Abstract
The improvements in outcomes associate with the use of preoperative therapy rather than postoperative treatment means that clinical teams are increasingly reliant on imaging to identify high-risk features of disease to determine treatment plans. For many solid tumours, including rectal cancer, validated techniques have emerged in identifying prognostic factors pre-operatively. In the MERCURY study, a standardised scanning technique and the use of reporting proformas enabled consistently accurate assessment and documentation of the prognostic factors. This is now an essential tool to enable our clinical colleagues to make treatment decisions. In this review, we describe the proforma-based reporting tool that enables a systematic approach to the interpretation of the magnetic resonance images, thereby enabling all the clinically relevant features to be adequately assessed.
Keywords: Rectal cancer staging, proforma, prognosis, pathology, preoperative treatment, magnetic resonance imaging, staging
Introduction
The last few years have seen improvements in survival of patients with a diagnosis of rectal cancer through improvements in surgical, oncological treatment, planning and follow-up. One of the key aspects of planning treatment is the staging information provided by radiologists to the multidisciplinary team. As with most solid malignancies, precise staging and anatomical delineation of the tumour enables preoperative treatment and radical surgery to improve outcomes in high-risk patients and equally prevent unnecessary and harmful over-treatment of patients with good prognosis or early stage tumours. We are now moving into an era of individualising treatments according to both risk of local and distant failure and the imaging is as crucially important as the tumour type and genetic susceptibility. The aim of this article is to consider proforma reporting as a tool that enables the systematic assessment of patients with newly diagnosed rectal cancer.
Why should we use proforma reporting?
We have traditionally relied on freeform text reporting to provide the radiological opinion of the tumour appearance and extent. However, when these reports are audited there is generally a lack of specific cancer staging detail that compromises the ability to consistently obtain the necessary staging data required for modern cancer management. In the past, free text reporting was also the standard for colorectal pathology reporting but audits of minimum data for staging of these freeform reports showed significant missing data. The pathology assessment of circumferential resection margin status in rectal cancers is a crucial prognostic factor strongly predicting for local recurrence and results in postoperative therapy. In the 1997 pathology audit, this was only reported in a third of patients[1]. Following the introduction of minimum data set reporting in histopathology, these rates improved to almost 100% resulting in better selection of patients who could benefit from postoperative adjuvant therapy. The same arguments can be applied to imaging with patients potentially benefiting from imaging-based preoperative treatments.
As has been shown in pathology audits, the absence of explicitly stated prognostic factors in reports can result in false-negative assumptions and can potentially lead to under staging and under treatment of patients[2]. In a recent audit of proforma versus non-proforma reporting in radiology, key staging data was missing in 97% of freeform reports; the introduction of proforma reporting resulted in missing data in only 3% of reports. Magnetic resonance imaging (MRI) assessment of the potential circumferential resection margin, which is the main factor that determines the use of preoperative chemoradiotherapy, was missing in 74% of non-proforma reports. With the introduction of proforma reporting this reduced to 4% (Mangat et al., Proceedings of RSNA 2009). Therefore, proforma reporting encourages:
accurate and reliable baseline documentation of disease
analysis of imaging-related prognostic data in the future may help better target patients in clinical trials receiving novel therapies and effective implementation based on such on imaging characteristics
the ability to compare baseline pre-treatment tumour phenotype images will provide us with much needed data regarding the effects of treatments on disease
There are other wider benefits for patients: national audits have demonstrated unacceptable variation in surgical practice and outcomes between regions and trusts for different cancers but without the imaging baseline and detailed and systematic documentation of the stage of tumours it is not possible to make any meaningful comparisons.
In 2002, we undertook a prospective European, multicentre, multidisciplinary study, with emphasis on quality control of imaging assessment, surgery, and pathology, to assess the diagnostic accuracy, feasibility, and reproducibility of MRI in predicting the final histopathological staging of tumour within 1 mm of the circumferential resection margin (MERCURY study). In the study, we used proformas, based on published national guidelines, to ensure quality control in collecting data. Before the launch of the study, all radiologists attended intensive training workshops, using comparative materials from MRI and histopathology. These workshops focused on interpretation of images and acquisition techniques, which require considerable specialist skill and were an important part of the success in achieving consistency and reproducibility in this multicentre trial.
In this article, we describe the rectal cancer staging proforma and image interpretation techniques to aid its completion.
Proforma reporting technique in rectal cancer
A proforma recommended for use in staging rectal cancers is illustrated in Appendix 1.
Tumour morphology
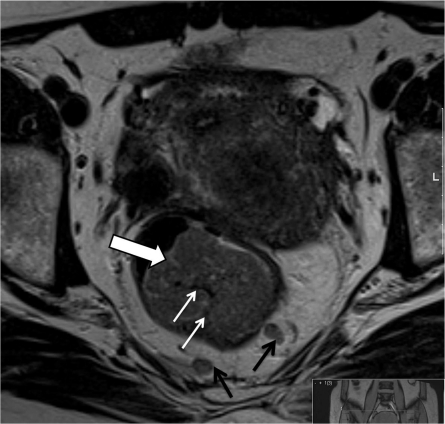
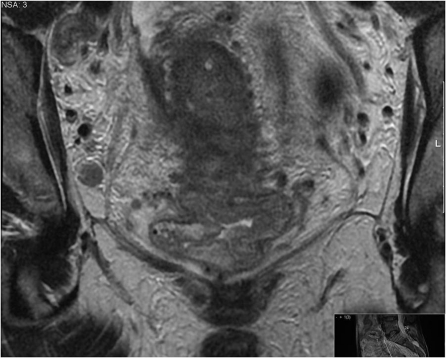
As with pathology reporting, it is helpful to the clinical teams to provide a general description of the morphology of the tumour. Rectal cancers can be polypoidal with a low signal intensity stalk that attaches the tumour to the rectal wall or annular with varying degrees of central ulceration. Polypoidal lesions tend to have a smaller invasive front and extend beyond the rectal wall through the stalk (Fig. 1). Annular ulcerating tumours are characterized by a central ulcer/crater with raised rolled edges. They invade at the ulcer crater with either a smooth or more nodular infiltrating border (Fig. 2). The latter is associated with a poorer prognosis and higher rate of metastatic failure [3–8]. It is very rare for rectal tumours to show intramural spread and this would be noteworthy for the surgeon in planning the distal resection margin. Current recommendation allows a 1-cm distal clearance[9–11]. Mucinous tumours are characterized by evidence of high signal intensity pools within the tumour [12] and are associated with a worse prognosis in the rectum. This is thought to be due to the fact that they infiltrate diffusely and unlike annular or polypoidal tumours they may spread intramurally[10,13].
Figure 1.
Polypoidal lesions tend to have a smaller invasive front and extend beyond the rectal wall through the stalk.
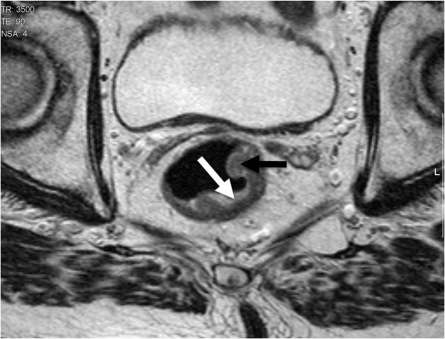
Figure 2.
Annular ulcerating tumours are characterized by a central ulcer/crater with raised rolled edges. They invade at the ulcer crater with either a smooth or more nodular infiltrating border. The latter is associated with a poorer prognosis and higher rate of metastatic failure[1–6].
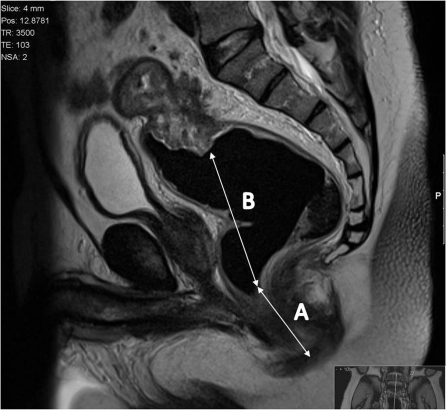
Tumour height
The height of the tumour is given from the anal verge as this is a useful reference point for surgeons. It is measured from the lowest point to the raised rolled edge of the tumour to the anal verge (Fig. 3). Traditionally the rectum has been divided into thirds, as outcomes and surgical management are affected by the height of the tumour:
Upper: the lowest edge of the tumour is higher than 10 cm from the anal verge. The anterior wall of the upper rectum is covered by the peritoneal reflection; the risk of peritoneal perforation of these tumours and the risk of trans-coelomic spread is high for such patients, and a warning to the surgeon will enable careful dissection and mobilisation of the rectum in such cases to minimize the risk of tumour spillage[14,15].
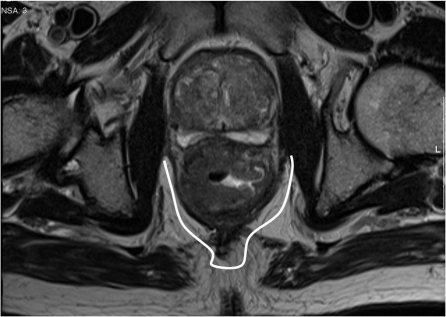
Middle: lowest point between 5 cm and 10 cm. This segment of the rectum lying below the peritoneal reflection is completely encircled by mesorectum and will therefore be suitable for total mesorectal excision (TME). The surgical margins will be formed by the mesorectal fascia which is the plane of dissection in TME surgery[16].
Lower: tumour below 5 cm from the anal verge, the area of rectum and mesorectum below the origin of the levators where the mesorectum tapers sharply, makes both the surgical approach and the interpretation of images challenging.
Figure 3.
The height of the tumour is given from the anal verge as this is a useful reference point for surgeons. It is measured from the lowest point to the raised rolled edge of the tumour to the anal verge.
T staging and depth of tumour spread beyond the muscularis propria
Within each T stage there is a heterogeneous survival range and there has been much interest in identifying poor prognostic groups within each stage. Both T1 and T2 tumours have high 5-year survival rates but the widest range in survival is demonstrated in patients with T3 tumours, which make up 80% of rectal tumours seen in clinical practice. The relationship between survival and the depth of extramural spread in millimetres is well established and it is independent of other prognostic factors including the circumferential margin status[17,18]. The more recent TNM classifications now take into account the body of evidence that enables the subclassification of T3 tumours as follows (T3a <1 mm; T3b 1–5 mm; T3c >5–15 mm; T3d >15 mm). Dukes’ original observations noted that with increasing depth of spread, there was an increased rate of nodal spread and distant failure. This is reflected in better disease-free survival for patients with T3 <5 mm (T3a, T3b) versus those with T3c or T3d tumours at presentation.
The MERCURY study group showed that depth of extramural tumour spread was accurate compared with the histopathology reference standard validating MRI as a method of accurate preoperative prognostication using depth of extramural spread.
On T2-weighted images, the mucosal layer is demonstrated as a fine low signal intensity line with the thicker, higher signal submucosal layer seen beneath this.
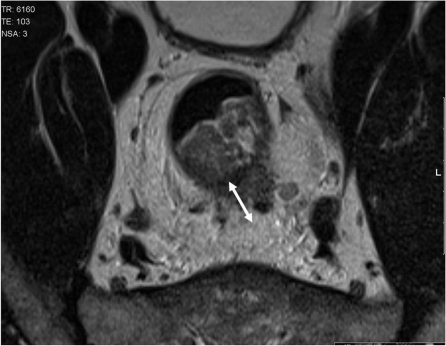
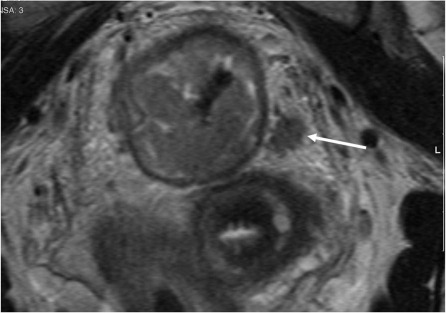
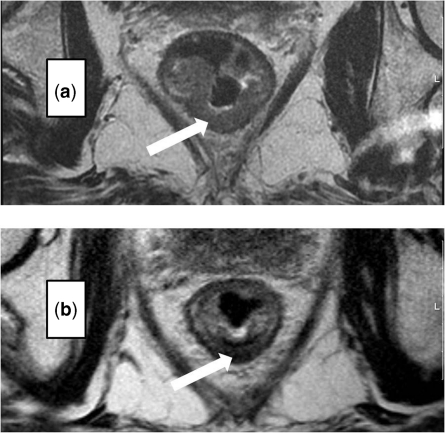
The muscularis propria is often visualised as two distinct layers: the inner circular layer and the outer longitudinal layer. The outer muscle layer has an irregular, somewhat corrugated appearance with interruptions due to vessels entering the rectal wall. The perirectal fat appears as high signal surrounding the low signal of the muscularis propria and contains signal void vessels. The mesorectal fascia is seen as a fine low signal layer enveloping the perirectal fat and rectum and it is this layer that defines the surgical excision plane in TME anterior resections[16]. MRI diagnosis of a tumour spread beyond the muscularis propria is based on the presence of tumour signal extending into the perirectal fat with a broad-based bulging or nodular configuration in continuity with the intramural portion of the tumour (Fig. 4). It is important to note the continuity with the intramural component as there can be disruption to the outer longitudinal layer as a result of small vessels penetrating the wall, not necessarily invaded by tumour. The depth of extramural spread is measured in millimetres beyond the outer edge of the longitudinal muscle coat.
Figure 4.
MRI diagnosis of a tumour spread beyond the muscularis propria is based upon the presence of tumour signal extending into the perirectal fat with a broad-based bulging or nodular configuration in continuity with the intramural portion of the tumour.
Low rectal tumours
The commencement of the puborectalis sling marks the start of the narrowest part of the mesorectum, which at this point tapers to form a thin segment of perirectal fat. Below the puborectalis sling lies the anal canal, which is formed of the mucosa and submucosa, the internal sphincter, the 1- to 2-mm intersphincteric plane and the external sphincter. At this level there is no mesorectum, which higher in the rectum acts as a protective barrier to contain tumour. Therefore any spread beyond the muscularis propria would result in exposure of tumour at the margins if an ultra-low TME or conventional plane abdominoperineal excision (APE) is undertaken (Fig. 5). This is because for the conventional plane of surgery, the muscularis propria rather than the mesorectal fascia forms the surgical margins. Therefore this plane can only be considered safe if a good thickness of preserved muscularis propria is visible deep to the invasive border of the tumour. If the muscularis propria is fully replaced by tumour or has minimal extension into the intersphincteric plane, then a more radical surgical approach is required in the form of an extralevator/extrasphincteric APE that removes the entire sphincter complex, levators and mesorectum en bloc.
Figure 5.
At this level there is no mesorectum, which, higher in the rectum, acts as a protective barrier to contain tumour. Therefore any spread beyond the muscularis propria would result in exposure of tumour at the margins if an ultra-low TME or conventional plane APE is undertaken.
Extramural venous invasion
Pathological studies of vascular invasion in the early 1980s[19–21] first established the body of evidence of the prognostic significance of vascular invasion. Amongst these histological studies, the rate of detection of vascular invasion was variable with reported incidences ranging from 17% to 70%[19,22–28]. Whereas lymph node status becomes less predictive of local failure in patients undergoing careful radical excision of the rectum and mesorectum, venous invasion remains an important independent prognostic factor[29,30]. Therefore extramural venous invasion (EMVI) is considered to be an important risk factor for both local recurrence[27], distant metastases[25,31–34] and death[25,27,35–40].
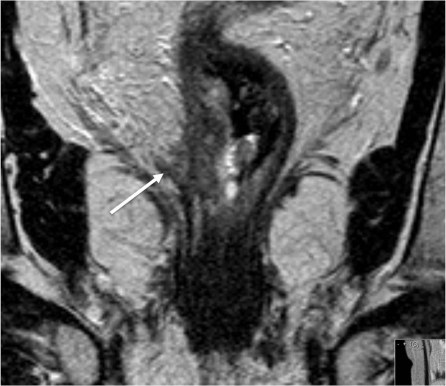
MRI is the only imaging modality that has been shown to consistently demonstrate EMVI in rectal cancer[41] and is depicted as discrete serpiginous or tubular projections of intermediate signal intensity into perirectal fat, following the course of a visible perirectal vein (Fig. 6). We have devised an MRI-EMVI grading score to evaluate the presence or absence of radiological features indicative of EMVI[42], and in a recent study carried out in our own unit we found that the proportion of patients with MRI-detected EMVI rectal and rectosigmoid cancers undergoing primary surgery was 26%, which was similar to the histologically proven proportion (28%). The sensitivity and specificity of MRI for detecting EMVI in this series was 62% and 88%, respectively. The relapse-free survival at 3 years was 35% for patients with an MRI-EMVI score of 3–4, compared with 74% for those with a score of 0–2 (P < 0.001); this was similar to values in patients with positive and negative histological EMVI status, respectively (34 vs 73.7%; P < 0.001). Therefore MRI assessment of EMVI is an important prognostic factor that can be readily detected and predicts outcome[43].
Figure 6.
MRI is the only imaging modality that has been shown to consistently demonstrate extramural vascular invasion in rectal cancer[7] and is depicted as discrete serpiginous or tubular projections of intermediate signal intensity into perirectal fat, following the course of a visible perirectal vein.
Nodal stage
Nodal staging has traditionally relied on size of the nodes on MRI criteria, however several studies have indicated the inaccuracy of using this technique alone. Criteria based on the outline of the node and features of signal intensity have been shown to be more reliable[44,45] It may be in the future that this will be augmented by using contrast agents as early results have been quite promising[46].
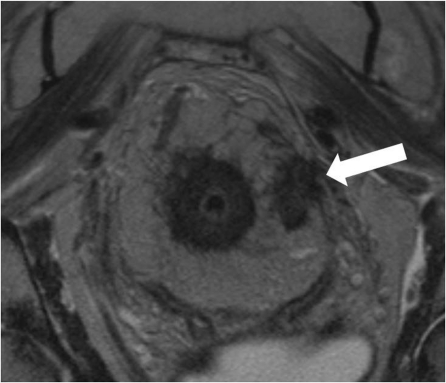
Lymph nodes should only be evaluated on high-resolution (minimum in-plane resolution 0.6 × 0.6 mm, slice thickness 3 mm). Nodes can then be classified according to their appearance. Uniform, homogenous signal intensity nodes are not considered to be suspicious. Nodes are judged suspicious if they have irregular borders or mixed signal intensity or both (Fig. 7)[44]. The prognostic importance of lymph nodes in patients undergoing TME is uncertain and most patients with 3 or fewer involved nodes have a good prognosis. However, patients with a heavier nodal burden (4 or more involved nodes) are known to have a worse prognosis but are also more likely to present with other more easily identifiable poor prognostic features on MRI, namely extramural venous invasion and increasing depth of extramural spread.
Figure 7.
Lymph nodes should only be evaluated on high resolution (minimum in plane resolution 0.6×0.6 mm, slice thickness 3 mm). Nodes can then be classified according to their appearance. Uniform, homogenous signal intensity nodes are not considered to be suspicious. Nodes are judged suspicious if they have irregular borders or mixed signal intensity or both[8].
Distance to circumferential resection margin
The mesorectal fascia represents the potential circumferential resection margin (CRM) in patients undergoing TME surgery. Bissett et al.[47,48] conclusively demonstrated by using markers that the mesorectal fascial plane seen with MRI corresponds to the fascia propria encasing the mesorectum and excised by TME. MRI can therefore be used to assess the distance from the tumour edge to the potential circumferential margin and thereby predict the final CRM status in patients undergoing TME surgery.
Studies have used different cut-off values of the measured distance to the mesorectal fascia to predict CRM status. The authors of one study concluded that CRM status could be predicted with a high degree of accuracy and consistency[49] when a cut-off of 5 mm MRI measured distance to the CRM was used. Other authors have been able to use more precise MRI measurements to predict CRM status. Our own prospective study involving 98 patients undergoing preoperative MRI staging predicted the CRM status to be positive when tumour was identified by MRI within 1 mm of the mesorectal fascia. Comparison with histology showed very good (92%, κ = 0.81) agreement[41] and in the MERCURY study, the 1-mm cut-off correctly predicted the CRM status[50].
Measurements are taken for the distance of tumour to the mesorectal fascia, the potential CRM of the tumour. A potentially positive margin is defined as tumour lying within 1 mm of the mesorectal fascia (Fig. 8). This is taken for:
the main tumour
suspicious lymph nodes
extramural vascular invasion
tumour deposit/satellite (a tumour deposit <3 mm is classed as a nodule and >3 mm classed as a node)
Figure 8.
Measurements are taken for the distance of tumour to the mesorectal fascia, the potential CRM of the tumour. A potentially positive margin is defined as tumour lying within 1 mm of the mesorectal fascia.
Pelvic sidewall lymph nodes
We record the location of the node, size, site and if there are any suspicious features according to the morphological criteria stated above (Fig. 9). This may be a further predictor of survival and local recurrence[51].
Figure 9.
We record the location of the node, size, site and if there are any suspicious features according to the morphological criteria stated above.
Post chemoradiotherapy assessment of tumour regression
For those patients who received preoperative chemoradiotherapy we used a tumour regression grade analysis, grade 1–5, modified from Dworak et al.[52]. This is known to be a better predictor for outcome after treatment compared with T stage (Fig. 10)[53].
Figure 10.
Post chemoradiotherapy assessment of tumour regression. For those patients who received preoperative chemoradiotherapy we used a tumour regression grade analysis, grade 1–5, modified from Dworak et al.[9] This is known to be a better predictor for outcome after treatment compared with T stage.
The universal adoption of proforma reporting will enable us to provide standardised comparisons to help in future national audits and improve treatment policies in cancer centres. A joint working group of the Royal College of Radiologists and the National Cancer Intelligence Network has been set up to develop web-based tools to support radiologists in their multidisciplinary team preparations so that they can effectively provide key data that is so crucial to the management of patients with cancer.
Appendix 1

References
- [1].Bull AD, Biffin AH, Mella J, et al. Colorectal cancer pathology reporting: a regional audit. J Clin Pathol. 1997;50:138–42. doi: 10.1136/jcp.50.2.138. doi:10.1136/jcp.50.2.138. PMid:9155695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morris EJ, Maughan NJ, Forman D, Quirke P. Who to treat with adjuvant therapy in Dukes B/stage II colorectal cancer? The need for high quality pathology. Gut. 2007;56:1419–25. doi: 10.1136/gut.2006.116830. doi:10.1136/gut.2006.116830. PMid:17494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cohen AM, Wood WC, Gunderson LL, Shinnar M. Pathological studies in rectal cancer. Cancer. 1980;45:2965–8. doi: 10.1002/1097-0142(19800615)45:12<2965::aid-cncr2820451213>3.0.co;2-m. doi:10.1002/1097-0142(19800615)45:12<2965::AID-CNCR2820451213>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [4].Bjerkeset T, Morild I, Mork S, Soreide O. Tumor characteristics in colorectal cancer and their relationship to treatment and prognosis. Dis Colon Rectum. 1987;30:934–8. doi: 10.1007/BF02554279. doi:10.1007/BF02554279. [DOI] [PubMed] [Google Scholar]
- [5].Michelassi F, Vannucci L, Montag A, et al. Importance of tumor morphology for the long term prognosis of rectal adenocarcinoma. Am Surg. 1988;54:376–9. [PubMed] [Google Scholar]
- [6].Grinnell RS. The grading and prognosis of carcinoma of the colon and rectum. Ann Surg. 1939;109:500–33. doi: 10.1097/00000658-193904000-00002. doi:10.1097/00000658-193904000-00002. PMid:17857341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spratt JA, Spjut HJ. Prevalence and prognosis of carcinoma of the colon and rectum. Cancer. 1967;20:1976–85. doi: 10.1002/1097-0142(196711)20:11<1976::aid-cncr2820201125>3.0.co;2-m. doi:10.1002/1097-0142(196711)20:11<1976::AID-CNCR2820201125>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [8].Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39:585–9. doi: 10.1136/jcp.39.6.585. doi:10.1136/jcp.39.6.585. PMid:3722412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hughes TG, Jenevein EP, Poulos E. Intramural spread of colon carcinoma. A pathologic study. Am J Surg. 1983;146:697–9. doi: 10.1016/0002-9610(83)90320-3. [DOI] [PubMed] [Google Scholar]
- [10].Madsen PM, Christiansen J. Distal intramural spread of rectal carcinomas. Dis Colon Rectum. 1986;29:279–82. doi: 10.1007/BF02553041. doi:10.1007/BF02553041. [DOI] [PubMed] [Google Scholar]
- [11].Andreola S, Leo E, Belli F, et al. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis Colon Rectum. 1997;40:25–9. doi: 10.1007/BF02055677. doi:10.1007/BF02055677. [DOI] [PubMed] [Google Scholar]
- [12].Kim MJ, Park JS, Park SI, et al. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J Comp Assisted Tomogr. 2003;27:48–55. doi: 10.1097/00004728-200301000-00010. doi:10.1097/00004728-200301000-00010. PMid:12544243. [DOI] [PubMed] [Google Scholar]
- [13].Sidoni A, Bufalari A, Alberti PF. Distal intramural spread in colorectal cancer: a reappraisal of the extent of distal clearance in fifty cases. Tumori. 1991;77:514–17. doi: 10.1177/030089169107700613. [DOI] [PubMed] [Google Scholar]
- [14].Salerno G, Daniels IR, Moran BJ, Wotherspoon A, Brown G. Clarifying margins in the multidisciplinary management of rectal cancer: the MERCURY experience. Clin Radiol. 2006;61:916–23. doi: 10.1016/j.crad.2006.06.005. doi:10.1016/j.crad.2006.06.005. PMid:17018303. [DOI] [PubMed] [Google Scholar]
- [15].Shepherd NA, Baxter KJ, Love SB. Influence of local peritoneal involvement on pelvic recurrence and prognosis in rectal cancer. J Clin Pathol. 1995;48:849–55. doi: 10.1136/jcp.48.9.849. doi:10.1136/jcp.48.9.849. PMid:7490320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brown G, Kirkham A, Williams GT, et al. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol. 2004;182:431–9. doi: 10.2214/ajr.182.2.1820431. [DOI] [PubMed] [Google Scholar]
- [17].Cawthorn SJ, Parums DV, Gibbs NM, et al. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer [see comments] Lancet. 1990;335:1055–9. doi: 10.1016/0140-6736(90)92631-q. doi:10.1016/0140-6736(90)92631-Q. [DOI] [PubMed] [Google Scholar]
- [18].Willett CG. Technical advances in the treatment of patients with rectal cancer [editorial; comment] Int J Radiat Oncol Biol Phys. 1999;45:1107–8. doi: 10.1016/s0360-3016(99)00329-6. doi:10.1016/S0360-3016(99)00329-6. [DOI] [PubMed] [Google Scholar]
- [19].Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins in cancer of the rectum. Br J Surg. 1980;67:439–42. doi: 10.1002/bjs.1800670619. doi:10.1002/bjs.1800670619. PMid:7388345. [DOI] [PubMed] [Google Scholar]
- [20].Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5:141–63. doi: 10.1111/j.1365-2559.1981.tb01774.x. doi:10.1111/j.1365-2559.1981.tb01774.x. PMid:7216178. [DOI] [PubMed] [Google Scholar]
- [21].Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. Spread of rectal cancer within veins. Histologic features and clinical significance. Am J Surg. 1981;141:15–7. doi: 10.1016/0002-9610(81)90004-0. doi:10.1016/0002-9610(81)90004-0. [DOI] [PubMed] [Google Scholar]
- [22].Tsuchiya A, Ando Y, Kikuchi Y, Kanazawa M, Sato H, Abe R. Venous invasion as a prognostic factor in colorectal cancer. Surg Today. 1995;25:950–3. doi: 10.1007/BF00312379. doi:10.1007/BF00312379. PMid:8640019. [DOI] [PubMed] [Google Scholar]
- [23].Lui KK, Enjoji M, Inokuchi K. Venous permeation of colorectal carcinoma. Jap J Surg. 1980;10:284–9. doi: 10.1007/BF02468789. doi:10.1007/BF02468789. PMid:7218608. [DOI] [PubMed] [Google Scholar]
- [24].Sunderland D. The significance of vein invasion by cancer of the rectum and sigmoid: a microscopic study of 210 cases. Cancer. 1949;2:429–37. doi: 10.1002/1097-0142(194905)2:3<429::aid-cncr2820020307>3.0.co;2-g. doi:10.1002/1097-0142(194905)2:3<429::AID-CNCR2820020307>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [25].Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer. 1988;61:1018–23. doi: 10.1002/1097-0142(19880301)61:5<1018::aid-cncr2820610527>3.0.co;2-h. doi:10.1002/1097-0142(19880301)61:5<1018::AID-CNCR2820610527>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- [26].Minsky BD, Mies C, Recht A, Rich TA, Chaffey JT. Resectable adenocarcinoma of the rectosigmoid and rectum. II. The influence of blood vessel invasion. Cancer. 1988;61:1417–24. doi: 10.1002/1097-0142(19880401)61:7<1417::aid-cncr2820610723>3.0.co;2-9. doi:10.1002/1097-0142(19880401)61:7<1417::AID-CNCR2820610723>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [27].Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–29. doi: 10.1002/1097-0142(19831001)52:7<1317::aid-cncr2820520731>3.0.co;2-6. doi:10.1002/1097-0142(19831001)52:7<1317::AID-CNCR2820520731>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [28].Knudsen JB, Nilsson T, Sprechler M, Johansen A, Christensen N. Venous and nerve invasion as prognostic factors in postoperative survival of patients with resectable cancer of the rectum. Dis Colon Rectum. 1983;26:613–7. doi: 10.1007/BF02552975. doi:10.1007/BF02552975. [DOI] [PubMed] [Google Scholar]
- [29].Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–82. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- [30].Bokey EL, Ojerskog B, Chapuis P, Dent O, Newland RC, Sinclair G. Local recurrence after curative excision of the rectum for cancer without adjuvant therapy: role of total anatomical dissection. Br J Surg. 1999;86:1164–70. doi: 10.1046/j.1365-2168.1999.01216.x. doi:10.1046/j.1365-2168.1999.01216.x. PMid:10504371. [DOI] [PubMed] [Google Scholar]
- [31].Ouchi K, Sugawara T, Ono H, et al. Histologic features and clinical significance of venous invasion in colorectal carcinoma with hepatic metastasis. Cancer. 1996;78:2313–7. doi: 10.1002/(sici)1097-0142(19961201)78:11<2313::aid-cncr7>3.0.co;2-n. doi:10.1002/(SICI)1097-0142(19961201)78:11<2313::AID-CNCR7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- [32].Gunther K, Dworak O, Remke S, et al. Prediction of distant metastases after curative surgery for rectal cancer. J Surg Res. 2002;103:68–78. doi: 10.1006/jsre.2001.6312. doi:10.1006/jsre.2001.6312. PMid:11855920. [DOI] [PubMed] [Google Scholar]
- [33].Shirouzu K, Isomoto H, Kakegawa T, Morimatsu M. A prospective clinicopathologic study of venous invasion in colorectal cancer. Am J Surg. 1991;162:216–22. doi: 10.1016/0002-9610(91)90073-m. doi:10.1016/0002-9610(91)90073-M. [DOI] [PubMed] [Google Scholar]
- [34].Horn A, Dahl O, Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum. 1991;34:798–804. doi: 10.1007/BF02051074. doi:10.1007/BF02051074. [DOI] [PubMed] [Google Scholar]
- [35].Harrison JC, Dean PJ, el-Zeky F, Vander Zwaag R. From Dukes through Jass: pathological prognostic indicators in rectal cancer [see comments] Hum Pathol. 1994;25:498–505. doi: 10.1016/0046-8177(94)90122-8. doi:10.1016/0046-8177(94)90122-8. [DOI] [PubMed] [Google Scholar]
- [36].Chapuis PH, Dent OF, Fisher R, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg. 1985;72:698–702. doi: 10.1002/bjs.1800720909. doi:10.1002/bjs.1800720909. PMid:4041728. [DOI] [PubMed] [Google Scholar]
- [37].Freedman LS, Macaskill P, Smith AN. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet. 1984;2:733–6. doi: 10.1016/s0140-6736(84)92636-9. [DOI] [PubMed] [Google Scholar]
- [38].Horn A, Dahl O, Morild I. The role of venous and neural invasion on survival in rectal adenocarcinoma. Dis Colon Rectum. 1990;33:598–601. doi: 10.1007/BF02052215. doi:10.1007/BF02052215. [DOI] [PubMed] [Google Scholar]
- [39].Mulcahy HE, Toner M, Patchett SE, Daly L, O'Donoghue DP. Identifying stage B colorectal cancer patients at high risk of tumor recurrence and death. Dis Colon Rectum. 1997;40:326–31. doi: 10.1007/BF02050424. doi:10.1007/BF02050424. [DOI] [PubMed] [Google Scholar]
- [40].Newland R, Dent O, Lyttle M, Chapuis P, Bokey E. Pathological determinants of survival associated with colorectal cancer with lymph node metastases. Cancer. 1994;73:2076–82. doi: 10.1002/1097-0142(19940415)73:8<2076::aid-cncr2820730811>3.0.co;2-6. doi:10.1002/1097-0142(19940415)73:8<2076::AID-CNCR2820730811>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [41].Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–64. doi: 10.1002/bjs.4034. doi:10.1002/bjs.4034. PMid:12594673. [DOI] [PubMed] [Google Scholar]
- [42].Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR. 2008;191:1517–22. doi: 10.2214/AJR.08.1298. doi:10.2214/AJR.08.1298. PMid:18941094. [DOI] [PubMed] [Google Scholar]
- [43].Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–36. doi: 10.1002/bjs.5917. doi:10.1002/bjs.5917. PMid:17932879. [DOI] [PubMed] [Google Scholar]
- [44].Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–7. doi: 10.1148/radiol.2272011747. doi:10.1148/radiol.2272011747. PMid:12732695. [DOI] [PubMed] [Google Scholar]
- [45].Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52:78–83. doi: 10.1016/j.ejrad.2003.12.005. doi:10.1016/j.ejrad.2003.12.005. PMid:15380850. [DOI] [PubMed] [Google Scholar]
- [46].Koh DM, Brown G, Temple L, et al. Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings – initial observations. Radiology. 2004;231:91–9. doi: 10.1148/radiol.2311030142. doi:10.1148/radiol.2311030142. PMid:14976266. [DOI] [PubMed] [Google Scholar]
- [47].Brown G, Richards CJ, Newcombe RG, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211:215–22. doi: 10.1148/radiology.211.1.r99ap35215. [DOI] [PubMed] [Google Scholar]
- [48].Bissett IP, Fernando CC, Hough DM, et al. Identification of the fascia propria by magnetic resonance imaging and its relevance to preoperative assessment of rectal cancer. Dis Colon Rectum. 2001;44:259–65. doi: 10.1007/BF02234302. doi:10.1007/BF02234302. [DOI] [PubMed] [Google Scholar]
- [49].Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497–504. doi: 10.1016/s0140-6736(00)04040-x. doi:10.1016/S0140-6736(00)04040-X. [DOI] [PubMed] [Google Scholar]
- [50].MERCURY. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ (Clinical Research Ed) 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sugihara K, Kobayashi H, Kato T, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006;49:1663–72. doi: 10.1007/s10350-006-0714-z. doi:10.1007/s10350-006-0714-z. [DOI] [PubMed] [Google Scholar]
- [52].Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. doi:10.1007/s003840050072. PMid:9112145. [DOI] [PubMed] [Google Scholar]
- [53].Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121–30. doi:10.1002/cncr.10327. PMid:11920483. [PubMed] [Google Scholar]