Abstract
Drug development continues to face challenges to successfully progress the most promising drug candidates through the stages of clinical trials. Given the increasing cost to develop a drug, methods are required to characterise early drug efficacy and safety. Imaging techniques are increasingly used in oncological clinical trials to provide evidence for decision making. With the application of conventional morphological imaging techniques and standardised response criteria based on tumour size measurements, imaging continues to be used to define key study end points. However, functional imaging techniques are likely to play an important role in the evaluation of novel therapeutics, although how these methods are to be optimally applied has yet to be clearly established. The specific challenges of standardising multi-centre imaging in the context of clinical trials are highlighted, including the processes for image acquisition, data analysis and radiological review.
Keywords: Imaging, clinical trials
Introduction
In the past 2 decades, expansion in the knowledge of molecular medicine and genomics has revealed an array of new molecular targets that are being harnessed for the design of new therapies in oncology. As the search for a cancer cure continues, the promise of new targeted therapies with more specific action and less toxicity compared with conventional chemotherapeutics is shifting the management paradigm towards more individualised treatment and personalised care.
As the number of new targeted treatments continues to grow, the challenge in the early phases of drug development is to accelerate the investigation of the most promising candidate drug compounds, while halting the development of others that are toxic or inefficacious. Radiological imaging in combination with specific molecular assays are seen as valuable tools to aid the go or no-go decision-making process in the preclinical and clinical setting. Furthermore, in late stages of clinical development, imaging forms the basis of robust response and progression criteria in order to interrogate the drug in a large number of clinical trial subjects. Data from this stage of development is typically used as the basis of a submission to regulatory bodies to seek marketing approval for a designated indication. Also, it is clear that regulatory approval is not the ultimate goal of drug development. Rather it is generating evidence that convinces payers that the drug provides cost-effectiveness and the required evidence is not always the same[1].
Throughout the oncology drug development process, imaging is a fundamental contributor to the generation of primary, secondary and exploratory study end points. To this end, non-invasive imaging using computed tomography (CT), magnetic resonance imaging (MRI) and [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT are delivering response biomarkers, providing evidence of drug effects and treatment efficacy. There is a view within the drug industry that the application of advanced imaging methods will enhance the clinical trial process by delivering earlier and more predictable measures of drug response. It may also be possible for imaging to prognosticate treatment outcome by identifying patient subgroups that are more likely to benefit from a therapy. In addition to the basic imaging methods commonly applied in clinical routine; an array of new structural, functional and metabolic methods promise to further support targeted drug development in the future.
In this article, we discuss the process of drug development and put into context the role of imaging at each stage. We compare and contrast some differences between routine clinical imaging and imaging performed specifically in the context of a clinical trial. The widely used response evaluation in solid tumours criteria (RECIST) for assessing tumour response to treatment is reviewed[2], highlighting the key criteria that are applied for solid tumour assessment, as well as the strengths and limitations of the system. We also discuss the functional imaging techniques that are being utilised for the assessment of novel therapeutics, the rationale for their deployment and the practical challenges of implementation.
The process of drug development
The discovery and development of new drugs for the market typically involve carefully coordinated partnerships between pharmaceutical companies, clinical hospitals, academic institutions and other partnering groups, such as contract research organisations (CROs). Drug development refers to the entire process required to bring a new drug to the market. This includes the discovery, preclinical and clinical systematic testing, regulatory filing and life cycle management of a drug product. The clinical phase is subdivided into 3 phases (Phase I, II and III), which form the basis for progressing a drug candidate through development towards regulatory submission. A drug that is finally approved will have passed the rigorous scrutiny in each phase but failure at any one of these stages is unfortunately common.
Clinical drug development is considered a costly exercise with estimates for taking a drug to regulatory approval in the order of $800 million USD[3]. Without doubt a high contributing factor to drug development costs is the expenditure in failed drug candidates. For oncology drugs specifically, DiMasi and Grabowski[4] highlight that more of the failures occurred in the expensive late stages of development relative to other drugs. Whilst all stakeholders have an ongoing desire to improve the efficiency of clinical trial conduct, it is clear that attrition of drug candidates continues to restrict productivity. In order to ensure trials are conducted quickly and efficiently to deliver the best medicines to the market, improved clinical trial methodologies such as imaging are required.
Preclinical phase
The primary objective of this phase of development is to establish the safety profile of the drug candidate before clinical testing, including evaluation of mutagenicity, carcinogenicity and teratogenicity. However, establishing pharmacokinetic and pharmacodynamic properties of the compound will also provide insight into the in vivo properties before clinical investigations. Pharmacodynamic studies may use preclinical models of disease despite the well-recognised limitations of such models in replicating the respective clinical conditions.
A range of imaging technologies may be used to elucidate and demonstrate the mechanistic actions of drugs in vivo. Imaging techniques used may include equivalents of those used for clinical studies, such as CT, MRI (including functional imaging techniques such as magnetic resonance spectroscopy (MRS), dynamic contrast-enhanced MRI (DCE-MRI), diffusion-weighted MRI (DW-MRI), PET imaging and ultrasound; but also technologies that are not readily translatable to humans such as optical imaging (fluorescence and bioluminescence) and microPET imaging with novel radiotracers.
Phase I clinical trial
Phase I clinical trials represent the first opportunity to evaluate the drug candidate clinically. Such studies aim to evaluate the drug pharmacokinetics and pharmacology across a range of drug doses. Evaluation of safety and tolerability through this dose range typically guides selection of a chosen dose for subsequent clinical testing. Although understanding the basic drug properties and establishing safety is a primary aim, such studies also enable evaluation of the effect of the drug on disease. In oncology Phase I trials in which subjects typically have refractory advanced solid tumours, imaging is routinely applied to either acquire any evidence of anti-tumour activity via lesion shrinkage on CT or interrogate pharmacodynamics that may relate to drug action. For example, DCE-MRI has been used to study the effects of anti-angiogenic agents, where reduction in vascular parameters such as inflow transfer constant (Ktrans), leakage space (ve) and rate constant (kep) can be taken as evidence of drug action. Functional imaging techniques using CT, MR and PET have been broadly utilised in such early clinical drug studies. In some instances, imaging results have helped to support the choice of a biologically active dose to be applied in later Phase II trials.
Imaging applications in Phase I trials represent some unique challenges. These are typically small trials, patients usually have advanced disease and responses across the cohort are unlikely. Also, disease may present heterogeneously and imaging will likely need to interrogate different organs. However, this setting represents a unique opportunity for imaging to begin to support drug candidate development particularly as it may be one of only a few opportunities where drug action can be studied across a range of doses. Because these studies are typically conducted across a small number of investigational centres, standardisation of imaging methods can be readily achieved, enabling more complex methods to be considered.
Phase II clinical trial
Once an acceptable safety profile has been established and drug dose and scheduling defined, a Phase II trial can be planned. These trials aim to evaluate drug efficacy and safety within a targeted patient population. It represents the first opportunity to establish a robust evaluation of efficacy for an intended indication.
These studies can be conducted across tens of clinical centres to enable recruitment of sufficient subjects towards timely completion. On completion, the efficacy data are used to gauge whether investment in a large, expensive trial in a wider population is likely to be successful.
In Phase II, clinical imaging is typically used and assessed using standard response criteria such as RECIST. Response is classified into complete response, stable disease, partial response or disease progression depending on the percentage reduction in tumour size. In addition to these categorised assessments of response, lesion sizes alone may also be studied by bi-dimensional or three-dimensional measurements. However, it is well known that many novel therapies may not result in significant reduction in tumour size despite clinical benefits. Therefore functional and metabolic imaging has significant potential to provide greater predictors of subsequent success.
Conducting Phase II trials that suitably predict Phase III success remains a high priority goal for drug developers. These trials need to result in sufficiently robust data to enable key decisions to be made regarding investment in further programs. Given many pharmaceutical companies are pursuing a range of drug mechanisms against many indications, prioritising the finite resource is paramount.
Phase III clinical trials
Phase III trials aim to provide substantive evidence regarding the safety and efficacy of a drug within a large population for which drug administration is indicated. The results of these trials are submitted to regulatory agencies in order to seek approval to market the drug for a proposed indication. These trials can typically span hundreds of centres across tens of countries to enable accrual of sufficient subject numbers within a reasonable time window. These studies are usually designed as double-blind with parallel arms comparing the new treatment with standard therapy. Study end points are designed to bridge efficacy of the drug with patient outcomes and time-to-event end points such as progression-free survival are commonly applied.
Currently, most Phase III trials in solid tumours utilise established RECIST criteria[2] based on established and routine imaging methods. For a typical Phase III solid tumour study, lesion size measurements as part of RECIST evaluation use mostly CT (>90% of evaluations) with the remainder provided by MRI. For some indications, bone scans or FDG-PET can supplement the anatomical methodologies, for example, to identify progression based on a new bone metastasis.
Many of the functional imaging techniques are not readily translatable into the Phase III setting because of:
Lack of evidence that a given functional imaging end point is sufficiently predictive of outcome
Inability to ascertain such linkage because of lack of methodological availability and/or standardisation
The significant cost of implementing functional imaging techniques across a large patient cohort
Partnerships between academia, industry and expert organisations are being forged to actively address the challenges that come with deploying functional imaging across multiple trial centres and generating evidence regarding linkage to outcome. However, there will continue to be debate as to what role such methodologies will have in later phase clinical trials. Is it possible to validate such end points broadly enough that they will be relevant across all indications, disease settings and treatment types? Or will our reliance upon simple structural methods for these trials continue?
Imaging in clinical trial setting compared with routine clinical practice
In oncological practice, although imaging is utilised on a day to day basis for evaluating disease response, the context in which imaging is used in clinical trials differs to some degree with how these are utilised in clinical trials. Some of the key differences are highlighted in Table 1.
Table 1.
Comparison of routine clinical imaging with imaging in the setting of a clinical trial
| Routine clinical imaging | Imaging in clinical trial |
|---|---|
| Assessment of response made using standard criteria, but often interpreted together with clinical and laboratory findings | Assessment of response made using strict objective criteria as set out in study protocol |
| Imaging performed when clinically indicated | Imaging performed as per study protocol, which is commonly more frequent than in clinical practice |
| Imaging studies are archived on picture archiving systems (PACS) in a clinical department according to hospital policy | In addition to local archiving, many clinical trial images will need to be exported to a single location for centralised radiological review |
| Imaging data are archived with patient identifiable information | Imaging data to be exported for analysis requires full anonymisation and should be identifiable only by a code |
| Imaging studies are reported as per clinical practice | Imaging studies are read using study criteria and clinical report forms have to be completed. In addition to reading by the site radiologist, centralised scans are often independently reviewed |
| Quantitative measurements not usually audited | Measurements are subject to an auditing process that may involve CROs |
The key consideration for imaging performed in a clinical trial setting is to ensure sufficient adherence to prescribed imaging and treatment response guidelines. Such guidelines need to be suitably pragmatic to account for the diversity of standard imaging practice and availability of hardware within many imaging centres across many countries. The balance between strict adherence and pragmatism needs to be considered on a per trial basis. For example, a Phase III trial across 100 centres will focus on some basic imaging requirements such as modality, slice thickness, anatomical coverage and use of intravenous contrast. Whereas a DCE-MRI study performed across 3 trial centres will likely require manufacturer-specific acquisition parameters, some quality assurance steps using phantoms and a detailed analysis process.
Centralisation of scans is commonly performed to provide a consistent application of response criteria or specialised image analysis. The former is commonly mandated by regulatory agencies for Phase III studies where site-based radiological interpretation could potentially be biased. To promote objectivity in the evaluation of response, scans are collected into one location and systematically presented to selected radiologists who are independent of the study and blinded to treatment and any clinical information. In addition to this so-called blinded independent review, scans may also be centralised in order to undertake specialised analysis where site analysis may either not be possible or would result in high variability.
Standardising imaging at trial centres and centralising these scans for analysis is a complex operational task. For this reason clinical trial sponsors often select specialised CROs to manage the trial imaging components. Responsibilities can include generating imaging guidance documents, managing quality assurance steps, setting up mechanisms to transfer scans to a central location, performing quality control on scans received and the organisation of independent radiological review and/or image analysis.
Whether collecting thousands of scan series for a multicentre Phase III study or using an advanced functional imaging method at 3 trial sites, a great deal of coordination is required. Although Phase III imaging typically requires very standard imaging, collecting scans efficiently from hundreds of participating centres during a lengthy trial is logistically complex. Smaller more specialised imaging studies will require greater interaction with site radiology departments, knowledge of specific scanner details and carefully prescribed quality assurance and quality control steps.
In the conduct of clinical trials, it is important to keep a clear audit trail within the participating imaging departments to enable the results to be recreated from the source data on demand. Such requirements may impose additional burdens on the department, and the radiologists should be aware of such potential resource implications before agreeing to participate in any clinical trial.
Utility of imaging in clinical trials
In the conduct of clinical trials, measurement of tumour size before and after therapy is still the most widely accepted method of assessing treatment response. The assumption is that tumour shrinkage equates to effective treatment. Currently, assessment of treatment response according to tumour size measurement is usually performed according to the RECIST and RECIST 1.1 criteria. However, many new drugs can have substantial clinical benefits without significantly reducing tumour size. Hence, other functional techniques are now being investigated that can provide other quantitative measurements, informing on different aspects of tumour pathophysiology such as vascularity, cellularity, metabolism and hypoxia. Table 2 summarises functional imaging techniques that are currently applied and the quantitative information that can be gained from such studies.
Table 2.
A summary of commonly used functional imaging techniques, their biological correlates and quantitative parameters that are derived
| Imaging technique | Biological correlates | Quantitative parameters derived |
|---|---|---|
| [18F]Fluorodeoxyglucose positron emission tomography (FDG-PET) | Glucose uptake and metabolism | Standardised uptake values (SUV), maximum SUV (SUVmax), minimum SUV (SUVmin) |
| Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) | Blood flow and vascular permeability | Transfer constant (Ktrans), leakage space (Ve), rate constant (kep) |
| Dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) | Blood flow and blood volume | Relative blood flow (rBF); relative blood volume (rBV) |
| Dynamic contrast-enhanced perfusion computed tomography (DCE-CT) | Blood flow and vascular permeability | Blood flow (F), permeability–surface area product (PS), mean transit time (MTT) |
| Diffusion-weighted magnetic resonance imaging (DW-MRI) | Cellularity, tortuosity of extracellular space, cell membrane integrity and fluid viscosity | Apparent diffusion coefficient (ADC) |
| Blood oxygenation level dependent (BOLD) magnetic resonance imaging | Blood flow and deoxygenated haemoglobin | Tissue R2* relaxivity |
| 1H-Magnetic resonance spectroscopy (1H-MRS) | Metabolism | Ratios of choline to other metabolites (e.g. N-acetylaspartate, citrate) |
RECIST criteria
The RECIST size measurement criteria, currently in version 1.1[2], are used for the assessment of treatment response in oncological imaging practices.
A formalised set of response criteria using tumour size measurement was not established until 1979, when the World Health Organization (WHO) guidelines were first drawn up to classify the degree of change of a tumour in response to therapy. The WHO criteria relied on the recording of bi-dimensional tumour size before and after treatment to assess response. In an attempt to further simplify these measurements, the RECIST criteria were proposed in the 1990s, by simplifying the measurement to the largest unidimensional tumour diameter. In an attempt to simplify the approach, it unfortunately also attracted some controversies[5]. In 2009, the RECIST criteria were revised to version 1.1[2], which helped to clarify some ambiguities that resulted from the original guidelines. Table 3 summarises the key features of current RECIST criteria used to categorise tumour treatment response.
Table 3.
Categorisation of tumour response according to RECIST criteria version 1.1
| Category of response | Lesion |
|
|---|---|---|
| Target lesion | Non-target lesion | |
| Complete response (CR) | Disappearance of all target lesions. Any pathological lymph nodes (whether target or non-target) must have reduction in short axis to <10 mm | Disappearance of all non-target lesions and normalisation of tumour marker level (where appropriate). All lymph nodes must be non-pathological in size (<10 mm short axis) |
| Partial response (PR) | ≥30% decrease in the sum of the longest diameter (SLD) of target lesions | |
| Progressive disease (PD) | >20% increase in the SLD of target lesions, taking as reference the smallest SLD recorded since the treatment started (nadir) and minimum of 5 mm increase over the nadir | |
| Stable disease (SD) | Small changes in target lesions that do not meet above criteria | Persistence of one or more non-target lesion(s) and/or maintenance of tumour marker level above the normal limits |
Some of the potential short-comings and issues of using the RECIST criteria should be discussed. First, the tumour response is gauged and categorised by RECIST purely by the percentage change in the unidimensional tumour diameter (commonly the sum of multiple lesions). Although it has been found that the tumour shrinkage usually implies efficacious treatment, this cannot be generalised to many new targeted treatments. Indeed, novel therapeutics can improve patient outcome without necessarily reducing tumour size[5]. Second, in order to avoid measurement errors, a lymph node has to be at least 15 mm in the maximum short axis diameter to be deemed measurable in the context of clinical trials[2]. This does imply that smaller lymph nodes less than 15 mm in diameter cannot be included for the assessment of therapy response, which may limit its utility in certain clinical settings where involved lymph nodes may be of small volume, measuring less than 1 cm in short axis diameter (e.g. testicular cancer). Third, the RECIST criteria were devised based upon CT imaging data, but they are now also applied to measurements obtained on MR imaging. MR imaging presents more challenges in terms of standardisation of scanning before and after treatment to ensure consistent disease assessment. Fourth, there are patterns and sites of disease involvement that cannot be objectively measured by RECIST criteria. This includes diffuse peritoneal disease, lymphangitis carcinomatosis and metastatic disease confined to the bones without associated soft tissue masses. Despite these potential limitations, RECIST measurement criteria remain the most widely used technique to assess disease burden before and after tumour treatment because tumour diameter measurements are robust, reproducible, relatively simple to perform and have been shown in a number of trials to have a relationship with patient outcome.
Functional imaging techniques
Functional imaging techniques such as PET/CT, CT perfusion imaging, DCE-MRI, DW-MRI and MRS have been utilised to inform on drug effects in clinical trials and have also sometimes provided unique prognostic information. Most of these techniques are currently only applied in early phase clinical trials (e.g. Phase I and II) as exploratory rather than primary or even secondary end points within these studies. Apart from FDG-PET/CT studies, it has been difficult to implement multicentre DCE-MRI, DW-MRI and MRS studies because of the lack of standardisation of these techniques, and the limited degree to which some of these have been validated as reliable biomarkers. Nevertheless, these techniques continue to be deployed consistently across centres within clinical trials, especially in centres with particular expertise in utilising these techniques. It is hoped that increasing utility will contribute to demonstrating the feasibility and qualification of such methods. Of the various functional imaging techniques, the most widely applied and perhaps the most promising are FDG-PET/CT, DCE-MRI and DW-MRI. These are discussed in a little more detail in the following sections.
FDG-PET/CT
The utility of FDG-PET/CT in clinical studies and research is well established. Fluorodeoxyglucose is a glucose analogue, which is phosphorylated and trapped within cells. Thus, increased tracer uptake is observed within tissues that show increased glucose utilisation (e.g. tumour cells). The degree of tracer uptake can be readily quantified by a semi-quantitative index known as the standardised uptake value (SUV). This represents the amount of tracer activity observed normalised to the injected dose and body weight of the patient.
There is compelling data from published literature that FDG-PET/CT is able to distinguish responders and non-responders to drug treatment at a relatively early time point (e.g. after one or two cycles of drug treatment), thus providing the opportunity for early intervention or change of treatment strategy[6–10] (Fig. 1). There are also data that demonstrate that responders identified by FDG-PET/CT in a variety of tumour types (e.g. lymphoma, gastric, oesophageal, gastrointestinal stromal tumours, lung) have a better survival compared with those who do not show a good initial response[6–12]. Thus, FDG-PET/CT appears to provide a useful imaging biomarker for treatment response, and is increasingly utilised broadly in clinical trials. The disadvantages of the technique include the relatively high cost of the study; and the potential radiation to the patient, which may be prohibitive if serial assessments are required before and throughout therapy.
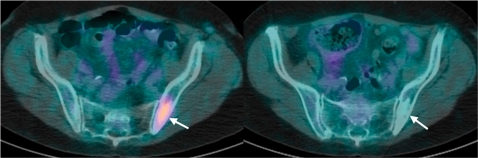
Figure 1.
A 48-year-old women with non-Hodgkin lymphoma. FDG-PET/CT axial images through the pelvis (a) before and (b) at 6 weeks after commencing chemotherapy. (a) A focus of bone marrow involvement (arrow) is noted in the left ilium, which shows moderately intense tracer uptake before treatment. (b) At 6 weeks after chemotherapy, there was complete absence of tracer uptake (arrow) in the affected left iliac bone in keeping with a complete metabolic response. Note area of increased sclerosis within the ilium (arrow) after treatment. (Courtesy of Dr Gary Cook, Royal Marsden Hospital, Sutton, UK.)
DCE-MRI
DCE-MRI informs on the perfusion and vascular properties of tumours. To perform imaging, low molecular weight gadolinium contrast medium is injected into a vein and the contrast is then carried by blood flow to the tumour tissue. Within the tumour tissue, contrast extravasates into the extracellular space, which results in signal enhancement on T1-weighted MRI. By performing MRI at a high temporal resolution (e.g. every 2 s) over the tumour of interest, the evolution of the signal change with time in the supplying blood vessel and within the tumour can be characterised by signal intensity–time curves. These signal intensity–time curves can be deconvolved by mathematical modelling to extract quantitative parameters that reflect tissue perfusion and vascular behaviour. For example, by applying the widely used Tofts model, parameters such as transfer constant (Ktrans), volume of leakage space (ve) and rate constant (kep) can be derived. It is also possible to derive a non-model-based semi-quantitative parameter known as the initial area under the gadolinium curve (IAUGC). The transfer constant Ktrans, is one of the most frequently reported using such a technique, and reflects both vascular flow and vascular permeability. Parameters such as Ktrans and IAUGC have been validated and shown to correlate with markers of angiogenesis, such as tissue mircovessel density count and expression of vascular endothelial growth factor[13–15].
DCE-MRI has been used widely in Phase I clinical trials to evaluate the effects of drugs that modulate tumour vasculature, such as antiangiogenic and antivascular therapies[16–19]. Studies have clearly shown that DCE-MRI can detect the effects of such treatment, by demonstrating a reduction in the quantitative parameters (e.g. Ktrans, IAUGC) after initiating therapy (Fig. 2). Based on the reported reproducibility of Ktrans values in the literature, it would appear that in a well-conducted research setting, the reproducibility of Ktrans is good to moderate (coefficient of repeatability ranges from about 20 to 40%)[20,21]. This suggests that in a well-conducted study, a change of Ktrans value of more than 40% is likely to indicate a significant drug effect. Some studies have also demonstrated a dose–response relationship between the drug and Ktrans value, which has helped the selection of a biologically active dose for translation into the later phase clinical trials[18,22]. More recently, DCE-MRI derived parameters have been shown to have predictive/prognostic values[23,24]. Studies on breast and renal cancers showed an inverse correlation between pretreatment Ktrans values and patient survival.
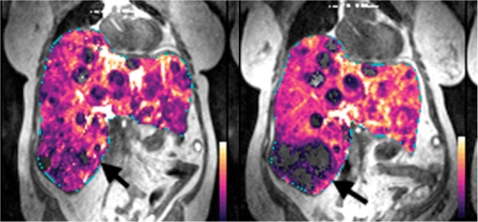
Figure 2.
Parametric transfer constant (Ktrans) maps overlaid on T1-weighted images in a woman with metastatic neuroendocrine tumour treated using a novel targeted agent (a) before and (b) after one cycle of treatment. A marker lesion at the inferior right tip of the liver (arrow) shows devascularisation with significant reduction in transfer constant after treatment. (Courtesy of Keiko Miyazaki, Institute of Cancer Research, UK.)
The disadvantages of the DCE-MRI technique currently include a lack of standardisation across multiple MR platforms and institutions, making it difficult to implement the technique robustly in a multicentre setting. However, substantial resources are being channelled to address this issue by several groups. There is also a lack of vendor software platforms that can be used to process DCE-MRI data with relative ease to extract the quantitative vascular parameters. This necessitates the operationally complex and expensive centralisation of scans for analysis on a single platform. Thus, developments in this area would also be helpful and welcomed. Nonetheless, DCE-MRI is an imaging technique that appears to provide quantitative and biologically relevant information related to tissue vasculature and perfusion, which can inform the drug development process.
DW-MRI
DW-MRI probes differences in the mobility of water protons between tissues. Cellular tissues (e.g. tumour tissues) impede the motion of water protons to a greater extent than normal tissues, and thus appear high signal on DW-MRI and return low apparent diffusion coefficient (ADC) values. The ADC is a quantitative measurement that reflects tissue diffusivity. Sequential ADC measurements before and after anticancer treatments can help to establish tumour response to therapy (Fig. 3)[25–27].
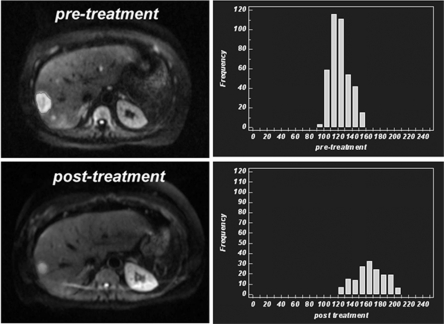
Figure 3.
A 46-year-old man with neuroendocrine liver metastasis. Diffusion-weighted MR images (b = 750 s/mm2) before and after targeted treatment shows significant reduction in tumour size of the lesion in the right lobe of the liver. However, corresponding histograms of the distribution of apparent diffusion coefficient (ADC) values within the tumour also show an increase in the median value with a shift of the histogram to the right, in keeping with treatment response.
Studies have shown that the ADC increases in response to a variety of chemotherapy and novel targeted treatments[25,27]. This ADC increase has been shown to relate to biological processes such as cellular necrosis[28] and apoptosis as a consequence of treatment. Significant ADC changes may be observed less than 1 week after initiating anticancer treatment, thus providing the opportunity to observe early treatment effects[29,30]. The pretreatment ADC values[30–32] and ADC response[33] may also be predictive or prognostic. A few studies have shown that a lower pretreatment ADC value may be associated with a better response to chemotherapy[31,32], although this is not uniformly observed[34,35].
In the clinical studies published thus far, ADC values calculated over a range of diffusion-weighting (b values) appear to be highly reproducible. Low coefficient of repeatability has been reported (e.g. 14%)[36], suggesting that the technique is likely to sensitive to treatment effects. However, the ADC derived from DW-MRI is still considered pre-biomarker and requires further validation to establish it as a reliable indicator of treatment response. This requires further exploration to link the ADC observation with biological changes occurring at the tissue level. Thus, more research detailing radiological–pathological changes in tumours with treatment should be encouraged.
DW-MRI is an attractive technique for a few reasons. First, the imaging is quick to perform and can be easily appended to any imaging protocol. Second, the technique relies on endogenous contrast between tissues and does not require the administration of any contrast medium. This makes it an attractive imaging option, especially at a time when the safety of MR contrast media are being scrutinised. However, there are also a number challenges that need to be addressed. These include technical standardisation across imaging platforms and multicentre studies, quality assurance of imaging platforms and better understanding of the optimum time for ADC measurements in relation to specific treatment[37].
Conclusions
Clinical trials enable the development, registration and ultimately access of new drugs to patients. Imaging can inform the drug development process by providing non-invasive assessment of drug action and tumour response to treatment. Although size measurement criteria are still the most widely used imaging-based assessment, functional imaging techniques are increasingly being used in early phase trials to study the early effects of drug action on aspects of tumour biology including vascularity, cellularity and metabolism. However, more work is needed to translate many of these functional imaging techniques into multicentre settings and the challenge is to standardise multicentre methodology and ensure good measurement reproducibility. There is increasing partnership between pharmaceutical industry, academic institutions and professional bodies to address these problems.
References
- [1].McCabe C, Bergmann L, Bosanquet N, et al. Market and patient access to new oncology products in Europe: a current, multidisciplinary perspective. Ann Oncol. 2009;20:403–12. doi: 10.1093/annonc/mdn603. doi:10.1093/annonc/mdn603. PMid:18854550. [DOI] [PubMed] [Google Scholar]
- [2].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- [3].DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–85. doi: 10.1016/S0167-6296(02)00126-1. doi:10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- [4].DiMasi JA, Grabowski HG. Economics of new oncology drug development. J Clin Oncol. 2007;25:209–16. doi: 10.1200/JCO.2006.09.0803. doi:10.1200/JCO.2006.09.0803. PMid:17210942. [DOI] [PubMed] [Google Scholar]
- [5].Michaelis LC, Ratain MJ. Measuring response in a post-RECIST world: from black and white to shades of grey. Nat Rev Cancer. 2006;6:409–14. doi: 10.1038/nrc1883. doi:10.1038/nrc1883. PMid:16633367. [DOI] [PubMed] [Google Scholar]
- [6].Zhao J, Qiao W, Wang C, Wang T, Xing Y. Therapeutic evaluation and prognostic value of interim hybrid PET/CT with (18)F-FDG after three to four cycles of chemotherapy in non-Hodgkin's lymphoma. Hematology. 2007;12:423–30. doi: 10.1080/10245330701393840. doi:10.1080/10245330701393840. PMid:17852456. [DOI] [PubMed] [Google Scholar]
- [7].Stroobants S, Goeminne J, Seegers M, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39:2012–20. doi: 10.1016/s0959-8049(03)00073-x. doi:10.1016/S0959-8049(03)00073-X. [DOI] [PubMed] [Google Scholar]
- [8].Wieder HA, Brucher BL, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–8. doi: 10.1200/JCO.2004.07.122. doi:10.1200/JCO.2004.07.122. PMid:14990646. [DOI] [PubMed] [Google Scholar]
- [9].Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604–10. doi: 10.1200/JCO.2003.06.574. doi:10.1200/JCO.2003.06.574. PMid:14673049. [DOI] [PubMed] [Google Scholar]
- [10].Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–7. doi: 10.1200/JCO.2003.12.004. doi:10.1200/JCO.2003.12.004. PMid:12860940. [DOI] [PubMed] [Google Scholar]
- [11].Spaepen K, Stroobants S, Verhoef G, Mortelmans L. Positron emission tomography with [(18)F]FDG for therapy response monitoring in lymphoma patients. Eur J Nucl Med Mol Imaging. 2003;30(Suppl 1):S97–105. doi: 10.1007/s00259-003-1166-5. [DOI] [PubMed] [Google Scholar]
- [12].Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102:53–9. doi: 10.1182/blood-2002-12-3842. doi:10.1182/blood-2002-12-3842. PMid:12609836. [DOI] [PubMed] [Google Scholar]
- [13].George ML, Dzik-Jurasz AS, Padhani AR, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg. 2001;88:1628–36. doi: 10.1046/j.0007-1323.2001.01947.x. doi:10.1046/j.0007-1323.2001.01947.x. PMid:11736977. [DOI] [PubMed] [Google Scholar]
- [14].Ikeda O, Yamashita Y, Takahashi M. Gd-enhanced dynamic magnetic resonance imaging of breast masses. Top Magn Reson Imaging. 1999;10:143–51. doi: 10.1097/00002142-199904000-00007. doi:10.1097/00002142-199904000-00007. PMid:10551629. [DOI] [PubMed] [Google Scholar]
- [15].Knopp MV, Brix G, Junkermann HJ, Sinn HP. MR mammography with pharmacokinetic mapping for monitoring of breast cancer treatment during neoadjuvant therapy. Magn Reson Imaging Clin N Am. 1994;2:633–58. [PubMed] [Google Scholar]
- [16].Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–77. doi: 10.1200/JCO.2005.03.4645. doi:10.1200/JCO.2005.03.4645. PMid:16391297. [DOI] [PubMed] [Google Scholar]
- [17].Herbst RS, Hong D, Chap L, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–65. doi: 10.1200/JCO.2008.19.6683. doi:10.1200/JCO.2008.19.6683. PMid:19546406. [DOI] [PubMed] [Google Scholar]
- [18].Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two Phase I studies. J Clin Oncol. 2003;21:3955–64. doi: 10.1200/JCO.2003.08.092. doi:10.1200/JCO.2003.08.092. PMid:14517187. [DOI] [PubMed] [Google Scholar]
- [19].Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–54. doi: 10.1200/JCO.2006.07.2066. doi:10.1200/JCO.2006.07.2066. PMid:17634482. [DOI] [PubMed] [Google Scholar]
- [20].Galbraith SM, Lodge MA, Taylor NJ, et al. Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: comparison of quantitative and semi-quantitative analysis. NMR Biomed. 2002;15:132–42. doi: 10.1002/nbm.731. doi:10.1002/nbm.731. PMid:11870909. [DOI] [PubMed] [Google Scholar]
- [21].Ng CS, Raunig DL, Jackson EF, et al. Reproducibility of perfusion parameters in dynamic contrast-enhanced MRI of lung and liver tumors: effect on estimates of patient sample size in clinical trials and on individual patient responses. AJR Am J Roentgenol. 2010;194:W134–40. doi: 10.2214/AJR.09.3116. doi:10.2214/AJR.09.3116. PMid:20093564. [DOI] [PubMed] [Google Scholar]
- [22].Thomas AL, Morgan B, Drevs J, et al. Vascular endothelial growth factor receptor tyrosine kinase inhibitors: PTK787/ZK 222584. Semin Oncol. 2003;30:32–8. doi: 10.1016/s0093-7754(03)00123-4. doi:10.1016/S0093-7754(03)00123-4. [DOI] [PubMed] [Google Scholar]
- [23].Pickles MD, Manton DJ, Lowry M, Turnbull LW. Prognostic value of pre-treatment DCE-MRI parameters in predicting disease free and overall survival for breast cancer patients undergoing neoadjuvant chemotherapy. Eur J Radiol. 2009;71:498–505. doi: 10.1016/j.ejrad.2008.05.007. doi:10.1016/j.ejrad.2008.05.007. PMid:18572340. [DOI] [PubMed] [Google Scholar]
- [24].Flaherty KT, Rosen MA, Heitjan DF, et al. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther. 2008;7:496–501. doi: 10.4161/cbt.7.4.5624. [DOI] [PubMed] [Google Scholar]
- [25].Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–35. doi: 10.2214/AJR.06.1403. doi:10.2214/AJR.06.1403. PMid:17515386. [DOI] [PubMed] [Google Scholar]
- [26].Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI–a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008;5:220–33. doi: 10.1038/ncponc1073. doi:10.1038/ncponc1073. PMid:18301415. [DOI] [PubMed] [Google Scholar]
- [27].Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 32:2–16. doi: 10.1002/jmri.22167. doi:10.1002/jmri.22167. PMid:20575076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lang P, Wendland MF, Saeed M, et al. Osteogenic sarcoma: noninvasive in vivo assessment of tumor necrosis with diffusion-weighted MR imaging. Radiology. 1998;206:227–35. doi: 10.1148/radiology.206.1.9423677. [DOI] [PubMed] [Google Scholar]
- [29].Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22:104–13. doi: 10.1002/nbm.1245. doi:10.1002/nbm.1245. PMid:18384182. [DOI] [PubMed] [Google Scholar]
- [30].Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248:894–900. doi: 10.1148/radiol.2483071407. doi:10.1148/radiol.2483071407. PMid:18710982. [DOI] [PubMed] [Google Scholar]
- [31].Koh DM, Scurr E, Collins D, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188:1001–8. doi: 10.2214/AJR.06.0601. doi:10.2214/AJR.06.0601. PMid:17377036. [DOI] [PubMed] [Google Scholar]
- [32].Dzik-Jurasz A, Domenig C, George M, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–8. doi: 10.1016/S0140-6736(02)09520-X. doi:10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- [33].Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA. 2005;102:5524–9. doi: 10.1073/pnas.0501532102. doi:10.1073/pnas.0501532102. PMid:15805192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nilsen L, Fangberget A, Geier O, Olsen DR, Seierstad T. Diffusion-weighted magnetic resonance imaging for pretreatment prediction and monitoring of treatment response of patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Acta Oncol. 2010;49:354–60. doi: 10.3109/02841861003610184. doi:10.3109/02841861003610184. PMid:20397769. [DOI] [PubMed] [Google Scholar]
- [35].Niwa T, Ueno M, Ohkawa S, et al. Advanced pancreatic cancer: the use of the apparent diffusion coefficient to predict response to chemotherapy. Br J Radiol. 2009;82:28–34. doi: 10.1259/bjr/43911400. doi:10.1259/bjr/43911400. PMid:19095814. [DOI] [PubMed] [Google Scholar]
- [36].Koh DM, Blackledge M, Collins DJ, et al. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol. 2009;19:2728–38. doi: 10.1007/s00330-009-1469-4. doi:10.1007/s00330-009-1469-4. PMid:19547986. [DOI] [PubMed] [Google Scholar]
- [37].Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–25. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]