Abstract
Imaging of neuroendocrine tumours (NET) poses significant challenges because of the heterogeneous biology of the tumours that are represented by this class of neoplasia. NET can range from benign lesions to highly aggressive cancers. Structural imaging techniques have suboptimal sensitivity in most published series and diagnosis is often delayed until metastatic disease is present. Current guidelines emphasise the importance of functional imaging for evaluating the extent of NET. The mainstay of this type of imaging has been somatostatin receptor scintigraphy (SRS) with [111In]diethylenetriaminepentaacetic acid-octreotide (Octreoscan™). Routine use of single-photon emission computed tomography (SPECT) and particularly of hybrid SPECT/computed tomography (CT) has significantly improved localisation of tumour sites and evaluation of somatostatin receptor (SSTR) expression, which is important for predicting the likelihood of response to somatostatin analogues (SSA). Positron emission tomography (PET) can also now be used for evaluating SSTR expression. There are a number of peptides that have been evaluated but [68Ga]tetraazocyclodecanetetraacetic acid (DOTA)-octreotate (GaTate) PET/CT, which has been shown to be significantly more sensitive for detecting small lesions than Octreoscan™, is now probably the preferred agent because high uptake in known sites of disease provides a diagnostic pair for assessing suitability of patients for [177Lu]DOTA-octreotate (LuTate) peptide receptor radionuclide therapy (PRRT). A range of other radiolabelled SSA has also been used for PRRT. Lesions without SSTR expression require alternative imaging and therapeutic strategies. Although fluorodeoxyglucose (FDG) uptake in low-grade NET is not generally increased relative to normal tissues, the loss of differentiation that often accompanies loss of SSTR expression may be associated with a significant increase in glycolytic metabolism and an accompanying improvement in the diagnostic sensitivity of FDG PET/CT. High FDG avidity is associated with a poorer prognosis but increases the likelihood of response to chemotherapy. Functioning tumours also require substrates for their secreted products. This can be exploited for NET imaging with amine precursor uptake being imaged using [18F]3,4-dihydrophenylalanine and serotonin-secreting tumours being sensitively detected using [11C]5-hydroxytryptamine. Both these agents are suitable for imaging with PET. [123I]meta-Iodo-benzyl-guanidine (MIBG) SPECT/CT may also be useful as a staging technique, particularly for NET of the sympathetic neuronal chain, and can identify patients who may be suitable for [131I]MIBG therapy. In the future, paradigms guided by clinical and biopsy features should allow personalised imaging paradigms aligned to therapeutic options.
Keywords: Neuroendocrine tumour, PET, radionuclide therapy, peptide receptor, FDG, [68Ga]octreotate
Introduction
There are at least 13 known neuroendocrine cells that can undergo malignant transformation, leading to a heterogeneous range of cancers that manifest disparate clinical symptoms[1]. These range from features of hormonal excess to more typical consequences of malignancy, including weight loss and pain.
Neuroendocrine cells signal to other cells, and thereby control various physiological processes, including digestion, through secretion of chemicals. These chemicals include peptide hormones that bind to stimulatory or inhibitory cell surface receptors. The most ubiquitous inhibitory receptor is the somatostatin receptor (SSTR), which has 5 known subtypes. Somatostatin is a peptide with 2 forms, containing 14 and 28 amino acids, respectively. Both bind to all subclasses of SSTR but are rapidly degraded in the blood by peptidases. Therefore, they tend to act in a paracrine manner, being secreted and acting locally. Various synthetic somatostatin analogues (SSA) have also been made to increase resistance to peptidases and thereby allow systemic delivery by virtue of longer circulation times. These SSA have varying affinity for the different SSTR subtypes. The most widely used is an 8-amino acid peptide, octreotide (Sandostatin®). This peptide has also been radiolabelled as [111In]diethylenetriaminepentaacetic acid (DTPA)-octreotide or Octreoscan™[2]. This agent has highest affinity for SSTR-2 and is suitable for imaging on a gamma camera. Despite malignant change, neuroendocrine tumours (NET) can retain, to a variable degree, the characteristics of the cell type from which they arose, including the capacity to secrete biological products. Whether they are functioning or not, most retain expression of the SSTR, most commonly of subtype 2. They are thus capable of being imaged using [111In]DTPA-octreotide[3], which can identify both the primary tumour and metastatic sites to soft tissue or bone (Fig. 1). This agent can also visualise SSTR expression on normal endocrine organs like the anterior pituitary and thyroid. More recently, octreotide and other SSA have also been labelled with positron-emitting radioisotopes, such as 68Ga, for positron emission tomography (PET).
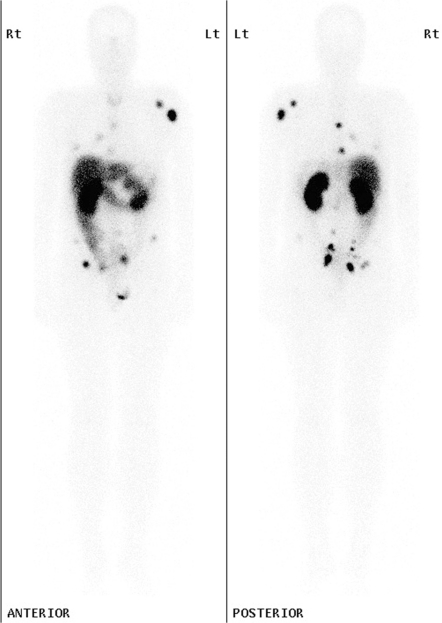
Figure 1.
Planar whole-body images in the anterior and posterior projection of a patient with metastatic rectal carcinoid demonstrate intense focal uptake in relationship to multiple bone metastases, particularly in the left scapula and humerus and in the pelvis as well as in soft tissue deposits in the lungs and abdomen, which were better localised on SPECT/CT (not shown).
Although the incidence of NET is less than 10 cases per 100,000 of population, this appears to have increased significantly in developed economies over the past 2 decades[4]. It is unclear whether this increase is real or reflects improved diagnostic methods. The relatively long survival of patients means that its prevalence is significantly higher than its incidence. Nevertheless, it remains a rare cancer and one with which many imaging specialists and oncologists consequently have little experience. Furthermore, the opportunity to perform large evaluation trials to validate new diagnostic methods or treatments is necessarily constrained leading to a relatively limited evidence base with which to inform patient management. Accordingly, many NET patients feel abandoned by conventional medicine, which they can often correctly accuse of providing delayed diagnosis and a lack of clarity regarding the appropriate investigation and management of their disease.
Despite their relative rarity, NET cause substantial morbidity in the community. Because many of the symptoms are innocuous early in the disease process and are often non-specific, they tend to be ignored by patients and medical practitioners alike, until advanced disease has developed. Also, because survival, even with advanced NET, can be relatively long, the duration of suffering can be protracted. Furthermore, the slow growth of these tumours leads to very low response rates using conventional cancer therapies including cytotoxic chemotherapy and radiotherapy that rely on actively proliferating cells. In primarily small and sometimes poorly designed trials, the therapeutic response rates have been disappointing and even the more successful treatments have objective response rates of only around 10–15%, which seldom justifies the attendant toxicity. This has led to a sense of nihilism amongst much of the oncology community regarding NET treatment. Accordingly, there is a critical need for effective diagnostic paradigms and personalised therapies[5]. In this review, the characteristics of NET and an explanation of why advanced molecular imaging is necessary for the detection, staging and characterisation of disease are discussed. How such imaging can inform and direct therapy, particularly the use of targeted therapy using radiopharmaceuticals, is also reviewed.
The classification of NET and its relevance to imaging
The various types of NET have been classified[6] and a TNM staging system has been agreed on by groups in Europe and North America[7]. The best-recognised NET is carcinoid with its associated syndrome of flushing, diarrhoea and endomyocardial fibrosis leading to right-sided heart failure. Siegfried Oberndorfer first described the characteristics of carcinoid tumorlets more than 100 years ago. However, he incorrectly considered them to be benign lesions. Most carcinoids are now thought to be at least potentially malignant. If large or found to be associated with regional lymph node metastases, imaging is appropriate to search for metastatic disease. Small bowel carcinoid can present with obstruction, intussusception or perforation; carcinoid of the appendix may lead to appendicitis. Most carcinoid tumours are slow growing and almost all express SSTR-2.
In the gastrointestinal tract, including the pancreas, there are numerous other neuroendocrine cell types, each producing different hormones, but which can be distinguished by the commonality of synaptophysin expression and the presence of dense-core granules containing chromogranin-A. Each of these cell types can form malignant tumours. The European Neuroendocrine Tumour Society (ENETS) have recommended a grading system based on the degree of proliferation in a tumour biopsy based on either the number of mitoses per 10 high-power fields (HPF) or the Ki-67 staining percentage. For either biomarker, 2 and 20 are important cut-offs for separating G1, 2 and 3 lesions[8]. This grading system has important prognostic implications but is constrained by the potential for sampling errors since there may be heterogeneity in the biology both within and between individual tumour deposits. Although most patients have indolent G1 lesions, some lesions can also behave in a highly malignant manner with rapid progression and death despite active treatment. An increase in tumour aggressiveness is generally associated with less well-differentiated histopathology, loss of SSTR expression and an increase in glucose utilisation, manifested by an increase in [18F]fluorodeoxyglucose (FDG) uptake on PET[9]. Lack of Octreoscan™ uptake has been shown to be associated with an adverse prognosis but does predict for higher responsiveness to chemotherapy. Combination therapies like carboplatin and etoposide, which are used for small cell cancers with neuroendocrine differentiation, can be quite effective in patients with poorly differentiated neuroendocrine carcinoma[10]. Patients with symptoms and biochemical features of NET or a biopsy-proven NET but a negative scan with a radiolabelled SSA should be considered for FDG PET.
After carcinoid-type tumours, endocrine tumours of the pancreas are the next most common subgroup. These may be benign and are problematic only insofar as they produce hormones that cause symptoms. Insulinoma, for example, is most often a benign pancreatic lesion but can produce life-threatening hypoglycaemic episodes. When pancreatic endocrine tumours become malignant, they tend to have a more aggressive natural history but higher response rates to available therapies compared with carcinoid tumours. Neuroendocrine carcinoma of the pancreas is most often represented by insulinoma (60%), followed by gastrinoma (20%), VIPoma and glucagonoma (around 5% each)[6]. Glucagonoma is associated with a typical rash called necrolytic migratory erythema. Up to 70% of these tumours are metastatic at diagnosis. Higher-grade tumours are more common with functioning pancreatic primary lesions than with small bowel NET and therefore, FDG PET positivity is also more common (Fig. 2). The exception to this statement is insulinoma, where the high insulin secretion drives FDG uptake in cardiac and skeletal muscles, limiting bioavailability of the tracer for tumour sites. Because these patients also tolerate fasting poorly, PET is not recommended for the staging of insulinoma. All but insulinoma have high SSTR-2 expression and therefore, the first-line molecular imaging technique is radiolabelled SSA. As with carcinoid syndrome, the indication for FDG PET is the presence of lesions that are negative on this scan.
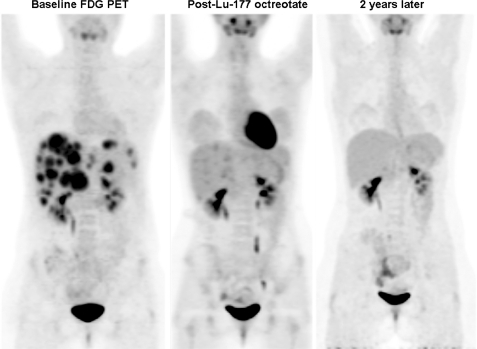
Figure 2.
This 18-year-old patient presented duodenal ulceration and was diagnosed with metastatic gastrinoma. Biopsy revealed a high Ki-67 and despite high [111In]octreotide uptake in her disease (not shown) a decision was made to treat her with chemotherapy. Unfortunately her disease progressed despite 2 lines of chemotherapy. An FDG PET prior to radionuclide therapy (left panel) confirmed progressive disease but a concordant distribution with [111In]octreotide uptake (not shown). Three months after completing 4 cycles of radionuclide therapy with Lu-177 octreotate the intensity of FDG uptake had dramatically decreased and 2 years later her FDG PET revealed no residual metabolic abnormality.
A further group of NET are characterised by lack of secretion of identified peptide hormones. These were formerly referred to as non-functioning islet cell tumours. They generally present with mechanical effects related to either the primary pancreatic tumour with pancreatitis or biliary obstruction, or its metastases. Although loss of hormone secretion could be construed to represent a loss of differentiation, most of these tumours still express SSTR-2 and have only variable FDG avidity. They also generally still secrete chromogranin-A (Cg-A).
Collectively NET of the gastrointestinal tract and pancreas are now termed as gastro-entero-pancreatic neuroendocrine tumours (GEP-NET). Most secrete Cg-A, which is now recognised as the most sensitive biomarker of disease, reflecting both disease burden and response to therapy[11]. It may not be increased in poorly differentiated, non-secreting tumours and increased levels are not specific for NET. The most common causes for increased Cg-A levels in the absence of NET are atrophic gastritis, use of proton-pump inhibitors and renal failure. The combination of symptoms suggestive of NET and increased Cg-A level should trigger scanning with a radiolabelled SSA, even in the absence of abnormality on structural imaging.
Although arising from different cellular origins, phaeochromocytoma[12], paraganglioma[13] and glomus tumours[14] represent a further group of NET that are known to express SSTR, primarily of subclass-2. Phaeochromocytomas are rare catecholamine-secreting tumours that arise from chromaffin tissue within the adrenal medulla and extraadrenal sites along the sympathetic chain, which extends from the thorax to the pelvis. They secrete hormones such as epinephrine, norepinephrine, and dopamine that can cause life-threatening and debilitating symptoms. Persistent and sometimes malignant hypertension is the most frequent manifestation leading to diagnosis. Discrimination between benign and malignant phaeochromocytoma is difficult on the basis of histology alone, and frequently the diagnosis of malignancy can only be made retrospectively once metastases have become evident. Indeed, the World Health Organization definition of a malignant phaeochromocytoma is made solely on the presence of metastases. In addition, there are currently no reliable markers to predict which patients are at risk of developing metastases. Before 2000 it was believed that 10% of phaeochromocytomas were associated with familial disease but it is now known that approximately 30% of patients with phaeochromocytoma carry a germ line (constitutional) mutation in one of the genes causative for specific familial cancer syndromes[15]. Most phaeochromocytomas take up amine as precursors for catecholamine production and can be visualised using radiolabelled meta-iodo-benzyl-guanidine (MIBG)[16]. Therefore, imaging with [123I]MIBG SPECT/CT can be useful for confirming the diagnosis of phaeochromocytoma and for diagnosing metastatic disease. In malignant phaeochromocytoma variable imaging characteristics may be observed with some lesions having higher avidity for MIBG than on SRS (Fig. 3) and vice versa.
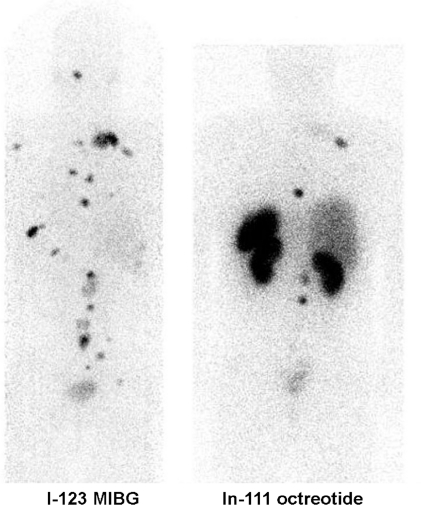
Figure 3.
Posterior projection images of a patient with metastatic phaeochromocytoma demonstrates more widespread disease on [123I]MIBG than [111In]octreotide scintigraphy and generally higher uptake.
The related head and neck paragangliomas, which are associated with the parasympathetic system, are generally not catecholamine producing but do express SSTR-2 as indicated by the high sensitivity of Octreoscan in these tumours[17].
The importance of imaging in determining therapeutic approaches to treatment of GEP-NET
There are 2 potential roles for imaging in the selection of therapy. The first is the conventional role of imaging for staging disease extent. The most favourable outcome would be to use a technique that could detect the early stages of disease when complete resection is feasible. However, the chance for surgical cure is often lost as a result of the delayed diagnosis of these tumours until they are already widely metastatic. Nevertheless, if they present as loco-regionally confined tumours, resection can be curative. Tumours that secrete active biological products tend to present earlier than those that are non-functioning. Insulinoma and gastrinoma, in particular, present with symptoms related to hypoglycaemia and increased gastric acid secretion, respectively and can be cured by surgery. Magnetic resonance imaging (MRI) and endoscopic ultrasound are useful techniques for identifying lesions in the pancreas; endoscopy, pill cameras or enteroclysis can be helpful for identifying bowel primaries. However, the ability to detect secondary deposits is often poor. Use of triple-phase CT or MRI can improve the detection of liver metastases[18].
The major biological product of carcinoid tumours is serotonin. This chemical is effectively degraded in the liver and therefore carcinoid syndrome does not generally occur until there are hepatic metastases or access to a draining venous system that can bypass the liver. Primary or secondary tumours of the ovary are sites of carcinoid lesions that can present with carcinoid syndrome in the absence of hepatic metastases. Again, if all sites of metastatic disease can be identified and resected, cure is possible.
In most other situations surgery is primarily palliative in intent and directed at preventing or relieving specific complications. Secretion of serotonin into the mesenteric venous system can lead to progressive mesenteric fibrosis with impaired perfusion to the small bowel. This can lead to a mesenteric angina syndrome with post-prandial pain being a common manifestation. Accordingly, resection of small bowel primaries, if recognised on imaging or endoscopy, is now advocated. Reduction in the burden of disease by palliative resection of large disease deposits can significantly improve symptoms and quality of life. Partial hepatic resection is the most common type of palliative surgery.
Synthetic chemicals that bind to SSTR have dramatically improved the quality of life of patients with hormone-related symptoms, and may delay tumour progression[19]. These are termed long-acting somatostatin analogues (SSA) and include octreotide (Sandostatin-LAR®, Novartis) and lanreotide (Somatuline® Autogel®, Ipsen). The recent PROMID study[20] suggests that even asymptomatic patients may benefit from these agents with demonstration of delayed progression compared with patients treated with placebo. Despite use of SSA many patients become refractory to these drugs with either uncontrolled symptoms or clear disease progression.
Improved techniques for molecular imaging characterisation of NET provide the ability to select and plan the most appropriate treatment of this important class of malignancy. Radioactive analogues of somatostatin, particularly [111In]DTPA-octreotide, are commonly used to detect and assess the extent of NET and provide a means to assess the possibility of delivering internal radiation to NET using agents labelled with particulate emitting radionuclides.
In patients with lesions that have little or no uptake of radiolabelled SSA, the likelihood of response to long-acting SSA is concomitantly reduced and these patients are also unsuitable for PRRT. Although the guidelines of the National Comprehensive Cancer Network (NCCN) suggest that FDG PET/CT is not indicated for the staging of NET because of relatively low sensitivity[21], there is evidence that it may provide complementary information to SRS by identifying lesions that have lost SSTR expression[9]. This is usually associated with more poorly differentiated tumours, especially higher-grade lesions as characterised by a high percentage of cells demonstrating the proliferation marker Ki-67. At my facility, we reserve FDG PET/CT for patients with biopsy indicating Ki-67 staining of >5% of cells, demonstrable progression on morphological imaging over a period of <6 months or the presence of lesions on CT or MRI that have low uptake on SSTR imaging.
Irrespective of SSTR expression, patients with any disease sites that have high FDG uptake are generally offered chemotherapy as first-line treatment, but may also be considered for external beam radiotherapy of isolated bone or soft tissue sites that could be treated with low morbidity. 5-Fluouracil (5-FU) and streptozocin is the most common regimen used for well-differentiated neuroendocrine carcinoma, and cisplatin/carboplatin and etoposide is used more often in more poorly differentiated neuroendocrine carcinomas based on efficacy in the related malignancy, small cell lung cancer and demonstrated efficacy in poorly differentiated tumours[10]. A platinum and etoposide doublet is the most common regimen for FDG-avid but SSTR-imaging negative disease in our institution. However, an improved understanding of the biology of NET has led to evaluation of novel molecular targeted therapies directed to potential mechanisms of disease in an attempt to develop treatment regimens with either greater efficacy or lower toxicity[22]. These include agents targeted at neo-vascularisation, such as antibodies like bevacizumab (Avastin®, Genentech/Roche), directed against secreted vascular endothelial growth factor (VEGF) and small tyrosine kinases, such as Sunitinib (Sutent®, Pfizer Inc.), which has activity against the VEGF receptor (VEGFR) amongst other targets and has shown some efficacy in pancreatic NET but less activity in carcinoid syndrome[23]. Other agents are directed at signalling pathways. The mammalian target of rapamycin (mTOR) complex is thought to be an important regulator of cell growth and proliferation in NET and there are several ongoing trials using the mTOR inhibitor everolimus (Afinitor®, Novartis)[24]. It is unclear whether particular subtypes of NET will be more responsive to these agents than others and whether these treatment approaches will be sufficiently efficacious to replace radionuclide therapy or more traditional chemotherapy combinations but there is clearly an expanding range of therapeutic options[25].
Irrespective of the initial type of treatment, patients who fail to demonstrate a metabolic response but who have a positive scan using a radiolabelled SSA are considered for peptide receptor radionuclide therapy. Conversely, patients who demonstrate failure following PRRT with primarily FDG-avid disease are considered for chemotherapy.
The treatment of GEP-NET using PRRT
There is a long experience in the treatment of NET using various forms of PRRT used alone or in combination with radio-sensitising chemotherapy. PRRT can provide significant symptomatic benefit, reduction in measurable hormone secretion, and either stabilisation or regression of previously progressive disease in most patients. These therapies were pioneered at the Erasmus Medical Centre in Rotterdam and most recent experience has focussed on [177Lu]tetraazocyclodecanetetraacetic acid (DOTA)-octreotate (LuTate) therapy[26].
At my own facility in Australia, we began using high-administered activity [111In]DTPA-octreotide therapy in 1996 and pioneered its use in combination with infusional 5-fluoro-uracil (5-FU) as a radiosenisitising chemotherapy[27]. Our institution was subsequently involved in a trial of Y-90 edotreotide therapy[28] and introduced LuTate therapy in 2005. We have recently established the safety of LuTate PRRT when combined with radiosensitising chemotherapy[29]. LuTate has also been shown to be associated with less marked reduction in renal function than Y-90 edotreotide therapy[30].
In our program, patients are considered eligible for treatment on compassionate grounds in the presence of unresectable disease that is either uncontrolled on maximum tolerated doses of long-acting SSA or is progressive over a period of less than 12 months. Patients are only treated if most of the known disease sites have uptake of greater intensity than the liver (grade 3 and 4 on the Krenning scale). In such patients, molecular imaging can demonstrate significant reduction in the extent and activity of disease sites after an induction therapy program comprising 3–5 doses given at 6–9 weekly intervals (Fig. 4). These are given in combination with an infusion of amino acids (25 g of arginine and 25 g of lysine in 1 L over 4 h) to decrease reabsorption of the LuTate by the proximal convoluted tubules of the kidney and thereby reduce the renal radiation dose[31]. Most patients also receive radiosensitising chemotherapy using infusional 5-FU at a dose of 200 mg/m2 per 24 h.
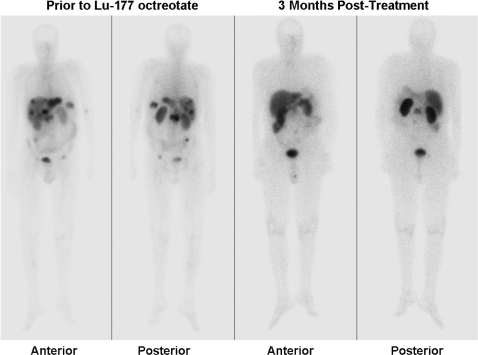
Figure 4.
Prior to radionuclide therapy with [177Lu]octreotate, widespread liver and focal bone disease in the lumbar spine and pelvis is apparent on whole-body planar [111In]octreotide imaging (left panels). At follow-up, 3 months following completion of 4 cycles of therapy (administered over a 6-month interval and therefore 9 months from the start of therapy), almost complete scintigraphic resolution was apparent (right panels). Residual disease in the lumbar region had been treated with external beam radiotherapy prior to radionuclide therapy.
A range of other compounds is being developed for PRRT[32] and will increase the scope for personalising the treatment of patients with various NET.
The use of molecular imaging to assess therapeutic efficacy in NET
The lack of robust methods of therapeutic response assessment represents a major limitation is establishing the efficacy of both PRRT and novel chemotherapy regimens. The evolution of structural changes following treatment may be highly discordant with the pattern of change in lesion metabolism or SSTR expression. For example, development of necrosis in previously highly octreotide-avid disease can be associated with loss of tracer uptake in these lesions but better visualisation on CT, particularly on the pre-contrast phase of imaging. Changes in vascularity can also change the pattern and timing of peak contrast enhancement. This is particularly relevant to anti-angiogenesis therapies.
Evaluation of the extent and activity of various subpopulations of malignant cells based on SSA imaging and FDG PET performed before and after treatment can demonstrate significant disparity in therapeutic response in different subpopulations of cells (Fig. 5). For example, even though the cells that concentrate LuTate are necessarily those with highest SSTR expression, these cells may be more radioresistant than FDG-avid cells by virtue of lower proliferation rates. By differentially depopulating cells with lower SSTR expression, the apparent intensity of lesion uptake may actually increase on Octreoscan SPECT/CT or GaTate PET/CT.
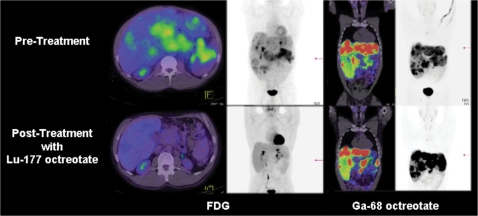
Figure 5.
Following chemotherapy that was not associated with morphological response, there was marked ongoing hepatomegaly and high FDG uptake in a primary pancreatic lesion (top left panels) but all sites of disease also had moderately intense uptake of [68Ga]octreotate (top right panels). At follow-up the patient’s clinical condition had improved markedly with resolution of previous abdominal distension, epigastric pain and fatigue. The FDG PET/CT demonstrated a marked reduction in hepatic size, development of multiple, low attenuation lesions with low metabolic activity and shrinkage of the pancreatic mass (bottom left panels). Despite this apparent complete metabolic response, multiple small but more intense lesions persisted in the liver and pancreas on [68Ga]octreotate. These findings suggest differential radiation sensitivity of the FDG-avid sub-population of cells compared with those with high somatostatin receptor expression and emphasise the advantages of cross-fire irradiation in heterogeneous tumour masses.
For phaeochromocytoma, paraganglioma and glomus jugulare tumours the traditional treatment has been MIBG. Although MIBG therapy can provide good biochemical and symptomatic responses and occasional impressive tumour regression, it generally has modest therapeutic effects. Most of these tumours also have significant SSTR-2 expression based on SRS and can therefore also be candidates for PRRT[33].
Novel molecular imaging approaches for NET
Functioning NET produce a characteristic array of peptide hormones and other neurosecretory products. The production of these secreted products requires facilitated uptake of various substrates. This can be exploited for NET imaging. Amine precursor uptake can be traced using [18F]3,4-dihydrophenylalanine (DOPA)[34] and serotonin-secreting tumours can be sensitively detected using [11C]5-hydroxytryptamine (HT)[35]. Both these agents are suitable for imaging with PET/CT. As with comparative studies comparing SRS and FDG PET, [18F]FDOPA and [11C]5-HT have both been shown to visualise lesions than are not seen on SRS and the converse can also apply[36]. Unlike the results of [123I]MIBG and SSTR imaging, which have direct therapeutic implications related to the biological characteristics of the tumours that are visualised, these newer agents are primarily of value for disease staging.
Conclusion
Although rare, NET can be associated with significant morbidity and decrease both the quality and duration of life of individuals who develop unresectable disease. Multi-modality imaging is increasingly recognised to be vital, not only in detecting and staging disease, but also in characterising biological features of lesions that may be relevant to the selection and delivery of therapy. In particular, the use of a range of biological probes can identify cellular populations with greater and lesser aggressiveness and thereby inform the choice between conventional cancer treatments such as chemotherapy and emerging treatment modalities, such as PRRT. Molecular targeted therapies may have different effects on subpopulations of cells within NET and therefore molecular imaging can also provide unique insights into therapeutic response to these agents. If the poor outcomes of these tumours is to be improved there will need to be a greater focus on designing diagnostic and therapeutic paradigms that are tailored to the individual characteristics of a given patient’s disease. Hybrid structural and molecular imaging techniques are providing new insights into the nature of this disparate group of neoplasms and will play a key role in developing and validating new therapies, including use of radiolabelled SSA. PRRT is an exciting emerging therapy that can provide significant benefit to well-selected patient populations.
References
- [1].Rindi G, Kloppel G. Endocrine tumors of the gut and pancreas tumor biology and classification. Neuroendocrinology. 2004;80(Suppl 1):12–15. doi: 10.1159/000080733. doi:10.1159/000080733. PMid:15477709. [DOI] [PubMed] [Google Scholar]
- [2].Krenning EP, Kwekkeboom DJ, Reubi JC, et al. 111In-octreotide scintigraphy in oncology. Metabolism. 1992;41:83–6. doi: 10.1016/0026-0495(92)90038-c. doi:10.1016/0026-0495(92)90038-C. [DOI] [PubMed] [Google Scholar]
- [3].Krenning EP, Kwekkeboom DJ, Oei HY, et al. Somatostatin receptor scintigraphy in carcinoids, gastrinomas and Cushing's syndrome. Digestion. 1994;55(Suppl 3):54–9. doi: 10.1159/000201202. doi:10.1159/000201202. PMid:7698538. [DOI] [PubMed] [Google Scholar]
- [4].Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. doi:10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- [5].Vilar E, Salazar R, Perez-Garcia J, Cortes J, Oberg K, Tabernero J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer. 2007;14:221–32. doi: 10.1677/ERC-06-0074. doi:10.1677/ERC-06-0074. PMid:17639039. [DOI] [PubMed] [Google Scholar]
- [6].Kloppel G, Rindi G, Anlauf M, Perren A, Komminoth P. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007;451(Suppl 1):S9–27. doi: 10.1007/s00428-007-0461-0. doi:10.1007/s00428-007-0461-0. [DOI] [PubMed] [Google Scholar]
- [7].Kloppel G, Rindi G, Perren A, Komminoth P, Klimstra DS. The ENETS and AJCC/UICC TNM classifications of the neuroendocrine tumors of the gastrointestinal tract and the pancreas: a statement. Virchows Arch. 2010;456:595–7. doi: 10.1007/s00428-010-0924-6. doi:10.1007/s00428-010-0924-6. PMid: [DOI] [PubMed] [Google Scholar]
- [8].Rindi G, Kloppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757–62. doi: 10.1007/s00428-007-0452-1. doi:10.1007/s00428-007-0452-1. PMid:17674042. [DOI] [PubMed] [Google Scholar]
- [9].Kayani I, Bomanji JB, Groves A, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–55. doi: 10.1002/cncr.23469. doi:10.1002/cncr.23469. PMid:18383518. [DOI] [PubMed] [Google Scholar]
- [10].Moertel CG, Kvols LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–32. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. doi:10.1002/1097-0142(19910715)68:2<227::AID-CNCR2820680202>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [11].Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A-biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1006-3. doi:10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- [12].Tenenbaum F, Lumbroso J, Schlumberger M, et al. Comparison of radiolabeled octreotide and meta-iodobenzylguanidine (MIBG) scintigraphy in malignant pheochromocytoma. J Nucl Med. 1995;36:1–6. [PubMed] [Google Scholar]
- [13].Schmidt M, Fischer E, Dietlein M, et al. Clinical value of somatostatin receptor imaging in patients with suspected head and neck paragangliomas. Eur J Nucl Med Mol Imaging. 2002;29:1571–80. doi: 10.1007/s00259-002-0939-6. doi:10.1007/s00259-002-0939-6. PMid:12458390. [DOI] [PubMed] [Google Scholar]
- [14].Muros MA, Llamas-Elvira JM, Rodriguez A, et al. 111In-pentetreotide scintigraphy is superior to 123I-MIBG scintigraphy in the diagnosis and location of chemodectoma. Nucl Med Commun. 1998;19:735–42. doi: 10.1097/00006231-199808000-00003. doi:10.1097/00006231-199808000-00003. PMid:9751927. [DOI] [PubMed] [Google Scholar]
- [15].Benn DE, Richardson AL, Marsh DJ, Robinson BG. Genetic testing in pheochromocytoma- and paraganglioma-associated syndromes. Ann N Y Acad Sci. 2006;1073:104–11. doi: 10.1196/annals.1353.011. doi:10.1196/annals.1353.011. PMid:17102077. [DOI] [PubMed] [Google Scholar]
- [16].Scholz T, Schulz C, Klose S, Lehnert H. Diagnostic management of benign and malignant pheochromocytoma. Exp Clin Endocrinol Diabetes. 2007;115:155–9. doi: 10.1055/s-2007-970410. doi:10.1055/s-2007-970410. [DOI] [PubMed] [Google Scholar]
- [17].Kwekkeboom DJ, van Urk H, Pauw BK, et al. Octreotide scintigraphy for the detection of paragangliomas. J Nucl Med. 1993;34:873–8. [PubMed] [Google Scholar]
- [18].Rockall AG, Reznek RH. Imaging of neuroendocrine tumours (CT/MR/US) Best Pract Res Clin Endocrinol Metab. 2007;21:43–68. doi: 10.1016/j.beem.2007.01.003. doi:10.1016/j.beem.2007.01.003. [DOI] [PubMed] [Google Scholar]
- [19].Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31:169–88. doi: 10.1111/j.1365-2036.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- [20].Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. doi: 10.1200/JCO.2009.22.8510. doi:10.1200/JCO.2009.22.8510. PMid:19704057. [DOI] [PubMed] [Google Scholar]
- [21].Clark OH, Benson 3rd AB, Berlin JD, et al. NCCN Clinical Practice Guidelines in Oncology: neuroendocrine tumors. J Natl Compr Canc Netw. 2009;7:712–47. doi: 10.6004/jnccn.2009.0050. [DOI] [PubMed] [Google Scholar]
- [22].Yao JC, Hoff PM. Molecular targeted therapy for neuroendocrine tumors. Hematol Oncol Clin North Am. 2007;21:575–81; x. doi: 10.1016/j.hoc.2007.04.001. doi:10.1016/j.hoc.2007.04.001. PMid:17548041. [DOI] [PubMed] [Google Scholar]
- [23].Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–10. doi: 10.1200/JCO.2007.15.9020. doi:10.1200/JCO.2007.15.9020. PMid:18612155. [DOI] [PubMed] [Google Scholar]
- [24].Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–8. doi: 10.1200/JCO.2008.16.7858. doi:10.1200/JCO.2008.16.7858. PMid:18779618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eriksson B. New drugs in neuroendocrine tumors: rising of new therapeutic philosophies? Curr Opin Oncol. 2010;22:381–6. doi: 10.1097/CCO.0b013e32833adee2. doi:10.1097/CCO.0b013e32833adee2. PMid:20473165. [DOI] [PubMed] [Google Scholar]
- [26].Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. doi: 10.1200/JCO.2007.15.2553. doi:10.1200/JCO.2007.15.2553. PMid:18445841. [DOI] [PubMed] [Google Scholar]
- [27].Kong G, Johnston V, Ramdave S, Lau E, Rischin D, Hicks RJ. High-administered activity In-111 octreotide therapy with concomitant radiosensitizing 5FU chemotherapy for treatment of neuroendocrine tumors: preliminary experience. Cancer Biother Radiopharm. 2009;24:527–33. doi: 10.1089/cbr.2009.0644. doi:10.1089/cbr.2009.0644. PMid:19877882. [DOI] [PubMed] [Google Scholar]
- [28].Bushnell DL, Jr, O'Dorisio TM, O'Dorisio MS, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol. 2010;28:1652–9. doi: 10.1200/JCO.2009.22.8585. doi:10.1200/JCO.2009.22.8585. PMid:20194865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hubble D, Kong G, Michael M, Johnson V, Ramdave S, Hicks RJ. (177)Lu-octreotate, alone or with radiosensitising chemotherapy, is safe in neuroendocrine tumour patients previously treated with high-activity (111)In-octreotide. Eur J Nucl Med Mol Imaging. 2010 doi: 10.1007/s00259-010-1483-4. doi:10.1007/s00259-010-1483-4. PMid: 20445977. [DOI] [PubMed] [Google Scholar]
- [30].Valkema R, Pauwels SA, Kvols LK, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med. 2005;46(Suppl 1):83S–91S. [PubMed] [Google Scholar]
- [31].Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging. 2003;30:9–15. doi: 10.1007/s00259-002-0982-3. doi:10.1007/s00259-002-0982-3. PMid:12483404. [DOI] [PubMed] [Google Scholar]
- [32].Reubi JC, Macke HR, Krenning EP. Candidates for peptide receptor radiotherapy today and in the future. J Nucl Med. 2005;46(Suppl 1):67S–75S. [PubMed] [Google Scholar]
- [33].Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging. 2008;52:334–40. [PubMed] [Google Scholar]
- [34].Jager PL, Chirakal R, Marriott CJ, Brouwers AH, Koopmans KP, Gulenchyn KY. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. J Nucl Med. 2008;49:573–86. doi: 10.2967/jnumed.107.045708. doi:10.2967/jnumed.107.045708. PMid:18344441. [DOI] [PubMed] [Google Scholar]
- [35].Orlefors H, Sundin A, Ahlstrom H, et al. Positron emission tomography with 5-hydroxytryprophan in neuroendocrine tumors. J Clin Oncol. 1998;16:2534–41. doi: 10.1200/JCO.1998.16.7.2534. [DOI] [PubMed] [Google Scholar]
- [36].Koopmans KP, Neels OC, Kema IP, et al. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J Clin Oncol. 2008;26:1489–95. doi: 10.1200/JCO.2007.15.1126. doi:10.1200/JCO.2007.15.1126. PMid:18349401. [DOI] [PubMed] [Google Scholar]