Abstract
Computed tomography (CT) and magnetic resonance imaging (MRI) are excellent modalities for the localization of mediastinal masses and there are often features that may allow the correct diagnosis to be made. However, CT and MRI cannot usually assess the aggressiveness of masses or identify viable tumour in residual masses after chemotherapy. Metabolic imaging using [18F]fluorodeoxyglucose (FDG)-positron emission tomography/CT, although not required in many cases, may be helpful for further characterization of masses and to guide the most appropriate site for biopsy.
Keywords: FDG-PET/CT, thymus, thymoma, malignant peripheral nerve sheath tumours, mediastinal germ cell tumours
Introduction
Computed tomography (CT) and magnetic resonance imaging (MRI) are excellent modalities for the localization of mediastinal masses and there are often features that may allow the correct diagnosis to be made. However, CT and MR cannot usually assess the aggressiveness of masses or identify viable tumour in residual masses after chemotherapy. Metabolic imaging using [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT, although not required in many cases, may be helpful for further characterization of masses and to guide the most appropriate site for biopsy.
The thymus
The thymus arises from the 3rd branchial pouch on each side, which fuse and migrate from the pharynx to the anterior mediastinum. A normal thymus may be seen anywhere along this path, and is usually seen anterior to the ascending arch. The thymus reaches its maximum weight at puberty and then undergoes fatty replacement with residual thymus identified in only 5% of people aged 40 years. CT identifies the normal thymus in virtually all children under 13 years of age and in most people under the age of 30 years.
Physiologic uptake of FDG is seen in the thymus in 28% of normal people aged less than 40 years, with the incidence dependent on the age of the patient, being identified in up to 73% of children under 13 years, decreasing to 12% of 20–40 year olds and up to 8% of people in their fourth decade[1]. There does appear to be a correlation between the degree of uptake and the attenuation of the gland suggesting that the disappearance of thymic uptake is related to fatty infiltration of the gland[2].
In children, the thymus is quadrilateral in shape initially and then becomes arrowhead shaped or bilobed and this is the pattern of uptake seen on FDG-PET/CT in 60% of cases. Unilateral extension to the right has been described in 25% and focal uptake in the midline in 16% of cases.
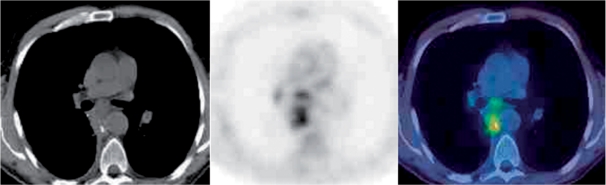
Changes in thymic size occur in response to sepsis, congenital heart disease, use of steroids and following chemotherapy and radiotherapy with re-growth occurring after recovery or following the termination of chemotherapy or steroids. The thymic volume may increase by 50% in thymic hyperplasia (Fig. 1) and is thought to be a rebound phenomenon with the thymus infiltrated by lymph follicles with large nuclei and plasma cells. Rebound thymic hyperplasia may last for several years after the completion of chemotherapy. It is important to differentiate thymic hyperplasia from ongoing or recurrent disease, especially in patients with lymphoma. The uptake in the normal thymus is variable with reported standardized uptake values (SUVs) ranging from 1.8 to 3.6; values greater than 4 are of concern. However, in a recent study 44% of patients with thymic hyperplasia following chemotherapy had an SUVmax of greater than 4[1].
Figure 1.
Thymic hyperplasia. Characteristic uptake on FDG-PET/CT.
Thymoma
Thymic epithelial tumours are the commonest tumour to originate within the thymus. They are uncommon with an incidence of 0.15:100,000. They usually occur in adults between 45 and 60 years of age and are often found incidentally and symptoms occur late. In 30–50% they are associated with myasthenia gravis and with red cell aplasia in 5%. Thymoma is also associated with autoimmune disorders such as systemic lupus, ulcerative colitis and hypogammaglobulinaemia. In 20% there is an associated malignancy including lymphoma, lung or thyroid cancer.
They may be classified as non-invasive when they are encapsulated, or invasive (15–37%) if there is extension beyond the capsule with spread along pleural and pericardial surfaces. Lymph node involvement occurs but is rare. Histologically it is difficult to differentiate benign from invasive tumours. They can be staged using the Masoaka classification (Table 1)[3]. However, in 1999 the World Health Organization proposed a histological classification, revised in 2004[4], which is widely accepted. In this classification thymomas are classified as A, AB, B1, B2 or B3 based on morphology and atypia of cells. Thymic carcinoma, including neuroendocrine and epithelial tumours, is a separate entity. Jeong et al.[5] stratified thymic tumours into 3 subgroups based on their prognosis with type A, AB and B1 low-risk thymomas, types B2 and B3 high-risk thymomas and thymic carcinoma.
Table 1.
Masaoka clinical staging system.
| Stage | 5-year survival (%) | |
|---|---|---|
| 1 | Encapsulated tumour with no gross or microscopic invasion | 96–100 |
| 2 | Macroscopic invasion into the mediastinal fat or pleura | 86–95 |
| 3 | Invasion into pericardium, great vessels or lung | 56–69 |
| 4a | Pleural or pericardial metastatic spread | Up to 50 |
| 4b | Lymphatic or haematogenous spread |
Thymic tumours are diagnosed with CT or MRI, and both modalities can define the anatomic extent of tumour but cannot always differentiate the subtypes. Most thymomas show mild uniform enhancement, with low-grade thymomas usually well defined and surrounded by clear fat planes and calcification, haemorrhage or necrosis may occur. In 34% there is capsular invasion with extension into adjacent structures. Thymic carcinomas may appear similar to thymomas but are aggressive and tend to metastasize to the lungs, liver, nodes and bone. They tend to have irregular margins with heterogeneous enhancement and vascular invasion.
The prognosis for low-risk (A, AB and B1) thymomas is very good following complete resection with a 100% 20-year survival. B3 thymomas have a complete resection rate of 92% with a 29% recurrence rate and a 36% 20-year survival. In thymic carcinoma complete resection is often not possible and debulking with adjuvant chemo-/radiotherapy is undertaken but the recurrence rate is high (36–77%)[6].
FDG-PET reflects glucose metabolism and may indicate tumour malignancy and therefore may be helpful in differentiation of thymoma types based on the SUV. Sung et al.[7] found that although there was a significant difference in uptake between the low-risk A, AB, B1 subtypes (SUVmax 4) and thymic carcinoma (SUVmax 10), there was no significant difference in uptake between low-risk and high-risk B2 and B3 subgroups (SUVmax 5.6). These authors noted that the uptake was homogeneous in 75% of thymic cancers and in 22% of high-risk tumours but not in low-risk tumours. Luzzi et al.[8] in a small study found the SUV could differentiate low-risk (A, AB, B1) from high-risk (B2, B3 and carcinoma) tumours and that the SUV in low-risk tumours was significantly less than that found in thymic lymphoma. Using a tumour/mediastinum ratio, Endo et al.[9] could differentiate the 3 groups and these authors suggested the degree of uptake in thymomas was related to the degree of atypia rather than the lymphocyte infiltration. El-Bawab et al.[10] found that the mean SUV was helpful in distinguishing thymic hyperplasia (SUV 1.89±0.58) from thymomas (SUV 4.75±0.88). Lee et al.[11] in a study of PET in the management of thymic tumours found that management was changed appropriately in only 7% of patients, with PET not influencing management in 88%. The effect of PET appears to be much less than in other tumours and this may be partly related to the comparative rarity of distant metastases with thymic tumours and also the variable, rather low uptake within thymic tumours making assessment of small volume disease difficult. In this study the negative predictive value (NPV) was 64.3%, which the authors suggested may limit the usefulness of PET in the follow-up of residual masses although the high positive predictive value (PPV) may allow for modification of management in a small group of patients.
Neurogenic tumours
CT and MRI are used to determine the site and extent of neurogenic tumours and their relationship to the spinal canal. In patients with neurofibromatosis type 1 (NF1), CT and MRI cannot always identify malignant degeneration and metabolic imaging may be of value.
NF1 is an autosomal dominant disease with an incidence of 1:2500–3000. Most patients have multiple neurofibromas but 30% also develop plexiform neurofibroma, which although composed of the same cell type as dermal neurofibromas grow along nerve roots and extend into surrounding structures. In 10% the plexiform neurofibromas undergo malignant transformation into malignant peripheral nerve sheath tumours (MPNST). The overall survival of patients with MPNST is poor (30% 5-year survival) with a high recurrence rate and metastases to lung, liver, nodes and the retroperitoneum. Clinically, malignant degeneration is suggested by increasing pain and deformity, and on imaging malignant transformation is suggested by interval growth or by the development of necrosis, haemorrhage or growth into adjacent structures. Biopsy may be difficult with sampling errors and complete surgical resection may not be possible in malignant transformation because of the local extent of the tumour.
FDG-PET has been found to be useful in other sarcomas[12] with the SUV used for risk assessment and as an independent predictor of survival and disease-free progression. In MPNST, FDG-PET is not only sensitive in the detection of malignant transformation (95% sensitivity, 72% specificity)[13] but also provides prognostic information with tumours with an SUV >3 having a shortened survival time compared with those with an SUV <3 (13 months vs 52 months)[14]. Wharby et al.[15] studied 69 patients with FDG-PET/CT with imaging performed at 90 and 240 min after injection. Using an SUVmax of 3.5 on delayed imaging for malignancy, these authors reported a sensitivity of 97% with a specificity of 87%. Lesions with an SUVmax <2.5 should be considered benign and lesions between 2.5 and 3.5 should be kept under review. These authors also found that the SUVmax of MPNSTs increased significantly with time unlike benign lesions, and that there was a correlation between SUVmax and grade of tumour (Fig. 2).
Figure 2.
MPNST. Non-homogeneous uptake on PET/CT. Biopsy should be directed to the posterior portion of the tumour.
Mediastinal germ cell tumours
Mediastinal germ cell tumours are usually benign and do not require imaging with FDG-PET. Germ cell tumours are classified based on cell type into (a) benign teratomas, (b) seminomas and (c) non-seminomatous germ cell tumours (NSGCT).
Mediastinal seminomas
These are uncommon but represent 25–50% of malignant germ cell tumours. They are usually diagnosed and staged with CT. Following chemotherapy a residual mediastinal mass may remain and FDG-PET has been used to identify residual viable disease. The early results were variable with one study finding no benefit[16]. However, Becherer et al.[17] found PET predicted residual viable tumour with a sensitivity of 80% and a specificity of 100% in masses greater than 3 cm, whereas for CT the sensitivity and specificity were 73%. In another multicentre trial the sensitivity was as high but the specificity was much lower at 47%[18].
NSGCT
These comprise a heterogeneous group of tumours and have a poorer prognosis than mediastinal seminomas (5-year survival 48% compared with 88%). They are usually diagnosed and staged with CT with markers helpful in diagnosis. Again these tumours show high uptake on FDG-PET, but it is not usually required for staging (Fig. 3). Following first-line treatment, residual masses remain in approximately 40% of patients consisting of necrosis in 40%, mature teratoma in 40% and viable tumour in 20%. Mature teratoma is chemotherapy resistant and surgical resection is required. In a large study of NSGCTs with residual masses and histological confirmation[19] the sensitivity and specificity of FDG-PET for viable tumour was 70% and 48% with a PPV of 59%, which was not dissimilar to CT, and FDG-PET/CT appears to offer no advantage over conventional imaging or markers.
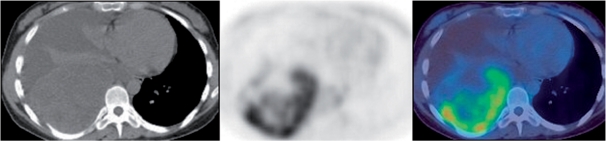
Figure 3.
NSGCT. Uptake on PET/CT identifies viable residual tumour in the posterior mediastinal mass.
Conclusions
FDG-PET/CT is not routinely used in mediastinal masses but is of value in assessing high-risk thymoma and thymic carcinomas. It is also important for the assessment of malignant transformation of plexiform neurofibromas. Its value in residual masses in mediastinal germ cell tumours is slightly more contentious. It appears to have value in seminomas but may not in NSGCTs.
References
- [1].Jerushalmi J, Frenkel A, Bar-Shalom R, Khoury J, Israel O. Physiologic thymic uptake of 18F-FDG PET in children and young adults: a PET/CT evaluation of incidence, patterns, and relationship to treatment. J Nucl Med. 2009;50:849–53. doi: 10.2967/jnumed.108.058586. doi:10.2967/jnumed.108.058586. PMid:19443604. [DOI] [PubMed] [Google Scholar]
- [2].Nakahara T, Fujii H, Ide M, et al. FDG uptake in the morphologically normal thymus: comparison of FDG positron emission tomography and CT. Br J Radiol. 2001;74:821–4. doi: 10.1259/bjr.74.885.740821. [DOI] [PubMed] [Google Scholar]
- [3].Masaoka A, Minden Y, Nakahara K, Tanioka T. Follow up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2458–92. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [4].Travis W, Brambilla E, Muller-Hermelink H, et al. Pathology and genetics of tumours of the lung thymus and heart. Paris: Oxford University Press; 2004. [Google Scholar]
- [5].Jeong YJ, Lee KS, Kim J, et al. Does CT of thymic epithelial tumours enable us to differentiate histologic subtypes and predict prognosis? AJR. 2004;183:283–9. doi: 10.2214/ajr.183.2.1830283. [DOI] [PubMed] [Google Scholar]
- [6].Srirajaskanthan R, Toubanakis C, Dusmet M, Caplin ME. A review of thymic tumours. Lung Cancer. 2008;60:4–13. doi: 10.1016/j.lungcan.2008.01.014. doi:10.1016/j.lungcan.2008.01.014. PMid:18343528. [DOI] [PubMed] [Google Scholar]
- [7].Sung YM, Lee KS, Kim B-T, et al. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumour subgroups. J Nucl Med. 2006;47:1628–34. [PubMed] [Google Scholar]
- [8].Luzzi L, Campione A, Gorla A, et al. Role of fluorine-fluorodeoxyglucose positron emission tomography/computed tomography in preoperative assessment of anterior mediastinal masses. Eur J Cardiothorac Surg. 2009;36:475–9. doi: 10.1016/j.ejcts.2009.03.055. doi:10.1016/j.ejcts.2009.03.055. PMid:19501523. [DOI] [PubMed] [Google Scholar]
- [9].Endo M, Nakagawa K, Ohde Y, et al. Utility of 18F-FDG PET for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer. 2008;60:4–13. doi: 10.1016/j.lungcan.2008.01.003. [DOI] [PubMed] [Google Scholar]
- [10].El-Bawab H, Al-Sugair A, Rafay M, et al. Role of Fluorine-18 fluorodeoxyglucose positron emission tomography in thymic pathology. Eur J Cardiothorac Surg. 2007;31:731–6. doi: 10.1016/j.ejcts.2007.01.024. doi:10.1016/j.ejcts.2007.01.024. PMid:17293120. [DOI] [PubMed] [Google Scholar]
- [11].Lee JW-Y, MacManus M, Hogg A, Hicks R, Ball D. Clinical influence of 18F-fluorodeoxyglucosepositron emission tomography on the management of primary tumours of the thymus. J Med Imag Rad Oncol. 2008;52:254–61. doi: 10.1111/j.1440-1673.2008.01955.x. doi:10.1111/j.1440-1673.2008.01955.x. PMid:18477120. [DOI] [PubMed] [Google Scholar]
- [12].Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol. 2003;32:133–8. doi: 10.1007/s00256-002-0586-9. [DOI] [PubMed] [Google Scholar]
- [13].Bredella MA, Torriani M, Hornicek F, et al. Value of PET in the assessment of patients with neurofibromatosis type-1. AJR. 2007;189:928–35. doi: 10.2214/AJR.07.2060. doi:10.2214/AJR.07.2060. PMid:17885067. [DOI] [PubMed] [Google Scholar]
- [14].Brenner W, Friedrich RE, Gawad KA, et al. Prognostic relevance of FDG PET in patients with neurofibromatosis type-1 and malignant peripheral nerve sheath tumours. Eur J Nucl Med Mol Imaging. 2006;33:428–32. doi: 10.1007/s00259-005-0030-1. doi:10.1007/s00259-005-0030-1. PMid:16404595. [DOI] [PubMed] [Google Scholar]
- [15].Warbey V, Ferner RE, Dunn JT, Calonje E, O’Doherty MJ. 18F-FDG PET/CT in the diagnosis of malignant peripheral nerve sheath tumours in neurofibromatosis type-1. Eur J Nucl Med Mol Imaging. 2009;36:751–7. doi: 10.1007/s00259-008-1038-0. doi:10.1007/s00259-008-1038-0. PMid:19142634. [DOI] [PubMed] [Google Scholar]
- [16].Ganjoo KN, Chan RJ, Sharma M, Einhorn LH. Positron emission tomography scans in the evaluation of postchemotherapy residual masses in patients with seminoma. J Clin Oncol. 1999;17:3457–60. doi: 10.1200/JCO.1999.17.11.3457. [DOI] [PubMed] [Google Scholar]
- [17].Becherer A, De Santis M, Karanikas G, et al. FDG-PET is superior to CT in the prediction of viable tumour in post chemotherapy seminoma residuals. Eur J Radiol. 2005;54:284–8. doi: 10.1016/j.ejrad.2004.07.012. doi:10.1016/j.ejrad.2004.07.012. PMid:15837411. [DOI] [PubMed] [Google Scholar]
- [18].Hinz S, Schrader M, Kempkensteffen C, et al. The role of positron emission tomography in the evaluation of residual masses after chemotherapy of advanced stage seminoma. J Urol. 2008;179:936–40. doi: 10.1016/j.juro.2007.10.054. doi:10.1016/j.juro.2007.10.054. PMid:18207171. [DOI] [PubMed] [Google Scholar]
- [19].Oechsle K, Hartmann M, Brenner W, et al. [18F]Fluorodeoxyglucose positron emission tomography in nonseminomatous germ cell tumours after chemotherapy: the German Multicentre Positron Emission Tomography Study Group. J Clin Oncol. 2008;26:5930–35. doi: 10.1200/JCO.2008.17.1157. doi:10.1200/JCO.2008.17.1157. PMid:19018083. [DOI] [PubMed] [Google Scholar]