the branched-chain alpha-keto acid decarboxylase (E1) component in lymphoblastoid cells derived from patients with maple syrup urine disease. J Clin Invest. 1987 Jul;80(1):63–70. doi: 10.1172/JCI113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Ohta S., Urata Y., Kagawa Y., Koike M. Cloning and sequencing of cDNAs encoding alpha and beta subunits of human pyruvate dehydrogenase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):41–45. doi: 10.1073/pnas.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. S., Fatania H. R., Randle P. J. Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett. 1982 Jul 19;144(1):57–62. doi: 10.1016/0014-5793(82)80568-1. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Griffin T. A., Hu C. W., Chuang D. T. Conservation of primary structure in the lipoyl-bearing and dihydrolipoyl dehydrogenase binding domains of mammalian branched-chain alpha-keto acid dehydrogenase complex: molecular cloning of human and bovine transacylase (E2) cDNAs. Biochemistry. 1988 Mar 22;27(6):1972–1981. doi: 10.1021/bi00406a025. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Phillips C. E., Randle P. J. Multi-site phosphorylation in ox-kidney branched-chain 2-oxoacid dehydrogenase complex. FEBS Lett. 1983 Aug 22;160(1-2):149–152. doi: 10.1016/0014-5793(83)80955-7. [DOI] [PubMed] [Google Scholar]
- Litwer S., Herring W. J., Danner D. J. Reversion of the maple syrup urine disease phenotype of impaired branched chain alpha-ketoacid dehydrogenase complex activity in fibroblasts from an affected child. J Biol Chem. 1989 Sep 5;264(25):14597–14600. [PubMed] [Google Scholar]
- Matsuda I., Yamamoto J., Nagata N., Ninomiya N., Akaboshi I. Lysosomal enzyme activities in cultured lymphoid cell lines. Clin Chim Acta. 1977 Nov 1;80(3):483–486. doi: 10.1016/0009-8981(77)90141-3. [DOI] [PubMed] [Google Scholar]
- Nobukuni Y., Mitsubuchi H., Endo F., Asaka J., Oyama R., Titani K., Matsuda I. Isolation and characterization of a complementary DNA clone coding for the E1 beta subunit of the bovine branched-chain alpha-ketoacid dehydrogenase complex: complete amino acid sequence of the precursor protein and its proteolytic processing. Biochemistry. 1990 Feb 6;29(5):1154–1160. doi: 10.1021/bi00457a009. [DOI] [PubMed] [Google Scholar]
- Nobukuni Y., Mitsubuchi H., Endo F., Matsuda I. Complete primary structure of the transacylase (E2b) subunit of the human branched chain alpha-keto acid dehydrogenase complex. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1035–1041. doi: 10.1016/0006-291x(89)91347-8. [DOI] [PubMed] [Google Scholar]
- Odessey R. Purification of rat kidney branched-chain oxo acid dehydrogenase complex with endogenous kinase activity. Biochem J. 1982 Apr 15;204(1):353–356. doi: 10.1042/bj2040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otulakowski G., Robinson B. H. Isolation and sequence determination of cDNA clones for porcine and human lipoamide dehydrogenase. Homology to other disulfide oxidoreductases. J Biol Chem. 1987 Dec 25;262(36):17313–17318. [PubMed] [Google Scholar]
- Paxton R., Harris R. A. Isolation of rabbit liver branched chain alpha-ketoacid dehydrogenase and regulation by phosphorylation. J Biol Chem. 1982 Dec 10;257(23):14433–14439. [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons G., Raefsky-Estrin C., Carothers D. J., Pepin R. A., Javed A. A., Jesse B. W., Ganapathi M. K., Samols D., Patel M. S. Cloning and cDNA sequence of the dihydrolipoamide dehydrogenase component human alpha-ketoacid dehydrogenase complexes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1422–1426. doi: 10.1073/pnas.85.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Schatz G. Mitochondrial presequences. J Biol Chem. 1988 Apr 5;263(10):4509–4511. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R. D., Wun T. C., Sadler J. E. cDNA cloning and expression in Escherichia coli of a plasminogen activator inhibitor from human placenta. J Biol Chem. 1987 Mar 15;262(8):3718–3725. [PubMed] [Google Scholar]
- Yeaman S. J. The 2-oxo acid dehydrogenase complexes: recent advances. Biochem J. 1989 Feb 1;257(3):625–632. doi: 10.1042/bj2570625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Crabb D. W., Harris R. A. Nucleotide and deduced amino acid sequence of the E1 alpha subunit of human liver branched-chain alpha-ketoacid dehydrogenase. Gene. 1988 Sep 15;69(1):159–164. doi: 10.1016/0378-1119(88)90390-3. [DOI] [PubMed] [Google Scholar]
- Zhang B., Edenberg H. J., Crabb D. W., Harris R. A. Evidence for both a regulatory mutation and a structural mutation in a family with maple syrup urine disease. J Clin Invest. 1989 Apr;83(4):1425–1429. doi: 10.1172/JCI114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



