Abstract
The evaluation of treatment response is an established role for imaging in oncologic research and clinical practice. In early phase trials, imaging response criteria are used to determine the presence and magnitude of the drug effect on tumour to aid decisions concerning progress to late phase trials, and to inform dose selection and scheduling. In late phase trials and clinical practice, the imaging response is used as a surrogate for clinical outcome. Due to the limitations of current anatomic response criteria, there is growing interest in the use of [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET) to assess treatment response. The technique is beginning to be adopted within mainstream approaches for evaluation of response in solid tumours and lymphoma. Difficulties with standardisation across PET centres and tumour types combined with uncertainty concerning the timing of assessment relative to treatment, have limited the use of quantitative measurements of FDG uptake to research applications. However, with a growing body of evidence that qualitative criteria such as the development of new PET lesions or complete metabolic response following treatment can provide surrogates marker for clinical outcome, [18F]FDG-PET is becoming established as a clinical technique for assessing tumour response, especially for FDG-avid lymphoma subtypes. Multimodality imaging using perfusion computed tomography/PET is an exciting novel technique with the potential to define treatment response in a new way.
Keywords: Fluorodeoxyglucose positron emission tomography, tumour response
Introduction
The evaluation of treatment response is an established role for imaging in oncology. The international method used to assess solid tumour response to treatment is currently based on the Response Evaluation Criteria in Solid Tumours (RECIST) which predominantly uses size criteria to evaluate tumour response[1]. However, RECIST has certain limitations. Tumour size measurements on computed tomography (CT) are often inconsistent. Observer variability in size measurement is illustrated by a study from Erasmus et al.[2] who assessed the consistency of readers measuring lung cancers using RECIST criteria on CT. They found the difference between measurements made by 2 readers was sufficiently large to be spuriously considered progressive disease in 29.75% of cases (range 17.5–50%) or spuriously considered partial response in 13.75% (range 2.5–27.5%). Even repeated measurements by the same observer were associated with significant variability with potential misclassification rates of 9.5% (range 2.5–17.5%) for progressive disease and 3% (range 0–5%) for partial response.
Tumour response evaluation by RECIST may also be limited by problems in defining the margins of ill-defined or irregular lesions and in some instances it is not possible to measure sites of disease involvement, e.g. bone marrow disease (Fig. 1). Size criteria also cannot distinguish reactive lymph node enlargement from enlargement due to nodal metastasis, or post-treatment residual masses from sites of active disease. Furthermore, current targeted drug treatments in oncology may alter the pathophysiology and pharmacokinetics of the tumour to produce functional changes associated with improved survival but without a measurable change in size of lesion.
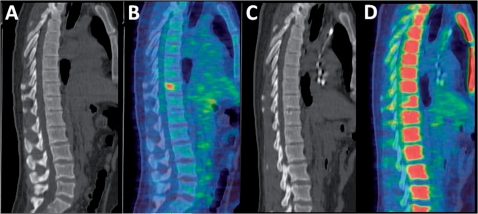
Figure 1.
Sagittal images of the thoracic and lumbar spine obtained by CT (A,C) and [18F]FDG-PET (B,D) in a patient with lymphoma before (A,B) and after (C,D) therapy. The vertebral lymphoma deposit is not visualised on CT before or after therapy and therefore CT cannot be used to assess response. The uptake of FDG before therapy has resolved after treatment indicating a complete metabolic response. On image (D), the remaining normal marrow shows increased physiological uptake in response to the therapy.
The limitations of anatomical response assessments such as RECIST have led to a growing interest in novel response criteria using functional imaging with positron emission tomography (PET). There are a number of PET response markers currently available, e.g. [18F]fluorothymidine, which assesses cellular proliferation, H215O for assessment of angiogenesis and 124I-labelled annexin V for apoptosis. [18F]Fluorodeoxyglucose (FDG) evaluates cellular glucose metabolism and is currently the most widely used PET tracer in clinical practice. This article illustrates the current status of FDG-PET as a response marker in oncology.
Application of imaging response markers in oncology
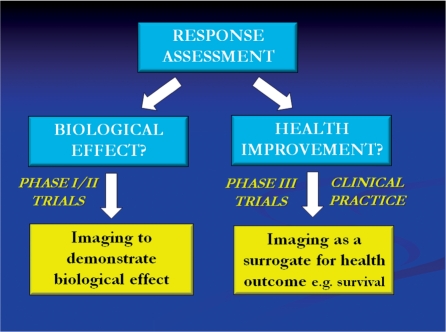
There are important differences in the application of imaging response markers in early phase oncology drug trials (phases I and II) compared with late phase trials (phase III) and clinical practice (Fig. 2). In early phase trials, the primary aim of using imaging is to determine whether the drug has any biological effect on tumour. This information can contribute to important decisions concerning whether the drug should proceed to late phase trials. The magnitude of the effect (if present) can also be used to inform dose selection and scheduling. However, in late phase trials and clinical practice, the imaging response is used as a surrogate for clinical outcome measures such as survival, and therefore there needs to be evidence of an association between the imaging change and the clinical outcome. This more stringent requirement is not necessary for the use of imaging in early phase trials. There is good evidence that the time to disease progression as assessed by RECIST correlates with survival for many cancer therapies; hence the acceptance of RECIST in clinical practice and as an imaging biomarker to support regulatory approval of novel cancer treatments. However, as discussed below, there is increasing evidence that other imaging biomarkers, including FDG-PET, can also be used as surrogates for clinical outcome.
Figure 2.
Summary of research and clinical uses of image-based tumour response assessment.
Technical considerations
An ideal marker of tumour response would distinguish accurately between responders and non-responders to treatment, should be reproducible, identify non-responders early in the course of treatment and provide sufficient benefit at a low cost. Consensus recommendations for the use of [18F]FDG-PET as an indicator of therapeutic response in patients in National Cancer Institute trials have been published with the aim of standardising protocols to allow quantitative assessments of tumour response in multicentre clinical trials[3]. These recommendations highlight the importance of consistency in patient preparation prior to scanning and of documentation of all current medication. Scanning protocols are also standardised, i.e. scans are performed at 60 ± 10 min after [18F]FDG administration and all patients must undergo a pre- and post-treatment scan, for quantitative analysis. It is recommended that post-treatment imaging is not performed within 2 weeks of chemotherapy treatment, however the timing after radiotherapy treatment is less established.
Tumour response analysis is performed using attenuation corrected PET images and by measuring the maximum and mean standardised uptake values (SUV), a semi-quantitative method in which FDG concentration is normalised to the amount of injected activity and total volume of distribution. Consideration should be given to the use of partial volume correction when using PET/CT[3]. The coefficient of repeatability for SUV measurements (16.9%)[4] is favourable compared with other imaging response markers, including automated CT measurements of volume (20.8%)[5] and dynamic contrast-enhanced magnetic resonance imaging (36.1%)[6].
Response criteria for [18F]FDG-PET
As a response marker in malignancy, a range of criteria for the interpretation of [18F]FDG uptake on PET studies has been defined. No visualised uptake after therapy implies cell death and hence a complete response and the presence of new lesions indicates progressive disease. These responses can be assessed qualitatively, including from PET acquisitions acquired in clinical practice. However, defining quantitative criteria for the change in [18F]FDG uptake in an individual lesion that reflects partial response, stable disease and progressive disease has proved challenging. Definitions for these responses have been proposed by the European Organisation for Research and Treatment of Cancer (EORTC) thresholds based on measurement of SUV[7] but these criteria have several limitations. The most significant issue relates to the timing of the post-therapy PET measurement as illustrated by application of the EORTC criteria to the serial PET data reported by Wieder et al.[8]. Measurements at 2 weeks after therapy showed 22 of 27 patients (81%) with a reduction of SUV of less than 25% (the EORTC threshold for response) comprising all 6 patients proceeding to complete response and 16 of 21 patients proceeding to various degrees of partial histological responses. However, at 4 weeks all but 2 of 38 (95%) patients had shown a greater than 25% reduction in SUV, regardless of histological response classification. Only the measurements made 2 weeks after therapy correlated with tumour response and survival. Thus, at this time there are no generally agreed quantitative [18F]FDG-PET criteria for the definition of treatment failure.
Other factors complicating assessment of metabolic response by PET include radiotherapy-induced local inflammatory responses, which may persist for up to 6 months and can result in a false-positive result. Treatment may also alter metabolic activity in normal tissues but the distribution of tracer uptake in these circumstances is frequently different (e.g. oesophagitis) and hence distinguishable from residual tumour uptake. Chemotherapy is thought to be able to suppress residual tumour activity and therefore post-treatment assessments should not be performed within 2 weeks of completion of treatment. Certain types of chemotherapy are recognised to result in diffusely increased bone marrow activity (Fig. 1).
The robustness of the appearance of new lesions on [18F]FDG-PET as a marker of progressive disease is recognised by inclusion of this criterion both within the latest version of RECIST (RECIST 1.1[9]) and within the revised response criteria for malignant lymphoma developed by Cheson et al. in 2007[10]. Similarly, the revised response criteria for malignant lymphoma have adopted no FDG uptake on completion of therapy as the sole criterion for complete response in predictably FDG-avid lymphoma subtypes (i.e. Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma and mantel cell lymphoma). Cheson et al. also strongly recommend pre-treatment PET to define sites of disease in these lymphoma subtypes. However, in the presence of a partial metabolic response, both RECIST 1.1 and the revised Cheson criteria maintain the use of tumour size measurements for determination of tumour response (Fig. 3). At present, there is also insufficient data to support [18F]FDG-PET for routine post-treatment surveillance of other lymphoma subtypes that are not predictably FDG-avid.
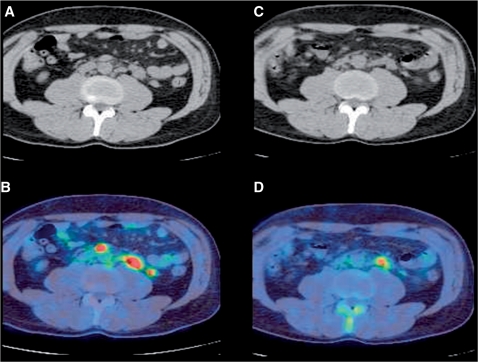
Figure 3.
Axial images of the mid-abdomen obtained by CT (A,C) and [18F]FDG-PET (B,D) in a patient with lymphoma before (A,B) and after (C,D) therapy. The presence of residual FDG uptake indicates only a partial metabolic response. In this circumstance, CT-based size criteria should be used for response assessment.
The new Cheson criteria also address the issue of mid-treatment [18F]FDG-PET for the assessment of lymphoma response. Such examinations have been shown to accurately predict the pathological response on completion of therapy. However, there is currently no change in therapy based on the results of mid-treatment [18F]FDG-PET that has been shown to improve health outcomes for lymphoma patients. Therefore, the guidelines recommend that mid-treatment PET should not be performed routinely.
Following completion of first-line therapy, failure of complete metabolic response on [18F]FDG-PET has high sensitivity of 80% and specificity of 89% for the diagnosis of treatment failure when residual masses are identified on CT, increasing to 81% and 95%, respectively, if CT positive and CT negative patients are included. These figures compare with a sensitivity of 75% and specificity of 45% for CT alone[11]. In an outcome study comparing CT-only response criteria to CT combined with complete metabolic response on PET (as used in the Cheson criteria), only the combined approach was a statistically significant independent predictor for progression-free survival (P = 0.008 vs P = 0.72 for CT alone)[12]. The superior diagnostic and prognostic power of [18F]FDG-PET translates to more cost-effective lymphoma management as assessed in the UK health care system[11]. Based on a 40-year-old male patient, the cost-effectiveness of CT alone was calculated at £339 per life year compared with £335 if [18F]FDG-PET was used in CT-positive disease and £311 per life year if only [18F]FDG-PET was used. Hence defining complete response on the basis of [18F]FDG-PET has become the standard of clinical care for FDG-avid lymphoma subtypes.
Complete metabolic response on [18F]FDG-PET has also been shown to be associated with improved survival for other forms of malignancy. MacManus et al.[13] evaluated the use of [18F]FDG-PET in non-small cell lung cancer after chemoradiotherapy treatment and found that patients failing to achieve a complete response on PET had an estimated death rate 4.1 times that for patients achieving a complete response (P < 0.0009). The predictive power of PET was superior to CT alone. In oesophageal cancer, Kim et al.[14] identified that a complete metabolic response on [18F]FDG-PET was a good predictor of pathological response and survival after chemoradiotherapy. In contrast, for colorectal liver metastases, Tam et al.[15] showed that chemotherapy-induced normalisation of FDG uptake did not reliably indicate complete pathologic response.
Combining [18F]FDG-PET with perfusion CT
With the advent of integrated PET/CT systems, [18F]FDG-PET can be combined with perfusion CT to provide simultaneous assessment of tumour metabolic and vascular responses to therapy[16]. Clinical studies have shown that a reduction in tumour metabolism after treatment is not necessarily paralleled by a reduction in tumour blood flow or other markers of vascularity, reflecting differential responses in tumour cell viability and angiogenesis[17]. By combining [18F]FDG-PET and perfusion CT, it is possible to envisage a novel approach to assessment of tumour response. Reductions in metabolism and perfusion would imply a complete response; no change in either metabolism or vascularity would represent no response to treatment. Mismatched reductions in these parameters would indicate partial responses with different biological characteristics. Reduced vascularity but with no change in metabolism may suggest tumour adaptation to hypoxia; reduced metabolism with no change in vascularity may represent residual angiogenesis (Table 1). Such a system could potentially allow personalisation of treatment on the basis of individual vascular-metabolic response[17].
Table 1.
Vascular and metabolic responses to therapy
| Vascularity | Metabolism |
|
|---|---|---|
| No change | Reduced | |
| No change | No response | Partial response ? residual angiogenesis |
| Reduced | Partial response ? adaptation to hypoxia | Complete response |
Summary
Assessment of tumour response with [18F]FDG-PET has a number of advantages over existing size-based approaches and the technique is beginning to be adopted within mainstream approaches for evaluation of response in solid tumours and lymphoma. Because of difficulties with standardisation across PET centres and tumour types combined with uncertainty concerning the timing of assessment relative to treatment, the use of quantitative measurements of FDG uptake for evaluating response primarily remains a research tool at present, most applicable to early phase drug trials. However, with a growing body of evidence that qualitative criteria such as the development of new PET lesions or complete metabolic response after treatment can provide surrogate markers for clinical outcome, [18F]FDG-PET is becoming established as a clinical technique for assessing tumour response, especially for FDG-avid lymphoma subtypes. Multimodality imaging using perfusion CT/PET is an exciting novel technique with the potential to define treatment response in a new way.
References
- [1].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. doi:10.1093/jnci/92.3.205. PMid:10655437. [DOI] [PubMed] [Google Scholar]
- [2].Erasmus JJ, Gladish GW, Broemeling L, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol. 2003;21:2574–82. doi: 10.1200/JCO.2003.01.144. doi:10.1200/JCO.2003.01.144. PMid:12829678. [DOI] [PubMed] [Google Scholar]
- [3].Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Institute Trials. J Nucl Med. 2006;47:1059–66. [PubMed] [Google Scholar]
- [4].Weber WA, Ziegler SI, Thödtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40:1771–7. [PubMed] [Google Scholar]
- [5].Wormanns D, Kohl G, Klotz E, et al. Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol. 2004;14:86–92. doi: 10.1007/s00330-003-2132-0. doi:10.1007/s00330-003-2132-0. PMid:14615902. [DOI] [PubMed] [Google Scholar]
- [6].Morgan B, Utting JF, Higginson A, Thomas AL, Steward WP, Horsfield MA. A simple, reproducible method for monitoring the treatment of tumours using dynamic contrast-enhanced MR imaging. Br J Cancer. 2006;94:1420–7. doi: 10.1038/sj.bjc.6603140. doi:10.1038/sj.bjc.6603140. PMid:16670720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82. doi: 10.1016/s0959-8049(99)00229-4. doi:10.1016/S0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- [8].Wieder HA, Brücher BL, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–8. doi: 10.1200/JCO.2004.07.122. doi:10.1200/JCO.2004.07.122. PMid:14990646. [DOI] [PubMed] [Google Scholar]
- [9].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. PMid:19097774. [DOI] [PubMed] [Google Scholar]
- [10].Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. doi:10.1200/JCO.2006.09.2403. PMid:17242396. [DOI] [PubMed] [Google Scholar]
- [11].Bradbury I, Bonell E, Boynton J, et al. Positron emission tomography (PET) imaging in cancer management. Health Technology Assessment Report 2. Glasgow: Health Technology Board for Scotland. 2002 [Google Scholar]
- [12].Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non-Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652–61. doi: 10.1200/JCO.2005.01.891. doi:10.1200/JCO.2005.01.891. PMid:15837965. [DOI] [PubMed] [Google Scholar]
- [13].MacManus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92. doi: 10.1200/JCO.2003.07.054. doi:10.1200/JCO.2003.07.054. PMid:12663716. [DOI] [PubMed] [Google Scholar]
- [14].Kim MK, Ryu JS, Kim SB, et al. Value of complete metabolic response by (18)F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer. 2007;43:1385–91. doi: 10.1016/j.ejca.2007.04.001. doi:10.1016/j.ejca.2007.04.001. PMid:17512192. [DOI] [PubMed] [Google Scholar]
- [15].Tan MC, Linehan DC, Hawkins WG, Siegel BA, Strasberg SM. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg. 2007;11:1112–19. doi: 10.1007/s11605-007-0218-8. doi:10.1007/s11605-007-0218-8. PMid:17623263. [DOI] [PubMed] [Google Scholar]
- [16].Miles KA. PET-CT in oncology: making the most of CT. Cancer Imaging. 2008;8:S87–93. doi: 10.1102/1470-7330.2008.9015. doi:10.1102/1470-7330.2008.9015. PMid:18852084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miles K, Williams RE. Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging. 2008;8:81–6. doi: 10.1102/1470-7330.2008.0011. doi:10.1102/1470-7330.2008.0011. PMid:18390391. [DOI] [PMC free article] [PubMed] [Google Scholar]