Abstract
Ephrins and Eph receptor(s) have recently been implicated in regulating neurogenesis in the adult subventricular zone (SVZ) and rostral migratory stream (RMS). Here, we examined the role of ephrinB3-EphB3 signaling in mediating the SVZ response to traumatic brain injury (TBI). Analysis of EphB3 expression showed co-localization with glial fibrillary acidic protein (GFAP)-positive neural stem progenitor cells (NSPCs) and doublecortin-positive neuroblasts, while ephrinB3 was expressed outside the neurogenic region. TBI resulted in a significant reduction in EphB3 expression, which coincided with enhanced NSPC survival and proliferation at 3 and 7 days post-injury. Analysis of mice lacking either ephrinB3 (ephrinB3−/−) or EphB3 (EphB3−/−) showed a significant increase in bromodeoxyuridine (BrdU) incorporation and Ki67 immunoreactivity in the SVZ. Interestingly, cell death was dissimilar between knockout mice, where cell death was reduced in EphB3−/− but increased in ephrinB3−/− mice. Lateral ventricle infusion of soluble pre-clustered ephrinB3-Fc reversed the proliferative and cell death defects in ephrinB3−/− but not EphB3−/− mice and prevented TBI-induced proliferation in wild type NSPCs. Coincidently, tumor suppressor p53 expression was increased following EphB3 stimulation and is reduced in the absence of either EphB3 or ephrinB3. Furthermore, pharmacological inhibition and siRNA knockdown of p53 attenuated ephrinB3-Fc mediated growth suppression while having no effect on cell death in cultured NSPCs. These data demonstrate that EphB3 signaling suppresses NSPC proliferation in a p53-dependent manner, induces cell death in the absence of ligand stimulation and is transiently reduced in the SVZ to initiate the expansion and survival of endogenous adult NSPCs following TBI.
Keywords: traumatic brain injury, ephrin, Eph, subventricular zone, p53
Introduction
The discovery of resident adult neural stem/progenitor cells (NSPCs) [1-5] and subsequent observations of increased survival, proliferation and migration in the subventricular zone (SVZ) following CNS damage [6-10] suggest that endogenous neural progenitor cells could be recruited to replace or support the recovery of damaged tissue. The adult SVZ is a highly vascularized [11, 12] and heterogeneous microenvironment occupied by multiciliated ependymal cells [13], astrocyte-like stem cells, transit-amplifying progenitor cells and immature migrating neuroblasts [14-18]. The SVZ niche represents an area of active neurogenesis that persists in both the adult rodent and human brain [19-23]. Under normal physiological conditions, the slowly proliferating neural stem cells (glial fibrillary acidic protein (GFAP)-positive; type B cells) can give rise to more rapidly dividing transit-amplifying progenitor cells (type C cells), which differentiate into neuroblasts (type A cells) [16] and undergo long-distance, tangential chain migration through the rostral migratory pathway (RMS) to the olfactory bulb (OB) [24-28].
Recent studies have begun to evaluate the mechanisms involved in the maintenance of the adult SVZ niche as a means to delineate the functional potential of NSPCs following CNS injury. In fact, many studies have now shown changes in the proliferative capacity and migration routes of cells residing in the SVZ and RMS after traumatic brain injury (TBI) and stroke [2, 8, 29-31]. For example, ischemia-induced CA1 pyramidal neuron death has been shown to mobilize and recruit caudal SVZ cells to the CA1 region of the hippocampus where they generated new neurons that altered learning and memory functions [10]. These and other studies demonstrate that SVZ-derived progenitor cells undergo increased proliferation, long-distance migration and multipotential differentiation. However, the exact mechanisms that initiate or promote this potentially therapeutic response to brain trauma are not well understood. Studies implicating factors that regulate the SVZ response to TBI are limited [32-35]; however, studies performed in ischemic rodent models suggest that many diffusible mitogens may be involved, including epidermal growth factors (EGF), basic fibroblastic growth factors (bFGF), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), stem cell factor (SCF), as well as nitric oxide and inflammatory cytokines [36-46]. Multiple growth promoting pathways may synergistically activate proliferation following ischemia, but it is still unclear whether they play a pathophysiological role through direct or indirect actions on NSPC functions. Alternatively, the activation of endogenous NSPCs may be influenced by local growth inhibitory molecules both in and around the SVZ.
The SVZ retains many developmental features including survival, proliferation and migration of NSPCs. The regulation of such events following brain injury may therefore require factors important during development. One family of membrane-bound growth and guidance molecules, that are especially important in diverse areas of the developing brain, are ephrins and their erythropoietin-producing hepatocellular (Eph) receptors [47-53]. We and others have recently described a novel role for ephrin-Eph signaling in the regulation of NSPCs in the adult SVZ [51, 52, 54, 55]. In particular, we have shown that ephrinB3 maintains proper adult NSPC numbers by reducing cell death through EphA4 dependence-receptor mechanisms [51, 56]. Immunohistochemical and RT-PCR analysis demonstrated that ephrinB3 is not expressed within the SVZ but lies outside the neurogenic region in the neighboring striatum and corpus callosum, while EphB1, B2, B3, B4, B6 and A4 as well as ephrin-B1 and B2 have been detected within the adult SVZ [51, 52, 57]. Therefore, Eph receptor-bearing NSPCs may be tightly regulated by the expression of ephrinB3 in the adult brain.
Here we examined the role of ephrinB3-EphB3 interactions in regulating proliferation and survival in the SVZ following unilateral controlled cortical impact (CCI) injury [58, 59]. We show that proliferation is augmented in mice deficient in either ephrinB3 or EphB3 following both sham and CCI injury and that ephrinB3-Fc infusion can reverse this effect in ephrinB3−/− but not EphB3−/− mice. EphB3 stimulation using ephrinB3-Fc infusion in wild type mice suppressed proliferation while inducing tumor suppressor p53 activation. Conversely, cell death is increased in ephrinB3−/− but decreased in EphB3−/− compared to wild type mice, suggesting that Eph receptors may also function as pro-apoptotic dependence receptors to regulate cell survival in the brains largest neurogenic region.
Materials and Methods
Animals
The generation of the mutant CD1 mice and genotyping using PCR analysis has been previously described [50, 60-62]. Animals were sacrificed by decapitation under anesthesia; the brain was immediately removed and frozen in OCT (Tissue Tek) then preserved at −80°C until further processing. Bromodeoxyuridine (BrdU) (Sigma) was administered as previously described [51]. Animal procedures were approved by the University of Miami Animal Use and Care Committee.
Controlled Cortical Impact and infusion
Male mice ages 2-4 months were anesthetized with ketamine and xylazine by i.p. injection and positioned in a stereotaxic frame. Body temperature was monitored with a rectal probe and maintained at 37°C with a controlled heating pad set. A 5mm craniotomy was made using a portable drill over the right parieto-temporal cortex (−2.5 mm A/P and 2.0 mm lateral from bregma). Injury was induced by moderate CCI using the eCCI-6.3 device (Custom Design & Fabrication) at a velocity of 6 m/s, depth of 0.5 mm, and 150 ms impact duration. Sham controls received craniotomy only. Infusions were performed as previously described [51]. Briefly, ephrinB3-Fc or control human Fc fragments (R&D Systems; 140 μg/ml in PBS) were pre-clustered with goat anti-human-Fc (ratio 1:5; Jackson Research Laboratories) for 2 hrs at RT and loaded into osmotic pumps (Alzet). Immediately after CCI or sham operation each infusion device was attached to the stereotactic holder and lowered 3mm into the contralateral ventricle at the following coordinates (from bregma: A/P −0.5 mm; lateral 0.7 mm) and infused over a 3 day period (100 μl volume, rate 0.5 μl/hr).
Tissue Culture
Adult NSPCs were isolated from the SVZ of adult wild type and EphB3−/− CD1 mice (2-4 months old) and grown as described by Scheffler and colleagues [63]. Cells were plated at 2000 cells/well and grown for 3 days in the presence of pre-clustered ephrinB3-Fc or Fc-control then assessed for cell proliferation and cell death. Cell death of NSPCs in culture was assessed by the addition of Sytox orange and Hoechst (LIVE/DEAD assay, Invitrogen) for staining dead and total nuclei. Proliferation was tested using BrdU (10 μM) added to each well for 1 hr, cells were fixed and stained with anti-BrdU (1:100 Roche). For quantification of BrdU and LIVE/DEAD assays, six images per well were acquired by using the Cellomics kinetic scan HCS reader and quantified as percentage of cell death by VHCS scan software. For p53 inhibition studies, pifithrin-α (10 μM; BIOMOL) was added 30 min prior and siRNA p53 (Cell Signaling; siRNA scramble, Dharmacon 25nM) used 24 hr prior to ephrinB3-Fc and Fc-control stimulations.
Immunohistochemistry and Western blot Analysis
Fresh frozen tissue sections were fixed with either 10% buffered formalin or cold acetone, permeabilized and incubated in primary antibodies (anti-GFAP, Dako; anti-p53, Novocastra; anti-EphB3, Abcam; anti-DCX, Santa Cruz; anti-PSA-NCAM, Chemicon, anti-BrdU, Chemicon) overnight at 4°C as previously described [51]. For BrdU staining, sections were first incubated in 2N HCl for 1 hr as previously described [64]. For Western blot analysis, tissue from the ipsilateral and contralateral wall of the ventricle was carefully dissected out and lysed in modified RIPA buffer as previously described [64]. Equal amounts (25 μg) of protein were resolved on 8% SDS-PAGE gels, transferred and probed using mouse anti-phospho (p)-p53 and anti-p53 (1:2000 Cell Signaling); mouse anti-β-actin (1:8000 Sigma); mouse anti-p-AKT and anti-AKT (1:4000 Cell Signaling); goat anti-EphB3 (1:4000 Abcam) and rabbit anti-ephrinB3 (1:500 Zymed) in 5% milk overnight at 4°C.
Stereology
Fresh frozen coronal, 30 μm-thick cryostat brain serial sections were collected beginning at the rostral extent of the lateral ventricle (total thickness of the examined tissue was 1500 μm). Cell death was assessed using TUNEL staining kit as previously described [64]. The total number of BrdU and TdT-mediated dUTP Nick-End Labeling (TUNEL)-positive cells within 1500 μm of SVZ tissue was analyzed using a motorized Axiophot (Zeiss) microscope, Optronix cooled video camera, and MicroBrightField StereoInvestigator software package. To perform non-biased cell number estimation, the optical fractionator method and optical dissector probe were used. The ipsilateral and contralateral SVZ contours were individually outlined at 20X magnifications using Hoechst to visualize/identify the cells lining the wall of the lateral ventricle in every fifth tissue section (30 μM). Next, a grid of 50 × 50 μm was placed over this area, and the number of TUNEL or BrdU-immunopositive cells with an identifiable nucleus was randomly counted using the optical fractionator function at 63X magnification (sampling box: 20 × 20 μm).
Statistical analysis
Student’s two-tailed t-test was used for comparison of two experimental groups. Multiple comparisons were done using one-way ANOVA followed by Tukey test for multiple pairwise examinations. Data was expressed as mean ± SEM.
Results
EphB3 expression is down-regulated in the adult SVZ after brain injury
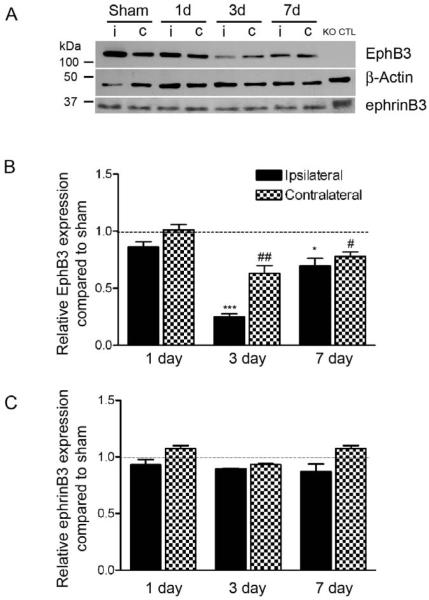
To evaluate the role of ephrinB3-EphB3 signaling in the adult SVZ, we first determined the cellular localization and relative expression levels of EphB3 receptor using immunohistochemistry and Western blot analysis. Previously we showed ephrinB3 staining to be localized outside the SVZ [51]. Here, we demonstrate using confocal image analysis co-localization of EphB3 receptor (red) with doublecortin (DCX; green)-positive neuroblasts (Fig. 1A-D) and glial fibrillary acidic protein (GFAP; green)-expressing NSPCs (Fig. 1E-H) in the SVZ. EphB3 immunostaining from the SVZ of EphB3−/− mice is shown as a staining control (Fig. 1I-L). EphB3 is also highly expressed within the rostral migratory stream (RMS) (Suppl. Fig. 1A) on GFAP-expressing cells (Suppl. Fig. 1B) and DCX-positive neuroblasts (Suppl. Fig. 1C). Next, we examined whether there were quantitative changes in ephrinB3 or EphB3 expression levels in the lateral wall of the lateral ventricle in response to moderate cortical damage. Tissue was dissected from both ipsilateral (i) and contralateral (c) sham- and CCI-injured wild type mice at 1, 3 and 7 days and evaluated for ephrinB3 and EphB3 protein expression using Western blot analysis. Our findings demonstrate a significant reduction in the ipsilateral and contralateral expression of EphB3 at 3 days and 7 days following CCI injury (Fig. 2A, 2B). No significant difference was observed in the level of ephrinB3 expression (Fig. 2A, 2C). This suggests that down-regulation of EphB3 on adult NSPCs and neuroblasts may play a role in the SVZ response to brain injury.
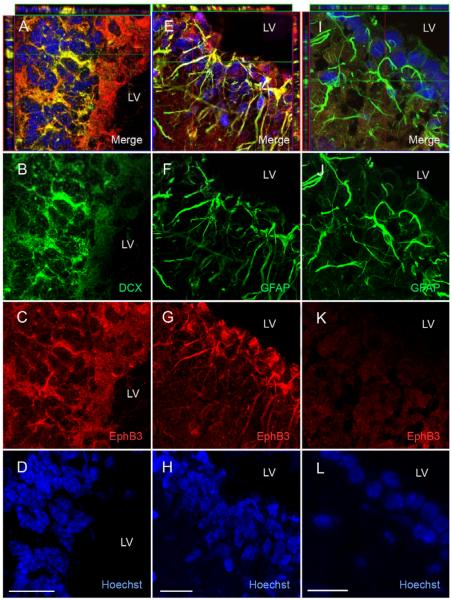
Figure 1.
EphB3 is expressed on GFAP-positive NSPCs and DCX-positive neuroblasts in the adult SVZ. (A-D) Confocal z-stack image analysis of EphB3 (red) using double-immunofluorescence showed co-labeling with doublecortin (DCX)-positive cells (green) in the lateral wall of the lateral ventricle. (E-H) GFAP-positive cells (green) also co-label with anti-EphB3 (red) in cytoplasmic extension in the adult SVZ. (I-L) Sagittal tissue sections from EphB3−/− mice do not show expression with anti-EphB3 antibody (red) and does not co-label with GFAP-expressing cells. Scale bar = 20 μm. LV, lateral ventricle.
Figure 2.
EphB3 expression is down-regulated in the SVZ following CCI injury. (A) Western blot analysis of EphB3 and ephrinB3 from the ipsilateral (i) and contralateral (c) SVZ in sham and CCI-injured wild type mice at 1, 3 and 7 days. EphB3 expression is significantly reduced at both 3 and 7 days but not 1 day after CCI injury. EphrinB3 expression was unchanged in the first 7 days after injury. Quantified (B) EphB3 and (C) ephrinB3 expression in the SVZ of CCI-injured mice (n=5) compared to sham-injured mice (n=5) at 1, 3 and 7 days. Quantified data was normalized to β-actin control levels then represented as relative expression compared to sham-injury at each time point. *P<0.05; ***P<0.001 compared to ipsilateral sham-injured mice. #P<0.05; ##P<0.01 compared to contralateral sham-injured mice. KO CTL=whole brain extract from EphB3−/− mice.
Cell proliferation is enhanced following CCI injury and in the absence of ephrinB3 and EphB3
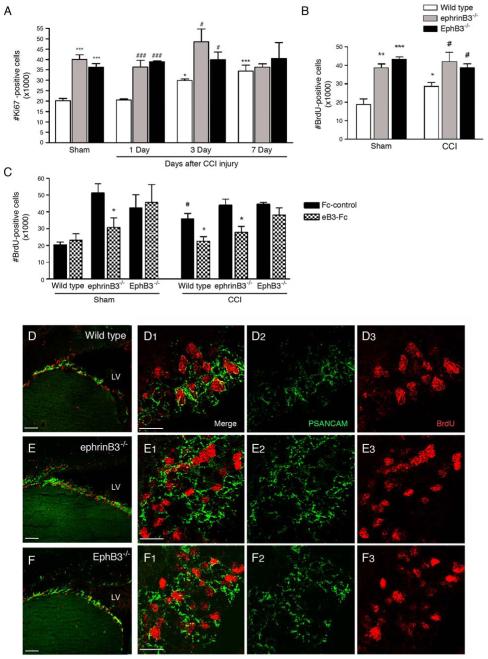
To examine whether changes in EphB3 expression correlate with proliferation in the SVZ after cortical damage, we initially measured the extent of Ki67 immunoreactivity, a marker for all cell cycle phases, at 1, 3 and 7 days after CCI injury in wild type, ephrinB3−/− and EphB3−/− mice. As previously reported in other mouse strains [35, 65, 66], stereological analysis of nuclear Ki67 staining showed proliferation in the SVZ to be increased in CCI-injured wild type mice at 3 days (29,858 ± 785) and 7 days (34,474 ± 2,813) compared to sham-injured (20,126 ± 1,126) or 1 day CCI-injured (20,455 ± 511) mice (Fig. 3A). Gene-targeted deletion of either ephrinB3 or EphB3 enhanced proliferation in sham-injured mice (40,078 ± 4,355 and 36,320 ± 4,146, respectively) which was not further augmented after CCI injury (Fig. 3A). Stereological analysis of BrdU incorporation performed at 3 days post-injury, the earliest significant increase in proliferation by Ki67 staining in CCI-injured wild type mice, showed a similar 33% increase in the number of BrdU-positive cells (Fig. 3B). This change was accompanied by decreased expression of EphB3 in Figure 2. EphB3−/− mice have a 232% increase in BrdU-positive cells, similar to Ki67, (43,551 ± 1,656) in the SVZ as compared to sham-injured wild type mice (18,750 ± 3,124). EphrinB3−/− mice also show a 184% increase (38,659 ± 2,083) in BrdU-positive cells (Fig. 3B). However unlike wild type mice, increased BrdU incorporation was not observed in the SVZ of CCI-injured ephrinB3−/− or EphB3−/− mice as compared to their sham-injured controls. The failure to induce further proliferation after CCI-injury in the absence of either ephrinB3 or EphB3 suggests that down-regulation of ephrinB3-EphB3 signaling may be an early event that stimulates a growth response in the SVZ following cortical damage.
Figure 3.
Proliferation in the adult SVZ after CCI injury in wild type, ephrinB3−/− and EphB3−/− mice. (A) Stereological counts of Ki67-positive cells at 1, 3 and 7 days post-injury. CCI increased proliferation at 3 and 7 days in wild type mice while having no effect in ephrinB3−/− and EphB3−/− ipsilateral SVZ. Greater numbers of Ki67-positve cells are found in sham- and CCI-injured (1 and 3 days) ephrinB3−/− and EphB3−/− mice as compared to wild type mice. (B) BrdU-incorporation at 3 days confirms the increase in proliferation in CCI-injured wild type ipsilateral SVZ, while loss of ephrinB3 and EphB3 enhances proliferation in both sham and CCI-injured mice. (C) CCI-induced proliferation is reversed in wild type and ephrinB3−/− but not EphB3−/− mice following 3 days infusion of pre-clustered soluble ephrinB3-Fc (eB3-Fc) compared to Fc-control infusion. (D-F) Confocal image analysis of BrdU (red) and PSA-NCAM (green) immunofluorescence from sagittal sections through the SVZ and RMS of wild type (D1-D3), ephrinB3−/− (E1-E3) and EphB3−/− (F1-F3). Co-expression of BrdU-positive cells is observed in PSA-NCAM-positive neuroblasts in the SVZ and greater PSA-NCAM staining is observed in ephrinB3−/− and EphB3−/− mice. (High-magnification images, scale bar = 20 μm; Low magnification: Scale bar = 100 μm). *P<0.05, **P<0.01, ***P<0.001 compared to wild type sham. #P<0.05, ###P<0.001 compared to corresponding wild type CCI. LV, lateral ventricle.
To confirm the role of EphB3 receptor in CCI-induced proliferation, we stimulated EphB3 in vivo by infusing soluble pre-clustered ephrinB3-Fc molecules (eB3-Fc) into the lateral ventricle of sham- and CCI-injured wild type, ephrinB3−/− and EphB3−/− mice for 3 days using ALZET osmotic pumps. Human-Fc fragments were used as a control (Fc control). Although EphB3 expression is significantly reduced in the SVZ of CCI-injured wild type mice (Fig. 2), EphB3 stimulation following infusion of eB3-Fc attenuated CCI-induced proliferation (35,659 ± 3,395 Fc control vs. 22,483 ± 2,705 eB3-Fc), and restored the number of BrdU-positive cells to sham-injured levels in wild type mice (23,097 ± 3,821 eB3-Fc sham) (Fig. 3C). It is unclear whether this effect is the result of an excessive presence of ephrinB3 or its promiscuous binding to other Eph receptors (i.e. EphA4). It is most likely the former, since eB3-Fc infusion had no effect on modifying proliferation in the SVZ of EphB3−/− mice (44,557 ± 1,051 CCI-injured Fc control vs. 38,097 ± 4,297 CCI-injured eB3-Fc). EphrinB3-Fc infusion did restore the number of proliferating cells in ephrinB3−/− to levels found in wild type mice (43,977 ± 3,589 CCI-injured Fc control vs. 27,869 ± 3,482 CCI-injured eB3-Fc) (Fig. 3B). Furthermore, this phenotypic rescue supports a direct effect on proliferation and not an indirect developmental defect in knockout cells that reside in the adult SVZ.
Previously, we showed that greater numbers of neuroblasts were present in the SVZ of naïve ephrinB3−/− compared to wild type mice [51]. Here, double immunofluorescence labeling of BrdU-positive cells also revealed enhanced PSA-NCAM expression in the lateral wall of both ephrinB3−/− (Fig. 3E) and EphB3−/− (Fig. 3F) mice following CCI injury as compared to wild type mice (Fig. 3D). These data confirm our previous findings in the naïve non-injured ephrinB3−/− mice [51], and further support a role for EphB3 in suppressing proliferation in the SVZ in response to CCI injury.
Cell death is reduced in the SVZ following CCI injury and in EphB3−/− mice
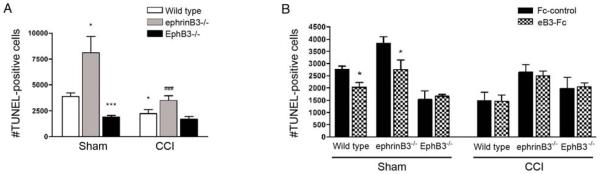
Neurogenesis and maintenance of proper NSPC numbers in the SVZ can also be regulated by cell death mechanisms [51]. To address whether ephrinB3-EphB3 interactions mediate CCI-induced proliferation by modulating cell death in the SVZ, we examined TUNEL labeling in the presence and absence of ephrinB3 and EphB3 at 3 days post-injury, when a significant effect on proliferation is initially observed. Coinciding with enhanced BrdU-incorporation, we observed a 28% reduction in the number of TUNEL-positive cells in the SVZ of wild type mice (1,800 ± 546) 3 days after CCI injury as compared to sham-injured mice (3,891 ± 375) (Fig. 4A). Analysis of EphB3−/− mice showed that the number of TUNEL-positive cells in sham-injured mice is significantly lower (1,909 ± 142) than wild type sham animals and is not further reduced after CCI injury (1,713 ± 242). Conversely, a 34% increase in cell death was observed in the absence of ephrinB3 (8,092 ± 1,586 in sham) as previously shown [51] but reduced by 40% following CCI injury (3,477 ± 477) (Fig. 4A). Infusion of pre-clustered eB3-Fc molecules into the lateral ventricles could attenuate cell death in sham-injured wild type (2,032 ± 188.1 vs. 2,764 ± 127 Fc control) and ephrinB3−/− (2,745 ± 398 vs. 3,845 ± 245 Fc control) but not EphB3−/− mice (1, 664 ± 81 vs. 1,527 ± 363 Fc control) (Fig. 4B). These data suggest that reduced cell death may account for greater numbers of proliferating cells in the SVZ after CCI injury and that down regulation of EphB3 may initiate this response. Interestingly, ephrinB3-Fc infusion did not further reduce the number of TUNEL-positive cells in the SVZ following CCI injury in wild type, ephrinB3−/− or EphB3−/− mice, suggesting that ephrin-independent mechanisms might also exist.
Figure 4.
EphB3 regulates cell death in the SVZ of sham and CCI-injured mice. (A) Stereological counts of TUNEL-positive cells in the adult SVZ of wild type, ephrinB3−/− and EphB3−/− mice. The number of TUNEL-positive cells is reduced in the ipsilateral SVZ 3 days after CCI injury in wild type and ephrinB3−/−; but remains significantly lower in both sham- and CCI-injured EphB3−/− mice. Interestingly, there is greater cell death in the absence of eprhinB3 but reduced cell death in the absence of EphB3 compared to wild type in sham-injured animals. (B) Infusion of pre-clustered eB3-Fc can restore the level of cell death in ephrinB3−/− mice to wild type levels and significantly reduce the basal level of cell death in sham-injured wild type mice. The number of TUNEL-positive cells in the SVZ of EphB3−/− mice is unaffected by eB3-Fc infusion nor is there any effect in CCI-injured SVZ tissue. *P<0.05, ***P<0.001 compared to wild type sham; ###P<0.001 compared to ephrinB3−/− sham in panel A and *P<0.05 compared to corresponding Fc-control in panel B.
p53 expression is reduced in the SVZ of ephrinB3−/− and EphB3−/− mice
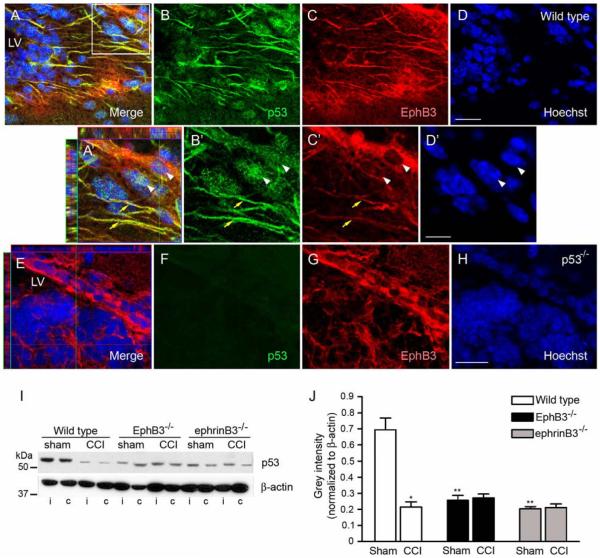
It is well established that p53 plays a pivotal role in regulating anti-proliferative and pro-apoptotic functions [67, 68]. Previous studies have demonstrated that p53 suppresses proliferation in the adult SVZ [69-71]. Here, we show co-localization by immunofluorescence of p53 with EphB3-expressing cells in the SVZ (Fig. 5A-D). p53 is expressed in both the NSPC processes (yellow arrows) and nuclei (white arrowheads) of EphB3-bearing cells (Fig. 5A’-5D’), which is absent in tissue isolated from p53−/− mice (Fig. 5E-H). Quantification by Western blot analysis showed that CCI injury resulted in a significant decrease in the total amount of p53 in the SVZ as compared to sham-injured wild type mice (Fig. 5I, 5J). This was in contrast to its expression in the cortex, which was robustly increased 3 days after injury (not shown). Down regulation of p53 expression in the SVZ may account for the enhanced proliferation and survival observed early in response to CCI injury. Our findings also indicate that in the absence of EphB3 there is a dramatic reduction in the basal level of p53 compared to sham-injured wild type mice and is unchanged in the SVZ following CCI injury. This strongly suggests that EphB3 may suppress proliferation in the SVZ by regulating p53 activity. Interestingly, ephrinB3−/− mice also display reduced p53 expression (Fig. 5I, 5J) combined with greater cell death (Fig. 4A), suggesting that both p53-dependent and p53-independent pathways could mediate EphB3 constraints on proliferation and survival.
Figure 5.
Tumor suppressor p53 expression is down-regulated in the wild type adult SVZ following brain injury and is expressed at lower levels in ephrinB3−/− and EphB3−/− mice. (A-D) Confocal images of p53 immunofluorescence in the SVZ of sagittal wild type tissue sections. (A’-D’) Confocal image analysis at 100X magnification. Co-localization of p53 (green) and EphB3 (red) is observed in cytoplasmic processes (yellow arrow). Nuclear staining of p53 is also seen in EphB3-expressing cells in the SVZ (white arrowheads). (E-H) Confocal images of p53 immunofluorescence in the SVZ of p53−/− mice. EphB3 (red) is expressed in the SVZ, while p53 staining is not observed in p53-null mice. (I) Western blot analysis of p53 expression 3 days after sham and CCI injury in wild type, EphB3−/− and ephrinB3−/− mice. P53 is reduced in the ipsilateral (i) and contralateral (c) SVZ after CCI injury, while lower levels of p53 remain in EphB3−/− and ephrinB3−/− mice. (J) Bar graph represents quantified data of p53 in the ipsilateral SVZ in sham- and CCI-injured wild type and knockout mice. Data represents relative p53 expression normalized to β-actin control levels. (n=4). *P<0.05; **P<0.01 compared to wild type sham. LV, lateral ventricle. (A-D and E-H) Scale bar = 20 μm, (A’-D’) Scale bar = 10 μm.
Stimulation of EphB3 inversely regulates p53 and p-AKT levels in the adult SVZ
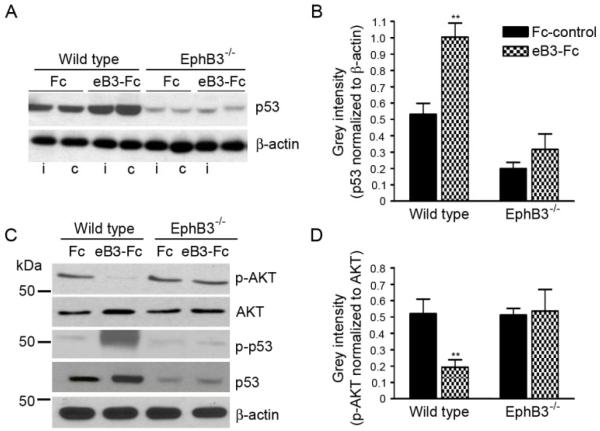
To examine whether p53 is directly regulated by EphB3 in the adult SVZ, we stimulated EphB3 directly by infusing soluble pre-clustered ephrinB3-Fc molecules into the lateral ventricle of naive wild type and EphB3−/− mice for 3 days. EphrinB3-Fc (eB3-Fc) infusion significantly enhanced the expression of total p53 (1.00 ± 0.09 eB3-Fc compared to 0.53 ± 0.07 Fc control (Fc)) (Fig. 6A, 6B) as well as phosphorylated (p)-p53Ser15 (Fig. 6C) in wild type mice; however, no significant increase was observed in EphB3−/− mice (0.32 ± 0.1 eB3-Fc compared to 0.2 ± 0.04 Fc) (Fig. 6A, 6B). We also examined the levels of phosphorylated AKT (p-AKT), since its activity has been shown to promote p53 degradation and is known to be suppressed downstream of Eph receptor activation [51, 72-75]. In the presence of pre-clustered eB3-Fc molecules, the level of p-AKT was significantly reduced (0.20 ± 0.05 eB3-Fc compared to 0.52 ± 0.08 Fc) (Fig. 6C, 6D). This effect was not observed following infusion in EphB3−/− mice (0.56 ± 0.12 eB3-Fc compared to 0.52 ± 0.04 Fc) suggesting that EphB3 may directly regulate p53 stability by limiting AKT activity in the adult SVZ.
Figure 6.
EphB3 directly regulates p53 and AKT phosphorylation in the SVZ. EphB3 was stimulated in vivo by infusing pre-clustered ephirnB3-Fc (eB3-Fc) or Fc-control (Fc) molecules into the lateral ventricle of wild type and EphB3−/− mice. (A) Western blot analysis of p53 protein expression 3 days after eB3-Fc and Fc infusions show an increase in p53 expression in the ipsilateral (i) and contralateral (c) SVZ of wild type but not EphB3−/− mice. (B) Bar graph representing quantified data of p53 normalized to β-actin control levels in the SVZ of wild type and EphB3−/− mice (n=4). (C) The level of phosphorylated AKT (p-AKT) is decreased in the ipsilateral SVZ 3 days after eB3-Fc stimulation as total p53 and phosphoSer15-p53 are increased in wild type while having no effect in EphB3−/− mice. (D) Bar graph representing quantified data of p-AKT levels normalized to total AKT levels in the SVZ of wild type and EphB3−/− mice (n=4). *P<0.05 and **P<0.01 compared to wild type Fc-control.
EphB3 suppresses neural stem/progenitor cell proliferation by regulating p53
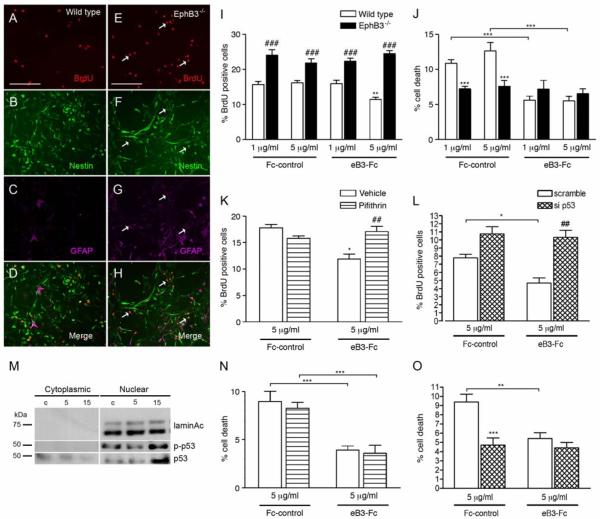
To determine whether the effects on adult NSPCs shown in vivo were not indirectly related to the overall loss of EphB3 receptor, we examined proliferation and survival in NSPC cultures harvested from the adult wild type and EphB3−/− SVZ (Fig. 7). When grown in vitro, primary NSPCs cultured from wild type (Fig. 7B) and EphB3−/− (Fig. 7F) mice retain an undifferentiated phenotype as defined by neural progenitor marker anti-nestin (green). Analysis of proliferation showed a significant 35% increase in the percentage of BrdU-positive cells in EphB3−/− NSPCs (Fig. 7E, 7I) compared to wild type (Fig. 7A, 6I). Interestingly, there are greater numbers of BrdU-positive cells expressing both nestin and GFAP in EphB3−/− cultures (Fig. 7E-H) compared to wild type (Fig. 7A-D). This confirmed our in vivo data and suggests that EphB3 suppresses proliferation of GFAP-expressing NSPCs and that expansion of the neural stem cell pool and subsequent neuroblast progeny occurs in the absence of EphB3. Direct stimulation of EphB3 in vitro, with soluble pre-clustered eB3-Fc at 5 μg/ml, could suppress proliferation (11.5 ± 0.6% eB3-Fc compared to 16.2 ± 0.6% Fc control) in wild type but not EphB3−/− NSPCs (21 ± 2% eB3-Fc compared to 24.5 ± 0.8% Fc control) (Fig. 7I).
Figure 7.
EphB3 regulates proliferation of cultured SVZ-derived adult NSPCs in a p53-dependent manner. (A-D) Triple immunofluorescence labeling of BrdU-positive (red) wild type NSPCs with nestin (green) NSPC marker and GFAP (purple). EphB3−/− NSPCs have more proliferating nestin-expressing, GFAP-positive cells compared to wild type cultures. (I) Proliferation of wild type and EphB3−/− NSPCs in the presence and absence of soluble pre-clustered ephrinB3-Fc (eB3-Fc) and Fc-control (Fc). The percentage of BrdU-positive cells is increased in EphB3−/− cultures. The addition of 5 μg/ml eB3-Fc molecules for 3 days suppressed proliferation in wild type NSPCs compared to Fc-control, while having no effect in EphB3−/− cells. (J) The percentage of basal level cell death is reduced in EphB3−/− compared to wild type NSPCs. The addition of either 1 μg/ml or 5 μg/ml eB3-Fc reduced the percentage of cell death in wild type NSPCs compared to Fc-control, while having no effect in EphB3−/− cells. (K) EB3-Fc induced growth suppression (white bars) is attenuated in the presence of 10μM p53 inhibitor, Pifithrin compared to PBS-vehicle control. (L) Pre-treatment with si p53, 24 hours prior to stimulation also attenuated eB3-Fc induced growth suppression compared to siRNA scramble control. (M) EphB3 stimulation induces nuclear translocation of total p53 and phosphoSer15-p53 following 15 min eB3-Fc exposures compared to Fc-control (c). Inhibiting p53 with 10 μM Pifithrin (N) or si p53 (O) had no effect on altering the percentage of cell death in the presence of 5 μg/ml ephrinB3-Fc. (**P<0.01, ***P<0.001 compared to corresponding wild type Fc-control; ###P<0.001 compared to wild type control in panel I and J), (*P<0.05 compared to Fc-control, ##P<0.01 compared to eB3-Fc plus vehicle in panel K, Scale bar = 200 μM).
Similar to the in vivo observations shown in Figure 4, cell death of cultured NSPCs was also reduced after exposure to eB3-Fc ligand and in the absence of EphB3 (Fig. 7J). Exposure to 1 and 5 μg/ml soluble pre-clustered eB3-Fc molecules attenuated the percentage of cell death in wild type NSPCs (5.6 ± 0.6% for 1 μg/ml and 5.5 ± 0.6% for 5 μg/ml) compared to Fc control (10 ± 0.5% for 1 μg/ml and 12.6 ± 1.2% for 5 μg/ml), while having no effect on EphB3−/− cells (7.8 ± 0.9% Fc control and 6.5 ± 0.7% eB3-Fc). These data show that cell death is reduced either in the presence of ephrinB3 or in the absence of EphB3, suggesting Eph receptors may function as pro-apoptotic dependence receptors [56].
Our in vivo data also suggests that EphB3 may exert its anti-proliferative effects on NSPCs by regulating tumor suppressor p53 (Fig. 5). Similar to p53 levels in the wild type and EphB3−/− SVZ, we found the basal level of p53 expression is also reduced in cultures of NSPCs-derived from EphB3−/− mice as compared to wild type NSPCs (not shown), which could explain the increase in the number of BrdU-positive cells. To examine the role of p53 in mediating EphB3 signaling, we initially stimulated NSPCs with either pre-clustered eB3-Fc or Fc-control to show that both total p53 and phosphorylated (p)-p53Ser15 increased in the nuclear fraction at 15 min, as total p53 is decreased in the cytoplasmic fraction (Fig. 7M). Next, we employed the pharmacological p53 inhibitor, Pifithrin, in combination with eB3-Fc stimulation. We show that the percentage of BrdU-positive cells was reduced following the addition of pre-clustered eB3-Fc (11.9 ± 0.9%) compared to Fc control (17.8 ± 0.6%), which was not observed when wild type NSPCs were first pre-treated with 10 μM Pifithrin (17.1 ± 1.0%) (Fig. 7K). These findings were confirmed using p53 siRNA (si p53), where NSPC cultures were pre-treated with si p53 24 hrs prior to eB3-Fc stimulation to knockdown endogenous p53. We show that application of eB3-Fc had no effect on proliferation when NSPCs were pre-treated with si p53 (11.3 ± 1.0%) in contrast to the reduction observed with scramble siRNA (5.1 ± 0.7%) (Fig. 7L). These data demonstrate that EphB3 suppresses NSPC proliferation in a p53-dependent manner.
Tumor suppressor p53 has been well studied for its ability to exhibit pro-apoptotic functions during times of cellular stress [68, 76-78]. To examine whether the anti-apoptotic effects of ephrinB3 function in a p53-dependent manner, we cultured NSPCs in the presence of Pifithrin or si p53 followed by eB3-Fc stimulation. The percentage of cell death with 5 μg/ml eB3-Fc (3.9 ± 0.4% compared to 9.2 ± 1.1% Fc control) was not augmented in the presence of p53 inhibitor, Pifithrin-α (3.6 ± 0.8% eB3-Fc compared to 8.3 ± 0.6% Fc control) (Fig. 7N) or following pre-exposure to si p53 (4.4 ± 0.6% eB3-Fc compared to 4.7 ± 0.8% Fc control) (Fig. 7O). In summary, these findings support our in vivo conclusions that EphB3 negatively regulates adult NSPC proliferation and survival in a cell autonomous fashion by regulating both p53-dependent and p53-independent mechanisms, respectively.
Discussion
Although a number of soluble mitogenic factors have been shown to participate in stimulating the SVZ response to brain injury, little is known about the regulation of local inhibitory molecules that maintain homeostasis in the SVZ niche. The current study shows that EphB3 immunoreactivity is prominent within GFAP-expressing NSPCs and neuroblasts in the SVZ and RMS, where EphB3 signaling suppresses NSPC proliferation and is pro-apoptotic in the absence of ephrinB3 ligand. Three days after CCI injury, the level of EphB3 expression was dramatically reduced in the ipsilateral SVZ and was partially restored at 7 days as proliferation and survival increased in wild type mice. Infusion of soluble pre-clustered ephrinB3-Fc molecules could reverse the proliferative changes in CCI-injured wild type and ephrinB3−/− but not EphB3−/− mice, demonstrating that ephrinB3 is required to suppress proliferation by mediating EphB3-dependent forward signaling. We also show that cell death is regulated by EphB3 and that its absence causes a reduction in the number of TUNEL-positive cells present in the SVZ. On the other hand, perturbation of cell death is observed in the absence of its primary ligand, which can be overcome by restoring ephrinB3. These data provide strong support for the role of EphB3 as a pro-apoptotic dependence receptor during adult neurogenesis in the SVZ. Finally, we show that EphB3 may exert its anti-proliferative actions on NSPCs by directly regulating the tumor suppressor protein p53 in a cell autonomous manner.
Although ephrins and their receptors have recently been shown to tightly regulate proliferation and migration in the SVZ [51, 52, 56, 75, 79], mechanisms controlling their expression are not fully understood and have not been studied in the context of brain injury. Removal of the inhibitory control on adult neurogenesis may be an early event that allows simultaneous expansion and migration of the NSPC population in response to injury-induced neurotrophic signals produced after trauma. Examination of the temporal change in EphB3 expression showed a dramatic yet transient decrease beginning at 3 days post-injury, which correlated with CCI-induced proliferation and cell survival in the SVZ. This supports our conclusion that changes in Eph expression may reflect the differences in NSPC proliferation. Although EphB3 expression appeared to be partially restored at 7 days, the number of proliferating cells remained high compared to sham-injured levels. This could reflect the expansion of the pool of neural progenitor cells since Ki67 was used to detect all phases of the cell cycle, and more than one week may be required for the restoration of homeostasis. In addition, we cannot rule out the possibility that there may be additional alterations in cell motility, differentiation, and other proliferative factors present after the first week following injury. It remains unclear what prior signaling events are occurring to elicit changes in EphB3 expression in the SVZ following TBI. Several pro-inflammatory cytokines have been shown to affect the expression of Eph receptors and their ligands in vitro [80-84]. EphB3 expression is modulated in vivo in response to mild febrigenic doses of LPS where EphB3 mRNA levels are significantly down-regulated. [84]. This suggests that Eph receptor expression in the SVZ could be regulated by post-injury pro-inflammatory cytokine production. It would be of interest to examine whether immediate application of anti-inflammatory drugs following CCI-injury could prevent the down-regulation of EphB3 expression in the SVZ.
Regulating homeostatic levels of NSPCs is dependent upon the regional expression of ephrinB3-EphB3 boundaries which control the level and activity of tumor suppressor protein p53 in the SVZ. It is well established that p53 initiates various cellular stress responses leading to cell-cycle arrest, cellular senescence, differentiation, and apoptosis [68, 76]. The presence of p53 in the naïve SVZ has been previously documented and is thought to thwart uncontrolled cellular division [69, 71, 85]. Here, we report that p53 is expressed in both the cytoplasmic and nuclear fractions of EphB3-bearing cells in the adult SVZ. Although p53 is typically absent or maintained at a lower level in most tissues and activated only in response to cellular stress, our data shows that p53 is active in the SVZ and highly responsive to EphB3 stimulation. The adult SVZ is a unique neurogenic zone where cells undergo bursts of proliferation, followed by cell cycle arrest, migration, differentiation and apoptosis. The regulation of such events in response to local environmental changes may, therefore, require strict control over p53. It is unclear what role either cytoplasmic or nuclear p53 plays in regulating these functions. Recently, cytoplasmic p53 was shown to regulate neural precursor cell apoptosis [86]. Although our studies do not implicate p53 in mediating EphB3 induced changes in cell survival, we observed a significant loss in the basal level of p53 in the absence of EphB3-ephrinB3 signaling and robust activation upon EphB3 stimulation which mediated growth suppression in SVZ-derived NSPCs.
Ephrins and their receptors have been well studied in the context of axon growth and guidance [87-89]. Several axon-guidance molecules have been shown to be inactivated in a number of human cancers [90] and are also thought to regulate cell survival by acting as dependence receptors [91-94]. Dependence receptors depend on their ligand to play supportive roles during development or homeostasis, but revert to pro-apoptotic functions in the absence of ligand stimulation [95-99]. The observation that there is increased apoptosis in the SVZ in the absence of ephrinB3 ligand, which could be reversed by replacing it back in soluble form through ventricle infusion, has lead to the conclusion that EphB3 may also function as a pro-apoptotic dependence receptor in the absence of ligand stimulation. This may explain the discrepancy between EphB3−/− mice (greater cell survival) versus ephrinB3−/− mice (greater cell death). A recent report by del Rio and colleagues implicate EphB3 as a possible dependence receptor based on a unique dependence-associated receptor transmembrane motif (DART) that is common to many described dependence receptors [100]. We also have recently implicated EphA4 as a dependence receptor [56] which may work in concert with EphB3 to regulate survival in the adult SVZ. Future studies will examine the combined EphB3/EphA4 double knockout mice to evaluate whether there are compensatory functions that regulate cell survival of adult NSPCs.
In conclusion, we demonstrate a novel mechanism controlling the SVZ response to TBI that involves suppressing the anti-proliferative and pro-apoptotic activities of EphB3 receptor, which is normally required for maintaining homeostasis in the SVZ. We also propose that EphB3 may function in a p53-dependent manner to limit the number of NSPCs and subsequent neuroblast progeny. Early expansion of the NSPC pool in response to brain injury requires down-regulation of the EphB3-p53 signaling pathway in the adult SVZ. Insights into the role of ephrins and their cognate receptors in regulating post-injury neurogenesis may be important for devising strategies that target endogenous progenitor cells for repair and regeneration in the injured CNS.
Supplementary Material
Acknowledgments
Thank you Drs. Mark Henkemeyer and Mike Xu for their generous gift of the mutant mice. This work was supported by NIH/NINDS NS049545 (DJL), NS30291 (DJL), NS007459 (MHT), NS064699 (MHT) and the Miami Project to Cure Paralysis.
Abbreviations
- NSPC
neural stem/progenitor cell
- SVZ
subventricular zone
- TBI
traumatic brain injury
- CCI
controlled cortical impact
- EGF
epidermal growth factors
- bFGF
basic fibroblastic growth factors
- GDNF
glial-derived neurotrophic factor
- SCF
stem cell factor
- GFAP
glial fibrillary acidic protein
- DCX
doublecortin
- BrdU
bromodeoxyuridine
- OB
olfactory bulb
- RMS
rostral migratory stream
- Eph
erythropoietin-producing hepatocellular
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Sohur US, et al. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1477–97. doi: 10.1098/rstb.2006.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempermann G, Gage FH. New nerve cells for the adult brain. Sci Am. 1999;280(5):48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 5.Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126(3):337–89. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 6.Salman H, Ghosh P, Kernie SG. Subventricular zone neural stem cells remodel the brain following traumatic injury in adult mice. J Neurotrauma. 2004;21(3):283–92. doi: 10.1089/089771504322972077. [DOI] [PubMed] [Google Scholar]
- 7.Arvidsson A, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26(24):6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundholm-Peters NL, et al. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64(12):1089–100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- 10.Nakatomi H, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–41. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 11.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snapyan M, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29(13):4172–88. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson CB, et al. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253(2):733–6. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- 14.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 15.Morshead CM, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13(5):1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doetsch F, et al. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36(6):1021–34. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 18.Mirzadeh Z, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–4. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 20.Kukekov VG, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156(2):333–44. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 21.Middeldorp J, et al. GFAPdelta in radial glia and subventricular zone progenitors in the developing human cortex. Development. 137(2):313–21. doi: 10.1242/dev.041632. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 23.Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–9. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 24.Luskin M, Parnavelas J, Barfield J. Neurons, astrocytes, and oligodendrocytes of the rta cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–1750. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betarbet R, et al. Dopaminergic and GABAergic interneurons of the olfactory bulb are derived from the neonatal subventricular zone. Int. J. Dev. Neurosci. 1996;14(7-8):921–30. doi: 10.1016/s0736-5748(96)00066-4. [DOI] [PubMed] [Google Scholar]
- 26.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93(25):14895–900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–8. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 29.Zhang RL, et al. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27(12):3157–62. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramaswamy S, et al. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053(1-2):38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 31.Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 37(2):267–74. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thau-Zuchman O, et al. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshiya K, et al. Profile of gene expression in the subventricular zone after traumatic brain injury. J Neurotrauma. 2003;20(11):1147–62. doi: 10.1089/089771503770802844. [DOI] [PubMed] [Google Scholar]
- 34.Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18(1):169–81. xi. doi: 10.1016/j.nec.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura S, et al. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest. 2003;112(8):1202–10. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dempsey RJ, et al. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87(3):586–97. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 37.Jin K, et al. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002;110(3):311–9. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai PT, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26(4):1269–74. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, et al. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35(7):1732–7. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, et al. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85(4):740–7. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- 41.Yan YP, et al. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24(1):45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura S, et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98(10):5874–9. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu DY, et al. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23(1):223–9. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiltrout C, et al. Repairing brain after stroke: a review on post-ischemic neurogenesis. Neurochem Int. 2007;50(7-8):1028–41. doi: 10.1016/j.neuint.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn HG, et al. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–9. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang R, et al. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50(5):602–11. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- 47.Liebl DJ, Henkemeyer M, Parada LF. Expression of Ephrins and Eph receptors in the neonatal and adult brain. J Comp Neurol. 2001 doi: 10.1002/jnr.10457. Submitted. [DOI] [PubMed] [Google Scholar]
- 48.Bergemann AD, et al. Ephrin-B3, a ligand for the receptor EphB3, expressed at the midline of the developing neural tube. Oncogene. 1998;16(4):471–80. doi: 10.1038/sj.onc.1201557. [DOI] [PubMed] [Google Scholar]
- 49.Blits-Huizinga CT, et al. Ephrins and their receptors: binding versus biology. IUBMB Life. 2004;56(5):257–65. doi: 10.1080/15216540412331270076. [DOI] [PubMed] [Google Scholar]
- 50.Cowan C, et al. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 51.Ricard J, et al. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol Cell Neurosci. 2006;31(4):713–22. doi: 10.1016/j.mcn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Conover JC, et al. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3(11):1091–7. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 53.Mendes SW, Henkemeyer M, Liebl DJ. Multiple Eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain. J Neurosci. 2006;26(3):882–92. doi: 10.1523/JNEUROSCI.3162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Depaepe V, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435(7046):1244–50. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- 55.Holmberg J, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125(6):1151–63. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 56.Furne C, et al. EphrinB3 is an anti-apoptotic ligand that inhibits the dependence receptor functions of EphA4 receptors during adult neurogenesis. Biochim Biophys Acta. 2009;1793(2):231–8. doi: 10.1016/j.bbamcr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Research Reviews. 2006;52(2):327–345. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Yu S, et al. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009;1287:157–63. doi: 10.1016/j.brainres.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi T, et al. Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res. 2009;1280:172–7. doi: 10.1016/j.brainres.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 60.Henkemeyer M, et al. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86(1):35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 61.Orioli D, et al. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. Embo J. 1996;15(22):6035–49. [PMC free article] [PubMed] [Google Scholar]
- 62.Yokoyama N, et al. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29(1):85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 63.Scheffler B, et al. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci U S A. 2005;102(26):9353–8. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theus MH, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–70. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Xiong Y, et al. Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res. 2007;1185:301–12. doi: 10.1016/j.brainres.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rola R, et al. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202(1):189–99. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 67.Roger L, Gadea G, Roux P. Control of cell migration: a tumour suppressor function for p53? Biol Cell. 2006;98(3):141–52. doi: 10.1042/BC20050058. [DOI] [PubMed] [Google Scholar]
- 68.Bose I, Ghosh B. The p53-MDM2 network: from oscillations to apoptosis. J Biosci. 2007;32(5):991–7. doi: 10.1007/s12038-007-0103-3. [DOI] [PubMed] [Google Scholar]
- 69.Medrano S, Scrable H. Maintaining appearances--the role of p53 in adult neurogenesis. Biochem Biophys Res Commun. 2005;331(3):828–33. doi: 10.1016/j.bbrc.2005.03.194. [DOI] [PubMed] [Google Scholar]
- 70.Medrano S, et al. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meletis K, et al. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133(2):363–9. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 72.Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27(9):462–7. doi: 10.1016/s0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- 73.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98(20):11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou BP, et al. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3(11):973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 75.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Zhou J, Schmid T, Brune B. HIF-1alpha and p53 as targets of NO in affecting cell proliferation, death and adaptation. Curr Mol Med. 2004;4(7):741–51. doi: 10.2174/1566524043359926. [DOI] [PubMed] [Google Scholar]
- 77.Kuribayashi K, El-Deiry WS. Regulation of programmed cell death by the p53 pathway. Adv Exp Med Biol. 2008;615:201–21. doi: 10.1007/978-1-4020-6554-5_10. [DOI] [PubMed] [Google Scholar]
- 78.Meulmeester E, Jochemsen AG. p53: a guide to apoptosis. Curr Cancer Drug Targets. 2008;8(2):87–97. doi: 10.2174/156800908783769337. [DOI] [PubMed] [Google Scholar]
- 79.Holmberg J, et al. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19(4):462–71. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beckmann MP, et al. Molecular characterization of a family of ligands for eph-related tyrosine kinase receptors. Embo J. 1994;13(16):3757–62. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li YY, McTiernan CF, Feldman AM. IL-1 beta alters the expression of the receptor tyrosine kinase gene r-EphA3 in neonatal rat cardiomyocytes. Am J Physiol. 1998;274(1 Pt 2):H331–41. doi: 10.1152/ajpheart.1998.274.1.H331. [DOI] [PubMed] [Google Scholar]
- 82.Rosenberg IM, et al. Epithelial cell kinase-B61: an autocrine loop modulating intestinal epithelial migration and barrier function. Am J Physiol. 1997;273(4 Pt 1):G824–32. doi: 10.1152/ajpgi.1997.273.4.G824. [DOI] [PubMed] [Google Scholar]
- 83.Li YY, et al. Differential effects of overexpression of two forms of ephrin-A5 on neonatal rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2001;281(6):H2738–46. doi: 10.1152/ajpheart.2001.281.6.H2738. [DOI] [PubMed] [Google Scholar]
- 84.Ivanov AI, et al. Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats. Physiol Genomics. 2005;21(2):152–60. doi: 10.1152/physiolgenomics.00043.2004. [DOI] [PubMed] [Google Scholar]
- 85.Gil-Perotin S, et al. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26(4):1107–16. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geng Y, et al. Cytoplasmic P53 and Activated Bax Regulate P53-dependent, Transcription-independent Neural Precursor Cell Apoptosis. J Histochem Cytochem. 2009 doi: 10.1369/jhc.2009.954024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baier H, Bonhoeffer F. Attractive axon guidance molecules. Science. 1994;265:1541–1542. doi: 10.1126/science.8079167. [DOI] [PubMed] [Google Scholar]
- 88.Orike N, Pini A. Axon guidance: Following the Eph plan. Curr Biol. 1996;6:108–110. doi: 10.1016/s0960-9822(02)00435-9. [DOI] [PubMed] [Google Scholar]
- 89.Grunwald I, Klein R. Axon guidance: receptor complexes and signaling mechanisms. Curr Opin Neurobiol. 2002;12:250–259. doi: 10.1016/s0959-4388(02)00323-9. [DOI] [PubMed] [Google Scholar]
- 90.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4(12):978–87. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 91.Ochi K, et al. Identification of semaphorin3B as a direct target of p53. Neoplasia. 2002;4(1):82–7. doi: 10.1038/sj.neo.7900211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dohn M, Jiang J, Chen X. Receptor tyrosine kinase EphA2 is regulated by p53-family proteins and induces apoptosis. Oncogene. 2001;20(45):6503–15. doi: 10.1038/sj.onc.1204816. [DOI] [PubMed] [Google Scholar]
- 93.Arakawa H. p53, apoptosis and axon-guidance molecules. Cell Death Differ. 2005;12(8):1057–65. doi: 10.1038/sj.cdd.4401601. [DOI] [PubMed] [Google Scholar]
- 94.Llambi F, et al. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. Embo J. 2001;20(11):2715–22. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mehlen P, Bredesen DE. The dependence receptor hypothesis. Apoptosis. 2004;9(1):37–49. doi: 10.1023/B:APPT.0000012120.66221.b2. [DOI] [PubMed] [Google Scholar]
- 96.Mehlen P, et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395(6704):801–4. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 97.Mehlen P, Thibert C. Dependence receptors: between life and death. Cell Mol Life Sci. 2004;61(15):1854–66. doi: 10.1007/s00018-004-3467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tulasne D, et al. Proapoptotic function of the MET tyrosine kinase receptor through caspase cleavage. Mol Cell Biol. 2004;24(23):10328–39. doi: 10.1128/MCB.24.23.10328-10339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tauszig-Delamasure S, et al. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. PNAS. 2007;104(33):13361–13366. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.del Rio G, et al. A novel motif identified in dependence receptors. PLoS ONE. 2007;2(5):e463. doi: 10.1371/journal.pone.0000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.