Abstract
Evaluation of the biliary tract by percutaneous transhepatic cholangiography (PTC) is often required in liver transplant patients with an abnormal postoperative course. Indications for PTC include failure of liver enzyme levels to return to normal postoperatively, an elevation of serum bilirubin or liver enzyme levels, suspected bile leak, biliary obstructive symptoms, cholangitis, and sepsis.
Over a 5-year period 625 liver transplants in 477 patients were performed at the University Health Center of Pittsburgh. Fifty-three patients (56 transplants) underwent 70 PTCs. Complications diagnosed by PTC included biliary strictures, bile leaks, bilomas, liver abscesses, stones, and problems associated with internal biliary stents.
Thirty-two percutaneous transhepatic biliary drainage procedures were performed. Ten transplantation patients underwent balloon dilatation of postoperative biliary strictures. Interventional radiologic techniques were important in treating other complications and avoiding additional surgery in many of these patients.
Keywords: Cholangiography, percutaneous transhepatic – Bile ducts, percutaneous drainage – Liver, transplantation
Liver transplantation has now become an acceptable therapy for advanced, irreversible liver disease in both children and adults [1, 2]. In patients with an abnormal posttransplantation course, a biliary tract complication is a primary diagnostic consideration. If serious biliary complications are not recognized and corrected promptly, there is no chance for the immunosuppressed liver transplant patient to survive [3].
In the early postoperative period, most liver transplant patients have a T-tube in the common bile duct that permits easy examination by T-tube cholangiography [4]. However, direct access to the biliary tree via a percutaneous tube is not always available, particularly in the late postoperative period. In many such patients, percutaneous transhepatic cholangiography (PTC) is the preferred, or only, means of evaluating the biliary tree.
A PTC procedure permits the diagnosis of a variety of posttransplant complications that may require early surgical intervention. In selected cases, percutaneous transhepatic biliary drainage (PTBD) and related interventions, such as balloon dilatation of strictures and transhepatic removal of internal stents, may be performed to avoid further complications and additional surgical procedures. In this paper, we report our experience with PTC, PTBD, and related techniques in 56 pediatric and adult liver transplants.
Subjects and Methods
During the 5 years from January, 1981 to January, 1986, 477 patients (204 children, 273 adults) received 625 orthotopic liver transplants. One hundred and forty-eight retransplants, including both first and second retransplants (78 in adults, 70 in children), were performed. There were 210 male and 267 female patients aged 4 months to 67 years.
Fifty-three patients (56 transplants) underwent 70 PTCs. There were 23 children and 30 adults (26 males and 27 females) aged 9 months to 54 years. The PTC was performed from 14 days to 10 years after liver transplantation. Twenty-four (34%) PTCs were performed 3 or more months after surgery.
Each PTC was performed from a standard right lateral intercostal approach with a 22-gauge Chiba needle. Most children were evaluated under general anesthesia. Patients not already on antibiotic coverage received a combination of ampicillin and gentamycin or cefoxitin. Abnormal clotting studies were corrected with fresh frozen plasma. Platelet transfusions were given when the platelet count was less than 50,000/mm3
Bile duct reconstruction was accomplished by 1 of several methods. Thirty-four transplants were performed with a hepaticojejunostomy or choledochojejunostomy in Roux-en-Y with internal biliary stent. Five transplants had biliary drainage established via a cholecystojejunostomy in Roux-en-Y. One underwent a cholecystoduodenostomy. In 1 patient, the donor gallbladder was used as a pedicle graft conduit between the donor and recipient common bile ducts. fifteen were reconstructed with a choledochocholedochostomy.
Twenty-six patients (27 transplants) underwent 32 percutaneous transhepatic biliary drainages (PTBDs). There were 10 children and 16 adults (12 male and 14 female) aged 9 months to 44 years.
Ten patients (11 transplants) with strictures underwent transhepatic biliary dilatation. There were 2 children and 8 adults (3 male and 7 female) aged 14 months–44 years. Balloon dilatation was usually begun a minimum of 2 days after initial catheter insertion to allow a tract to form. In general, strictures were dilated every other day for up to 3 sessions. During each session, the balloon was inflated 3 separate times for 15–20 minutes each time. Balloon size was progressively increased, from 4–6 mm to 8–15 mm, depending on the size of the bile ducts.
Following dilatation, a transhepatic catheter (10–14 Fr) was inserted and capped for internal drainage. The catheters remained for a minimum of 2 months, at which time follow-up cholangiography was performed. If there was no evidence of recurrent stricture and the patient was asymptomatic, the catheter was removed.
Results
Percutaneous Transhepatic Cholangiography
Of 70 PTCs attempted, the biliary tree was successfully opacified in 55 (79%). The biliary tree was normal in 17 (31 %) of 55.
Complications diagnosed at PTC are listed in Table 1. Anastomotic strictures were diagnosed in 9 patients from 2 weeks to 20 months after operation. They occurred at the choledocho- or hepaticojejunostomy in 4 patients and at the choledochocholedochostomy in 5 patients.
Table l.
Results of 70 PTCs in 53 patients (56 transplants)
| Children | Adults | |
|---|---|---|
| Normal | 4 | 13 |
| Biliary stricture | 2 | 17 |
| Cystic duct obstruction | 1 | 0 |
| Cystic duct obstruction and hepatic abscess | 0 | 1 |
| Anastomotic obstruction due to internal stent |
1 | 1 |
| Choledocholithiasis and stricture | 0 | 1 |
| Choledocholithiasis | 2 | 1 |
| “Pseudo”-stone (extrinsic impression on common hepatic duct by hepatic artery) |
0 | 1 |
| Intrahepatic biloma and peritoneal bile leak |
1 | 0 |
| Intrahepatic biloma | 4 | 0 |
| Biliary-cutaneous fistula | 0 | 1 |
| Biliary-portal vein fistula | 1 | 0 |
| Hepatic abscess | 3 | 0 |
| Unsuccessful | 9 | 6 |
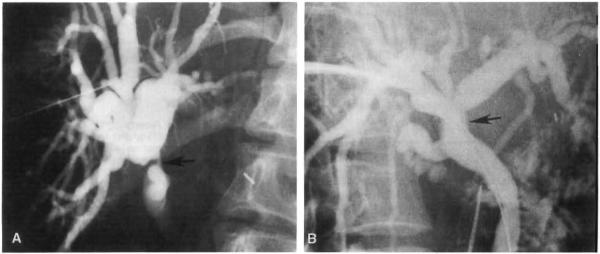
Nonanastomotic focal strictures in the donor biliary tree occurred in 5 subjects. Four of the 5 strictures were in the donor common hepatic duct, and were detected at 4, 5, 9, and 12 weeks after transplantation (Fig. 1 A). The patient who developed a stricture at 12 weeks was also found to have strictures within an intrahepatic right duct at 7 months (Fig. 2). One of the 5 subjects developed a left hepatic duct stricture (in addition to an anastomotic stricture) 14 weeks after operation.
Fig. 1.
Transhepatic balloon dilatation of biliary stricture. A Stricture in donor common hepatic duct (arrow) demonstrated on PTC 7 months after transplant. In this patient, the stricture was a recurrence after balloon dilatation 5 months earlier. B Patent donor common hepatic duct (arrow) after balloon dilatation to 15 mm. The patient remained asymptomatic for another 25 months when the stricture recurred with choledocholithiasis.
Fig. 2.

Intrahepatic biliary strictures (arrows) in right hepatic duct seen on PTC 7 months after transplantation.
Multiple intrahepatic biliary strictures were observed in 3 patients. In 1, a choledochojejunostomy stricture was also present. In another, transplantation was performed for end-stage primary sclerosing cholangitis; the cholangiographic findings within the transplant liver were consistent with recurrent disease.
Obstruction developed in 2 patients with a cholecystojejunostomy. In both, obstruction occurred at the cystic duct. The patient in whom the gallbladder was utilized as a pedicle graft conduit developed partial obstruction, probably secondary to the internal biliary stent.
Intrahepatic abscesses communicating with the biliary tree were observed in 4 patients, 2 of whom had a cholecystojejunostomy. In 1, a large fungal abscess developed in a right hepatic lobe infarct (Fig. 3).
Fig. 3.

Intrahepatic abscess (arrows) within the right lobe of the liver demonstrated on PTC 2 weeks after transplantation. The patient was successfully treated with a second transplant. Pathologic examination of the first graft revealed a large fungal abscess communicating with the right hepatic duct.
Intrahepatic bilomas secondary to bile duct necrosis were seen in 5 patients (Fig. 4). All had angiographically documented hepatic artery thrombosis. In 1, the biloma communicated with the portal vein. In another, a peritoneal bile leak originating from the biloma was seen.
Fig. 4.

Intrahepatic biloma (arrows) due to bile leakage from necrotic central bile ducts seen on PTC 3 months after transplantin a patient with hepatic artery thrombosis.
Stones were diagnosed in four patients at 2, 5, 32, and 36 months postoperatively (Fig. 5). In 2, the stones were due to inspissated bile. Partial biliary obstruction occurred in 3. At the time of diagnosis, 1 of the 4 also had a stricture in the common hepatic duct above a nonobstructing stone in the distal common bile duct. The stricture represented a recurrence after successful balloon dilatation 25 months previously.
Fig. 5.

Large stone (arrow) within common hepatic duct causing partial biliary obstruction, especially of left hepatic ducts, seen on PTC 14 months after transplant.
A biliary cutaneous fistula occurred in 1 patient.
Percutaneous Transhepatic Biliary Drainage
Twenty-six patients (27 transplants) underwent 32 PTBDs. It was the initial procedure to treat all patients with obstruction due to strictures or stones.
All 4 patients with stone disease underwent re-operation. In 3, revision of the biliary anastomosis was performed. In 2 patients, the stones were removed. In 1 of the 4, a 9-year-old boy, monooctanoin was infused into the bile ducts via the transhepatic catheter in an unsuccessful attempt to dissolve the stones. The liver subsequently underwent rejection and retransplantation was performed successfully.
In 1 patient, a presumed filling defect was seen within the common hepatic duct thought to represent a stone. A PTBD was performed because of symptoms of possible intermittant obstruction. The filling defect represented a “pseudo” -stone, probably due to an extrinsic impression from the hepatic artery. The catheter was subsequently removed. The patient was found to have rejected the transplant.
In the 5 patients with a biloma (Fig. 4), transhepatic external drainage of the biloma was performed until a suitable liver was obtained for retransplantation. In 1 patient with sepsis due to obstruction and intrahepatic abscess, a catheter was placed to control sepsis preoperatively. The patient subsequently underwent biliary revision and drainage of the abscess.
One patient developed a choledochocholedochostomy stricture 16 months after transplantation for intrahepatic cholangiocarcinoma. A transcatheter brush biopsy of the stricture was positive for adenocarcinoma. The patient was treated with an iridium-192 wire inserted through the transhepatic catheter. The patient died of metastatic disease after 4 months. Another patient with multiple intrahepatic strictures and a choledochojejunostomy stricture underwent surgical revision of the biliary–enteric anastomosis after failure of balloon dilatation, and was also found to have cholangiocarcinoma
Hyperbilirubinemia and fever developed 2 months after transplantation in a 3-year-old girl, in whom the gallbladder was utilized as a pedicle graft conduit between the donor and recipient common bile ducts. A PTe study demonstrated partial obstruction at the anastomosis, probably secondary to the internal biliary stent. The stent was pushed into the jejunum when PTBD was performed. The patient became afebrile and the serum bilirubin level returned to normal. In the patient with probable recurrent sclerosing cholangitis, an obstructed stent was removed percutaneously via a transhepatic tract using a Dormia basket. The stent was obstructed by encrusted bile.
Two patients with strictures and obstruction underwent biliary reconstruction following PTBD. The remainder were initially treated with transhepatic balloon dilatation.
Percutaneous Transhepatic Biliary Dilatation
A total of 13 dilatation procedures in 10 patients (11 transplants) were performed. Four patients are asymptomatic at 21, 23, 43, and 44 months after dilatation. In 1 patient, a stricture recurred in the donor common hepatic duct 5 months after initial dilatation and was successfully redilated (Fig. 1). The patient remained asymptomatic for another 25 months when abnormal liver function tests were demonstrated. The PTC demonstrated recurrent stricture with choledocholithiasis. Because of the stone disease, revision of the biliary anastomosis was performed. Because of the high location of the stricture, separate anastomoses of the right and left ducts to a Roux limb of jejunum were performed. To facilitate this difficult reconstruction, transhepatic catheters were placed into the right and left hepatic ducts preoperatively to enable positive identification of the ducts at operation.
In another patient, biliary dilatation was successfully performed for a stricture in the donor common hepatic duct. The patient returned after 10 weeks with symptoms of cholangitis. A PTC study demonstrated obstruction of the anterior segmental right hepatic ducts due to intrahepatic biliary strictures (Fig. 2). The previously dilated stricture in the common hepatic duct was patent. Dilatation of the intrahepatic strictures was subsequently performed with an excellent initial result.
A patient with multiple intrahepatic strictures was dilated with a good cholangiographic result. However, because of rejection, retransplantation is planned.
Three subjects underwent early surgical revision of the biliary anastomosis after dilatation. In 1, a recurrent stricture was found and the biliary anastomosis was revised. In another patient, multiple intrahepatic strictures were present in addition to an anastomotic stricture at the choledochojejunostomy. After balloon dilatation of the biliary enteric anastomosis failed, reexploration demonstrated cholangiocarcinoma at the anastomosis. In the third patient, the serum bilirubin level continued to rise after apparent successful dilatation. Surgical revision of the anastomosis was performed; however, the bilirubin continued to rise postoperatively due to severe rejection. Retransplantation was performed.
Complications
Two patients developed a hemothorax that required a chest tube. Hemobilia occurred in 1 other patient in whom the portal vein was inadvertently entered in an attempt to drain a cavity near the hilum of the liver. At exploration, the portal vein was incorporated into a central necrotic cavity. The cavity functioned as a cloaca between a necrotic common bile duct and the jejunum. Retransplantation was considered but the patient died from massive hemorrhage from the portal vein. There were no cases of intraabdominal bleeding in our series.
Discussion
The first human liver transplant was performed in 1963 [5]. Over the following 3 years, 6 more patients received liver transplants. However, all died early, the longest survival being 23 days [6]. The first successful extended survival (13 months) was achieved in 1967 [7]. Survival rates have continued to improve especially as a result of the success of cyclosporine as an immunosuppressive drug [8, 9]. From 1980 to 1984, the 1-year survival rate has risen from 50 to 85% [8]. Four-year survival is 75% in children and 50% in adults [8].
Other factors responsible for improved survival in liver transplantation include newer surgical techniques, changes in patient selection, and better postoperative care [10, 11]. One aspect of care that has evolved is the frequent use of cholangiography [6, 12, 13]. There is no chance for survival in the immunosuppressed transplant patient if serious biliary complications are not diagnosed and corrected promptly [3]. For this reason, cholangiography is performed whenever biliary complications are suspected.
In most adult liver transplant patients, biliary reconstruction is accomplished by a choledochocholedochostomy. A T-tube is inserted into the recipient common bile duct to stent the anastomosis and monitor the quantity and quality of bile out-put. Cholangiography may be performed through the T-tube [4]. The tube remains for a minimum of 2–3 months and is then removed if the patient’s clinical condition permits.
After T-tube removal, radiographic evaluation of the biliary tree in patients with a choledochocholedochostomy is carried out by either PTC or endoscopic retrograde cholangiography. In patients without a normal native common bile duct (e.g., primary sclerosing cholangitis, biliary atresia), biliary reconstruction consists of a Roux-en-Y hepaticojejunostomy or choledochojejunostomy and internal stent. No T-tube is used. Thus, in these patients, PTC is required to assess the biliary tree when postoperative complications are suspected.
Sonography is frequently obtained first if obstruction or liver abscess is suspected. However, false-negative examinations can occur with early biliary obstruction [14]. For this reason, PTC may still be performed even if normal-size ducts are detected by ultrasound.
Both PTC and PTBD are generally safe procedures in transplant patients. The technique and guidelines for performance are similar to those in patients who have not received transplants. Because of the immunosuppressed patient’s susceptibility to infection, antibiotics are given prophylactically in all instances. Abnormal clotting studies are corrected with fresh frozen plasma, and platelet transfusions are given for thrombocytopenia. With these precautions, major bleeding complications occurred in 3 subjects.
The types of posttransplant abnormalities one is likely to encounter in performing PTC are summarized in Table 1. Postoperative complications unique to liver transplant patients are related to bile duct anastomoses, particularly the choledochocholedochostomy, and to ischemia. Bile duct strictures at an anastomosis or in the donor biliary tree (Figs. 1, 2), and bile leaks and bilomas (Fig. 4) secondary to bile duct necrosis, are examples. Common causes of biliary obstruction that prompt PTC in nontransplant patients are less frequently encountered. Thus, choledocholithiasis is uncommon and obstructing neoplasms are rare.
If biliary obstruction is diagnosed by PTC, prompt treatment must be initiated. Since many of these patients present with cholangitis or sepsis, the preferred initial step is transhepatic external biliary drainage to control infection. Biliary sepsis can be life-threatening in these immunosuppressed patients. Once the patient is stabilized, a decision is made as to how best to treat the patient depending on the cause of the obstruction. As demonstrated in 1 of our patients, the late development of a biliary stricture in a patient receiving a transplant for cholangiocarcinoma should be evaluated for possible recurrent carcinoma.
Biliary obstruction due to stricture formation was the most frequently encountered surgical complication at PTC. Most anastomotic strictures can probably be explained on the basis of scar formation with retraction and narrowing, although ischemia may also be an important factor [15, 16]. Strictures in the donor biliary system, away from the anastomosis (Fig. 1), are of unknown cause but are probably due to ischemia. The donor bile duct is completely dependent on the hepatic artery (usually the right) for its blood supply [17]. One subject in our series who had strictures of the donor common hepatic duct and main left hepatic duct had angiographically documented hepatic artery thrombosis. Ischemia can also occur if there is an excessively long lime between donor organ removal and transplantation.
During the early years of liver transplantation, biliary drainage was usually performed utilizing the donor’s gallbladder. Of the first 59 transplanted patients with a cholecystoduodenostomy, 25% were found to be obstructed [18]. Obstruction usually occurred at the cystic duct, as seen in 2 patients in our series. Besides obstruction and liver abscess, cholecystojejunostomies have resulted in biliary abdominal sepsis [19]. Because of the high incidence of complications, simple cholecystoenteric anastomosis is rarely performed today for biliary reconstruction in liver transplantation. However, utilization of the donor gallbladder as a pedicle graft conduit between the donor common bile duct and the recipient common bile duct or jejunum has been advocated by Calne in England [20].
Regardless of the location and cause of the stricture, transhepatic balloon dilatation is usually the preferred initial method of treatment (Fig. 1). Balloon dilatation of postoperative biliary strictures in nontransplant patients is safe, with reported success rates of 85–89% [21, 22]. Similarily, we have found the procedure to be safe and effective in transplants. Of the 5 patients with adequate follow-up, 4 patients are asymptomatic up to 3-1/2 years following dilatation. The fifth patient was asymptomatic for 5 and 25 months after initial and repeat dilatations, respectively. If dilatation fails, biliary revision can be undertaken. In this circumstance, the transhepatic catheter is generally left in place to facilitate locating the duct at operation.
Because the blood supply of the distal donor bile duct is tenuous [15, 16], minor bile leaks may occur due to bile duct necrosis at the anastomosis. However, only 2 such patients, 1 with a biliary-cutaneous fistula and another with an intraperitoneal bile leak, have been seen on PTC. Instead, anastomotic bile leaks have been diagnosed most frequently with T-tube cholangiography [4]. This is so in part because anastomotic leaks occur more commonly at choledochocholedochostomies, a type of anastomosis usually stented with a T-tube [4]. Also, donor bile duct necrosis at the anastomosis appears to occur relatively early in the postoperative course when the T-tube is likely to remain in place. When such anastomotic breakdowns occur, surgical revision of the anastomosis is necessary.
Major bile leaks have resulted from necrosis of the donor bile ducts secondary to hepatic artery thrombosis. Accompanying conditions have included bile peritonitis, subhepatic fluid collections, drainage of bile through abdominal drains, and bacteremia [17]. In order for these patients to survive, retransplantation, rather than biliary reconstruction, is always necessary [4, 17]. Five such patients presented with intrahepatic bile leaks and bilomas. Because of recurrent fevers, transhepatic catheters were inserted via the biliary tree to drain these secondarily infected collections until a suitable liver became available for retransplantation. In 1 patient, the transhepatic catheter has been in place for scveral months. It is hoped that this catheter can eventually be removed without the need for retransplantation. A detailed analysis of the cholangiographic findings in hepatic artery thrombosis in liver transplant patients is currently under-way and will be the subject of a later report.
Acknowledgment
We thank Donna Scahill for manuscript preparation.
References
- 1.Gartner JC, Jr, Zitelli BJ, Malatack JJ, Shaw BW, Iwatsuki S, Starzl TE. Orthotopic liver transplantation in children: two-year experience with 47 patients. Pediatrics. 1984;74:140–145. [PMC free article] [PubMed] [Google Scholar]
- 2.Van Thiel DH, Schade RR, Gavaler JS, Shaw BW, Jr, Iwatsuki S, Starzl TE. Medical aspects of liver transplantation. Hepatology. 1984;4(suppl. 1):79S–83S. doi: 10.1002/hep.1840040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Putnam CW, Hansbrough JF, Porter KA, Reid HAS. Biliary complications after liver transplantation: with special reference to the biliary cast syndrome and techniques of secondary duct repair. Surgery. 1977;81:212–221. [PubMed] [Google Scholar]
- 4.Zajko AB, Campbell WL, Bron KM, Lecky JW, lwatsuki S, Shaw BW, Jr, Starzl TE. Cholangiography and interventional biliary radiology in adult liver transplantation. AJR. 1985;144:127–133. doi: 10.2214/ajr.144.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Marchioro TL, von Kaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW, Jr, Hakala TR, Rosenthal JT, Porter KA. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Jr, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–414. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, lwatsuki S, Shaw BW, Jr, Gordon RD. Orthotopic liver transplantation in 1984. Transplant Proc. 1985;17:250–258. [PMC free article] [PubMed] [Google Scholar]
- 9.Malatack JJ, Zitelli BJ, Gartner JC, Jr, Shaw BW, Iwatsuki S, Starzl TE. Pediatric liver transplantation under therapy with cyclosporin-A and steroids. Transplant Proc. 1983;15:1292–1296. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Koep LJ, Halgrimson CG, Hood J, Schroter GPJ, Porter KA, Weil R., III Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 11.Calne RY, Williams R, Lindop M, Farman JV, Tolley ME, Rolles K, Macdougall B, Neuberger J, Wyke RJ, Raftery A, Duffy TJ, Wight DGD, White DJG. Improved survival after orthotopic liver grafting. Br Med J. 1981;283:115–118. doi: 10.1136/bmj.283.6284.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE. Liver transplantation. Johns Hopkins Med J. 1978;143:73–83. [PubMed] [Google Scholar]
- 13.Starzl TE, Putnam CW, Ishikawa M, Picache R, Husberg B, Halgrimson CG, Schroter G. Current policies in hepatic transplantation: candidacy of patients with alcoholic liver disease or preformed antidonor antibodies and in reappraisal of biliary duct reconstruction. Ann NY Acad Sci. 1975;252:145–158. doi: 10.1111/j.1749-6632.1975.tb19151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrucci JT, Jr, Adson MA, Mueller PR, Stanley RJ, Stewart ET. Advances in the radiology of jaundice: a symposium and review. AJR. 1983;141:1–20. doi: 10.2214/ajr.141.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Northover J, Terblanche J. Bile duct blood supply. Transplantation. 1978;26:67–69. [PubMed] [Google Scholar]
- 16.Northover JMA, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379–384. doi: 10.1002/bjs.1800660603. [DOI] [PubMed] [Google Scholar]
- 17.Tzakis AG, Gordon RD, Shaw BW, Jr, Iwatsuki S, Starzl TE. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporin era. Transplantation. 1985;40:667–671. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martineau G, Porter KA, Corman J, Launois B, Schroter GT, Palmer W, Putnam CW, Groth CG, Halgrimson CG, Penn I, Starzl TE. Delayed biliary duct obstruction after orthotopic liver retransplantation. Surgery. 1972;72:604–610. [PMC free article] [PubMed] [Google Scholar]
- 19.Iwatsuki S, Shaw BW, Jr, Starzl TE. Biliary tract complications in liver transplantation under cyclosporin–steroid therapy. Transplant Proc. 1983;15:1288–1291. [PMC free article] [PubMed] [Google Scholar]
- 20.Calne RY. A new technique for biliary drainage in ortho-topic liver transplantation utilizing the gallbladder as a pedicle graft conduit between the donor and recipient common bile ducts. Ann Surg. 1976;184:605–609. doi: 10.1097/00000658-197611000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallacher DJ, Kadir S, Kaufman SL, Mitchell SE, Kinnison ML, Chang R, Adams P, White RI, Jr, Cameron JL. Non-operative management of benign postoperative biliary strictures. Radiology. 1985;156:625–629. doi: 10.1148/radiology.156.3.4023219. [DOI] [PubMed] [Google Scholar]
- 22.Molnar W, Stockum AE. Transhepatic dilatation of choledochoenterostomy strictures. Radiology. 1978;129:59–64. doi: 10.1148/129.1.59. [DOI] [PubMed] [Google Scholar]