Abstract
Aims
Reflex contractions of the external urethral sphincter (EUS) are a major component of voiding dysfunction after neurological injury or disease. Aberrant urethral reflexes can prevent voiding and cause serious medical complications. Characterizing these urethral reflexes during genitourinary studies is necessary for evaluating novel pharmacological or neuroprosthetic approaches. The objectives of the present study were to generate urethral reflexes in the acute spinal feline, to quantify these reflexes, and to suppress them with electrical stimulation of the sacral dermatomes.
Methods
This study comprised eight male cats. Anaesthesia was maintained with alpha-chloralose or sodium pentobarbital. The spinal cord was transected between T10 and T12, and nerve cuff electrodes were placed on the extradural S2 sacral roots to provide bladder activation. Bladder and urethral pressures were recorded during and after bladder contractions. Electrical stimulation was applied non-invasively to the sacral dermatomes with commercial surface electrodes.
Results
Urethral reflexes were elicited consistently in six cats. The corresponding urethral pressure spikes were quantified. Putative metrics of urethral reflex activity such as the rate and average magnitude of reflex pressure spikes correlated significantly with standard urodynamic variables. Electrical stimulation of the sacral dermatomes suppressed urethral reflexes in three cats.
Conclusions
These findings in an acute spinal feline preparation demonstrate a non-invasive means of suppressing undesirable urethral reflexes. Translation of this work to clinical use could improve neuroprostheses for restoring bladder function and enhance treatment of aberrant urethral reflexes in humans.
Introduction
In healthy adults, evacuation of the urinary bladder can be initiated and terminated voluntarily. In infants and young children (Fowler et al., 2008) and in many animals, the bladder empties involuntarily by reflex activity. In some neurological disorders, coordination between the somatic and autonomic nervous systems required for bladder emptying is lost. Such loss of coordination can produce pathological reflexes that impair voiding and continence.
Normally, voiding occurs when the smooth muscle of the bladder contracts and the skeletal muscle of the external urethral sphincter (EUS) relaxes. Loss of descending cortical input can lead to unopposed parasympathetic tone and uncoordinated viscero-somatic reflexes of the lower urinary tract (Craggs et al., 2006). These aberrant reflexes cause detrusor-sphincter dyssynergia (DSD), in which the bladder and EUS contract simultaneously, preventing voiding (Watanabe et al., 1996). Bladder hyperreflexia may also occur (Craggs et al., 2006). Chronically, these effects lead to clinically significant problems including urine reflux into the ureters and kidneys and life-threatening autonomic dysreflexia (Creasey et al., 2001). Individuals with spinal cord injury (SCI) place great emphasis on restoration of bladder function as a recovery priority (Anderson, 2004) for this costly condition (Creasey and Dahlberg, 2001).
Current treatment approaches for voiding dysfunction have marked disadvantages. The Brindley procedure using sacral anterior root stimulation requires invasive surgery and irreversible dorsal rhizotomy, which has undesirable side effects (Creasey et al., 2001). Without rhizotomy, urethral reflexes prevent voiding in both humans (Kirkham et al., 2002) and animals (Bhadra et al., 2002; Bhadra et al., 2006a).
However, under certain conditions without rhizotomy in the canine, phasic reflex EUS activity and bladder voiding were observed, suggesting that neural signals from the EUS provided “feedback” to central spinal circuits to produce voiding. This observation led to application of afferent stimulation to interrupt the aberrant EUS reflexes and allow voiding without rhizotomy in the canine (Bhadra et al., 2006b).
Recent work in the feline demonstrates that electrical (Boggs et al., 2005; Boggs et al., 2006a; Boggs et al., 2006b; Gustafson et al., 2003) and perigenital mechanical (Fowler et al., 2008; Tai et al., 2008) afferent stimulation can produce bladder excitation via a spinal micturition reflex. Peripheral afferent stimulation can also produce reflex bladder contractions in cats (Shefchyk and Buss, 1998) and humans (Gustafson et al., 2004). In addition, neuromodulation by stimulating afferent pathways has been explored in other contexts, including alleviating congenital nystagmus in humans (Sheth et al., 1995) and reducing extensor spasticity during treadmill walking in incomplete SCI patients (Bajd et al., 2002).
This study had three main goals. An acute spinal feline preparation exhibiting aberrant urethral reflexes was developed. A method for quantifying the urethral reflex contractions was devised, allowing correlation of the reflexes to standard urodynamic variables. Finally, these urethral reflexes were suppressed by non-invasive electrical stimulation of the sacral dermatomes.. Our approach for quantifying and suppressing these undesirable urethral reflexes will aid comparison of emerging therapies, fulfilling an unmet clinical need.
Materials and Methods
Eight sexually and neurologically intact adult male cats 3.7–5.4 kg were used. All procedures were approved by the Case Western Reserve University institutional animal care and use committee. Cats are sufficiently complex to serve as a neurophysiological model of the urogenital system with better than previously appreciated neuroanatomical homology to the human (Mariano et al., 2008). The surgical preparation was substantially similar to that previously described (Boger et al., 2007). Briefly, surgery was performed under isoflurane anaesthesia (dosed to effect). Experimental testing occurred under either α-chloralose (65 mg/kg IV, n = 6 cats) or sodium pentobarbital (25 mg/kg IV, n = 2 cats) anaesthesia. A 6 F dual lumen (DLC-6D, Life Tech, TX) or 7 F Foley (Rochester Medical, MN) catheter was implanted in the bladder suprapubically and connected to an external transducer (Deltran I, Utah Medical Devices, UT) to monitor bladder pressure (Pves). Urethral pressure (Pur) was measured with a 3.5 F urethral perfusion catheter or a 2 F or 4 F microtransducer-mounted catheter (FT-21C-ABOG, Scisense, ON; CTC/4F-2, Gaeltec, UK) placed at the location of the EUS as determined by a urethral pressure profile. Recorded responses were consistent regardless of catheter type. This catheter obstructed the urethra, preventing direct voiding measurements. A laminectomy of vertebral levels T10–T12 was then performed, and the exposed spinal cord was transected with micro-scissors and the aid of a surgical microscope (Storz Urban US-1, Urban Engineering, Inc., CA) usually after instillation of 0.1 mL bupivacaine. Post-mortem dissection verified spinalization between T10 and T12 with two of eight spinalizations incomplete. One of these cats was a non-responder excluded from the analysis. The other animal (Cat 1) exhibited urethral reflexes despite the ventral 20% of the white matter being intact. A sacral laminectomy allowed implantation of custom tripolar cuff electrodes bilaterally on the extradural sacral roots (S1 or S2) eliciting the greatest electrically evoked bladder contractions.
The acute phase of spinal shock resolves quickly in cats (McCouch et al., 1935) and passed after approximately one hour in this study, signaled by return of the anal stretch or bulbospongiosus reflexes (or both). Experimental testing began after an additional 3.5 hours, allowing significant recovery of evoked lower urinary tract and bladder responses (Boggs et al., 2005). The bladder was then filled incrementally with warm saline from a syringe infusion pump (Genie YA-12, Kent Scientific, CT) at 0.5–3 mL/min to 15–45 mL; filling was ended when the bladder became reflexive. Electrical stimulation (continuous 20 Hz 100 µs biphasic cathodic-first current-controlled rectangular pulses, 600 µA – 3 mA) was then applied to the extradural sacral roots (Pulsar 6bp or 6bp-as, FHC, ME; DS8000, World Precision Instruments, FL) to elicit bladder contractions. Urethral pressures were monitored for post-stimulus reflex activity.
Intermittent surface stimulation (primarily 20 Hz, 0.5 s on / 0.5 s off, 200 µs monophasic, cathodic current-controlled, rectangular pulses, 4 – 20 mA) was applied (DS7A, Digitimer, UK) to shaved, depilated, and cleaned skin of the S1–S3 sacral dermatomes (Brown and Fuchs, 1975; Kuhn, 1953) through 5 cm by 5 cm self-adhesive electrode pads (Re-Ply 655, Tyco/Uni-Patch, MN). An identical return electrode was placed on the upper back or ipsilateral thigh.
To ensure that observed urethral reflexes were not an artifact of bladder stimulation modality—wherein both sensory and motor roots were activated by the extradural electrodes— an additional male cat (3.5 kg) was tested. This animal only received stimulation of the left pudendal nerve (primarily 33 Hz 100 µs biphasic cathodic-first current-controlled rectangular pulses, 1–5 mA), which produced bladder contractions via an afferent-mediated micturition reflex (Boggs et al., 2005). No sacral root access or stimulation occurred. The remainder of the experimental setup was as described for the sacral drive animals.
The data acquisition hardware was as previously described (Boger et al., 2007), consisting of a computer-based system (NI DAQ 6024E, National Instruments Corporation, TX) with customized software interface (LabVIEW 7.1, National Instruments Corporation). Data processing was performed with MATLAB (R2007a, The MathWorks, MA) using code developed for this study. The end of an experimental trial was taken to be when Pur returned to within 10% of baseline.
The algorithm extracted the urethral reflex metrics Nspk (reflex pressure spike count), Pspk,ave (average spike amplitude), and Pspk,ave,norm (normalized average spike amplitude, Pspk,ave / Pur,ave). Both are useful as Pspk,ave is a primary measure and the normalization of Pspk,ave,norm is more robust for comparing across cats. Nspk was calculated per trial and was therefore biased due to varying trial length and to pooling Nspk data across cats, as trial blocks were not balanced. Therefore, an additional measure, the spike rate (events per second) was calculated as Rspk = Nspk/[(trial duration) − (bladder stimulus duration)], with all durations in seconds.
Extracted urodynamic predictor variables were Pves,ave (average bladder pressure), Pves,end (end-of-stimulus bladder pressure), Pves,end,norm (normalized end-of-stimulus bladder pressure, Pves,end / Pves,ave), Pur,ave (average urethral pressure), Pur,max (maximum urethral pressure), Pur,end (end-of-stimulus urethral pressure), and Pur,end,norm (normalized end-of-stimulus urethral pressure, Pur,end / Pur,ave).
Correlation matrices were computed using Pearson product-moment correlation coefficients and two-tailed significances. Analyses of variance (ANOVAs) and t-tests were also performed (SPSS 16.0.2 GP, SPSS, IL and JMP 5.1, SAS Institute, NC). Separately fit two-way ANOVAs and independent samples t-tests were chosen because trial blocks were unbalanced in this preliminary study.
Results
Twitch- or spike-like urethral reflexes were produced and quantified in an acute spinal feline model. Surface stimulation of the sacral dermatomes significantly affected these urethral reflexes, suppressing them in three of six animals.
Generation of Urethral Reflexes
Six of eight cats (designated as Cats 1–6) exhibited consistent and robust post-spinalization urethral reflexes that were observed after direct stimulation ended (Figure 1). The other two cats exhibited only occasional slow sustained rises in Pur and therefore were not analyzed further. In total, 214 trials occurred without sacral dermatome surface stimulation applied (3–42 trials per cat), and 127 trials occurred with surface stimulation (2–35 trials per cat). Cat 1 with incomplete spinalization yielded the fewest trials. In each trial, urethral reflex activity typically ceased within 45–60 seconds, except for Cat 4, in which urethral reflex activity extended for approximately three minutes.
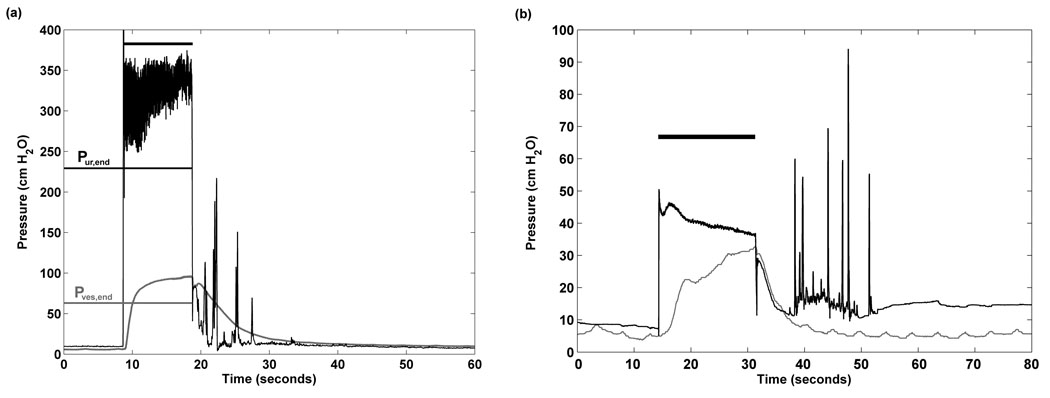
Figure 1.
(a) Representative trial from Cat 5 exhibiting aberrant urethral reflex pressure spikes following sacral root bladder drive. The horizontal black bar indicates the duration of application of sacral drive. (b) Representative trial with reflex spikes derived from the cat with reflex bladder drive by pudendal nerve stimulation. Pur is urethral pressure and Pves is bladder pressure. Note the different y-axis scaling.
Quantification of Urethral Reflexes
To assess the quantitative predictors and metrics, correlations were computed for trials without sacral dermatome surface stimulation, pooled across all six responder cats (Table I). This matrix included n = 214 trials, except for Pves,ave and Pves,end,norm (n = 211). The urethral reflex metrics Rspk, Pspk,ave, and Pspk,ave,norm exhibited the greatest number of significant correlations to urodynamic predictor variables representing bladder and urethral pressures. These metrics were therefore included in the ANOVAs.
Table I.
The correlation matrix was computed for trials without surface stimulation, pooled from Cats 1–6. Cell values are Pearson product-moment correlation coefficients; cell shading indicates two-tailed significances of p < 0.05.
| Metrics | |||||
|---|---|---|---|---|---|
| Nspk | Rspk | Pspk,ave | Pspk,ave,norm | ||
| Urodynamic predictors | Pur,ave | −0.156 | −0.211 | 0.358 | −0.226 |
| Pur,max | −0.271 | −0.274 | 0.244 | −0.265 | |
| Pur,end | −0.067 | −0.168 | 0.083 | −0.258 | |
| Pur,end,norm | 0.400 | 0.042 | −0.377 | −0.128 | |
| Pves,ave | 0.057 | 0.238 | 0.343 | 0.249 | |
| pves,end | 0.048 | 0.031 | 0.331 | 0.027 | |
| pves,end,norm | 0.008 | −0.178 | 0.076 | −0.202 | |
 P < 0.05
P < 0.05
Suppression of Urethral Reflexes
Surface stimulation of the sacral dermatomes affected urethral reflex activity in all six cats. Surface stimulation significantly reduced urethral reflex activity in three cats, increased Rspk in one cat (Cat 4), and increased reflex activity in two cats. An example from Cat 4 of suppression with extended reflex duration appears in Figure 2.
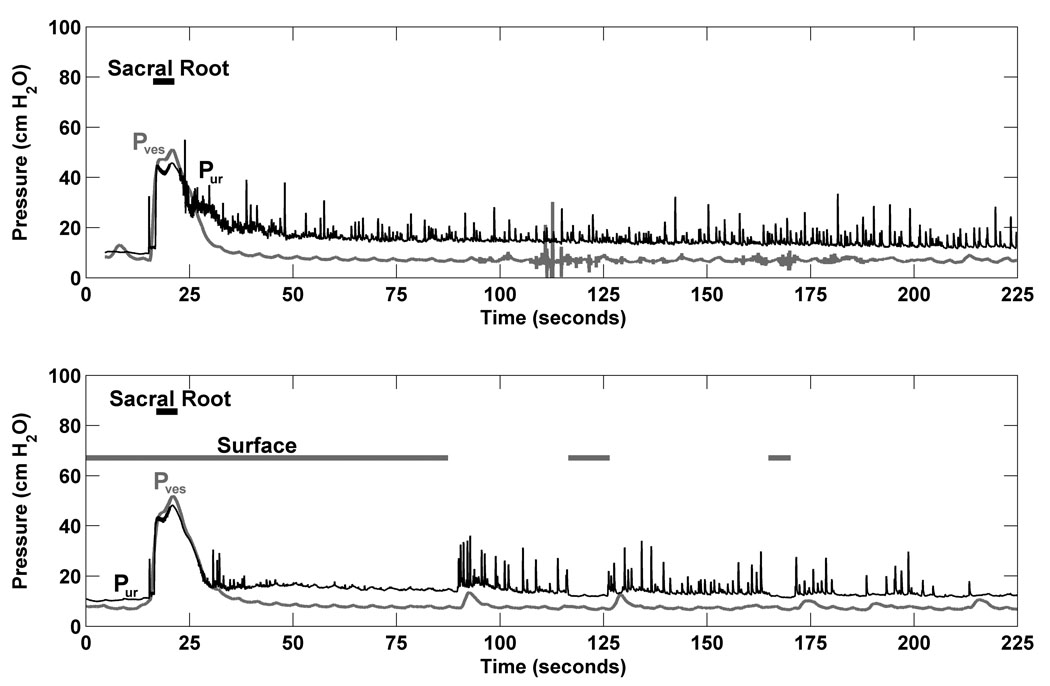
Figure 2.
Dermatome stimulation reduced urethral reflex activity. The top panel is from a trial with sacral root bladder drive and no surface stimulation; the bottom panel is a repeat trial with surface stimulation commencing at 0 sec. Both trials were taken from Cat 4. Pur is urethral pressure and Pves is bladder pressure.
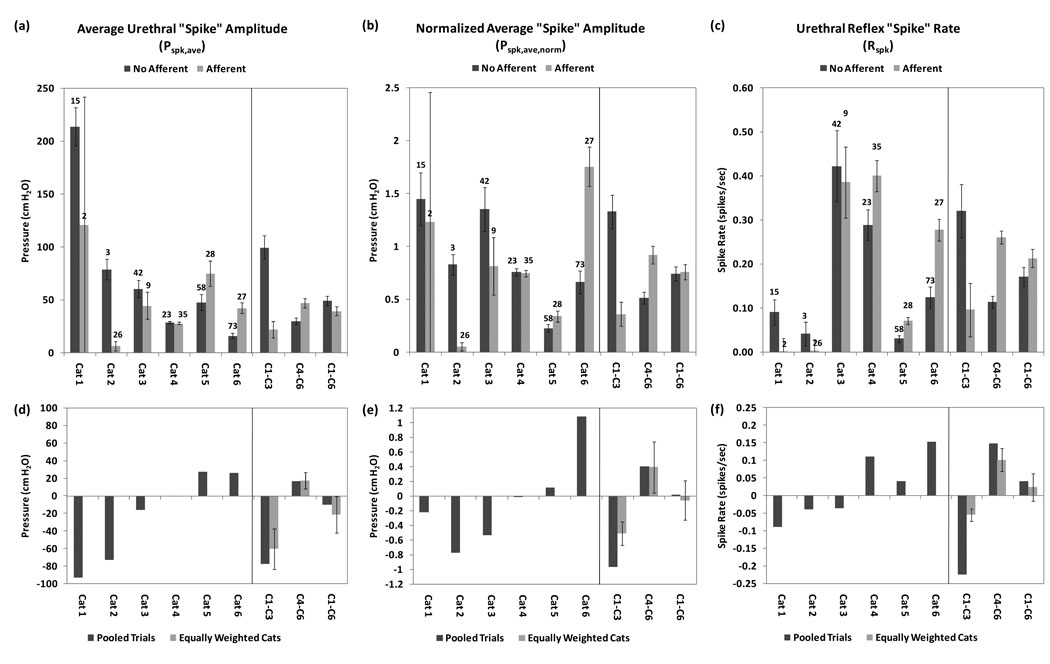
The analyzed metrics (Rspk, Pspk,ave, and Pspk,ave,norm) were examined for each cat, both without and with sacral dermatome surface stimulation (Figure 3a–3c). Animals were categorized as (1) positive responders (Cats 1–3), in which surface stimulation decreased urethral reflex activity, and (2) negative responders (Cats 4–6), in which surface stimulation exacerbated these reflexes (though only for Rspk with Cat 4). The positive responder group comprised 60 trials without surface stimulation (3–42 trials per cat) and 37 trials with surface stimulation (2–26 trials per cat). The negative responder group comprised 154 trials without surface stimulation (23–73 trials per cat) and 90 trials with surface stimulation (27–35 trials per cat). Panels d–f of Figure 3 present the same data as directly calculated differences between averages of pooled trials without and with surface stimulation (dark bars). The lighter bars are a calculated measure that weights each cat equally to eliminate pooling bias from unbalanced trial blocks.
Figure 3.
Histograms for urethral reflex metrics (given as a mean ± 1 SEM error bars), presented both for individual cats, positive responders (Cats 1–3), and negative responders (Cats 4–6). The top panels (a–c) indicate non-normalized average reflex spike amplitude, normalized average spike amplitude, and spike rate, respectively. The numbers above the bars for individual cats represent the n of trials for each condition (without or with surface stimulation applied). The lower panels (d–f) represent the same data using directly calculated differences between averaged pooled trials without and with surface stimulation (dark bars). The lighter bars are a measure that weights each cat equally to eliminate pooling bias caused by unbalanced trial blocks. Error bars represent ± 1 SEM.
One-way ANOVAs assessed reflex suppression in the positive responders, the negative responders, and all cats (Table II). In the positive responder group, Rspk, Pspk,ave, and Pspk,ave,norm were significantly reduced (all F > 7, all p < 0.01) when sacral dermatome surface stimulation was applied. In the negative responder group, all metrics increased significantly (all F > 9, all p < 0.0025) with surface stimulation. When Cats 1–6 were analyzed together, no significant differences were found (all F < 2.25, all p > 0.10).
Table II.
The degree of reflex suppression was assessed with one- and two-way ANOVAs. The factors for the two-way ANOVA were individual animal and application of surface stimulation. The number of trials for each condition is indicated at the top.
| Positive Responders (C1–C3) |
Negative Responders (C4–C6) |
All Cats (C1–C6) |
|||
|---|---|---|---|---|---|
| n of trials | No Surface | 60 | 154 | 214 | |
| Surface | 37 | 90 | 127 | ||
| 1-Way ANOVA |
Rspk (spikes/sec) |
F | 7.1718 | 25.3137 | 1.5660 |
| P | 0.0087 | 0.0001 | >0.20 | ||
|
Pspk,ave (cm H20) |
F | 24.8902 | 9.4640 | 2.2392 | |
| P | 0.0001 | 0.0023 | >0.10 | ||
| Pspk,ave,norm | F | 19.0683 | 17.4391 | 0.0269 | |
| P | 0.0001 | 0.0001 | >0.85 | ||
| 2-Way ANOVA |
Rspk (spikes/sec) |
F | 0.1731 | 14.2478 | 5.4065 |
| P | >0.65 | 0.0002 | 0.0207 | ||
|
Pspk.ave (cm H20) |
F | 7.1429 | 13.3104 | 2.4178 | |
| P | 0.0089 | 0.0003 | >0.10 | ||
| Pspk,ave,norm | F | 1.5990 | 23.3082 | 8.7860 | |
| P | >0.20 | < 0.0001 | 0.0033 | ||
To account for the multiple comparisons inherent in one-way ANOVA, a series of two-way ANOVAs was performed with separate model fits for each urethral reflex metric (Table II). For positive responder cats (Cats 1–3), there was a significant main effect of the between-subjects factor of cat for all metrics (all F > 4, all p < 0.02), but no significant interactions. Only the within-subjects factor of Pspk,ave showed a significant reduction (F = 7.1429, p < 0.01) when sacral dermatome surface stimulation was applied. For negative responder cats (Cats 4–6), all metrics showed a significant main effect of cat (all F > 20, all p < 0.0001), but no significant interactions. Surface stimulation significantly increased all reflex metrics (all F > 13, all p < 0.0004). For all cats, there again was a significant main effect of cat for all reflex metrics (all F > 19, all p < 0.0001) but no significant interactions. Both Rspk and Pspk,ave,norm showed significant increases (all F > 5, all p < 0.03).
Pudendal Nerve Stimulation
In the additional animal that received bladder drive via only the pudendal nerve, urethral reflexes similar to those elicited with sacral drive occurred (Figure 1b). In 42 pooled trials without (n = 32) and with (n = 10) surface stimulation, independent samples t-tests revealed that sacral dermatome surface stimulation significantly reduced Pspk,ave (p = 0.034, equal variances assumed) and Pspk,ave,norm (p < 0.005).
Discussion
This study demonstrated production of aberrant urethral reflexes in an acute spinal feline preparation. These data are the first demonstration of suppression of such reflexes with surface stimulation of the sacral dermatomes. The approach has significant clinical potential for undesirable urethral reflex activity in people with SCI or other neurological disorders, and it could be incorporated into future bladder neural prostheses.
Urethral reflexes following acute spinal transection may not be neurophysiologically equivalent to detrusor-sphincter dyssynergia (DSD) resulting from chronic neurological changes after SCI. Therefore, although this acute preparation has considerable experimental utility, further study in chronic preparations is required to demonstrate effectiveness for DSD.
Generation of Urethral Reflexes
In this study, an acute SCI feline preparation was developed that exhibited robust post-spinalization urethral reflexes. Although post-SCI urethral reflexes are usually seen chronically and may result from urethral flow (Galeano et al., 1986), acute SCI excitatory afferent models have been used to examine spinal neural circuits and to obtain insight (Boggs et al., 2005; Boggs et al., 2006a; Boggs et al., 2006b) before validation in chronic SCI (Tai et al., 2008) and human genitourinary studies.
Urethral reflexes could not be observed during bladder drive due to simultaneous direct sphincter activation from extradural sacral root stimulation. Therefore, urethral reflexes were observed only after bladder pressure was decreasing. Nonetheless, these reflexes are similar to DSD. The reflexive EUS activity observed after sacral stimulation (Figure 1), presumably due to urethral fluid flow, is similar to that observed in humans with chronic SCI after sacral stimulation (Figure 8 of Kirkham et al., 2002). In humans, this post-stimulus reflexive EUS activity (DSD) prevents voiding and requires a dorsal rhizotomy to attain voiding. Canine studies have shown a similar result (Bhadra et al., 2006a). The observed urethral reflexes are also consistent with urethral-flow-activated dyssynergia.
Bladder excitation modality did not affect urethral reflexes; they could also be elicited with pudendal nerve mediated bladder contractions and then suppressed with surface stimulation. Therefore, the urethral reflexes were not an artifact during extradural sacral root activation, as that modality could indirectly activate afferent pathways. Anaesthetic agents also did not materially affect responses. The reflexes were absent in spinal-intact animals and occurred at physiologically normal bladder volumes. It is possible that sacral root and pudendal stimulation activated other reflex pathways that contributed to the urethral activity.
Urethral pressure responses include non-EUS activity from the pelvic floor and urethral smooth muscle. However, the time constant of the repeated, rapid, spike-like contractions is consistent with skeletal muscle. Pelvic floor contractions were also observed in synchrony with urethral pressure spikes. Future studies could eliminate smooth muscle effects with blocking agents. Nonetheless, the non-EUS contributions in the present study are functionally relevant as they could also impede voiding.
Quantification of Urethral Reflexes
The choice of recorded values for analysis was motivated by urodynamic variables that would be easily obtainable in a clinical setting with existing equipment and techniques, although these quantities usually are not examined in this fashion. Quantification was consistent and unbiased using an automated computer algorithm. The resulting correlation matrix indicated that the best urethral reflex metrics were Rspk, Pspk,ave and Pspk,ave,norm. Similarly, the best urodynamic predictors of urethral reflex activity were Pur,ave and Pur,max (Table I).
Suppression of Urethral Reflexes
Electrical surface stimulation delivered non-invasively to the sacral dermatomes suppressed urethral reflex contractions in three of six cats with sacral bladder excitation and in the one cat with pudendal nerve bladder excitation. Rspk, Pspk,ave and Pspk,ave,norm were sensitive metrics as they showed significant decreases and increases in the positive and negative responders (with the exceptions of Rspk and Pspk,ave,norm in the positive responder two-way ANOVA). Even in the negative responders, there was a statistically significant increase in all urethral reflex metrics. Although the opposite of what occurred in Cats 1–3, the statistical significance of these results demonstrates that surface stimulation nevertheless modulated the neurophysiologic response in these animals. It is hypothesized that these effects could be afferent-mediated, although additional control tests would be necessary to rule out other influences.
The parameter space for surface stimulation of the sacral dermatomes was not fully explored; therefore, the observed reflex suppression effect was not optimized. Some variation of surface stimulation duty cycle did occur, although the majority of trials followed the 0.5 s on / 0.5 s off intermittent paradigm. Surface stimulation amplitude also varied but for most trials remained below the threshold for visible muscle fasciculations in the tested animal. Higher stimulation amplitudes increased the urethral reflexes—perhaps by an afferent-mediated reflex—and would therefore impede voiding. Although feline dermatomes are not well defined and may overlap (Brown and Fuchs, 1975; Kuhn, 1953), there is evidence to suggest a dermatomal specificity for the suppression effect. In Cat 6, a control test was performed, comprising a series of 12 trials in which only surface stimulation was applied at random to two identical electrodes placed over lumbar (L3–L4) or sacral (S1–S3) dermatomes. For each trial, the stimulus amplitude was slowly increased from 2 mA to 15 mA, and the amplitude at which urethral responses first appeared was noted. This control test showed that the lumbar dermatomes required greater stimulation (2.4 times the visible muscle fasciculation threshold of 5.6 mA) to evoke urethral responses than did the sacral dermatomes (1.4 times the fasciculation threshold of 8.5 mA), suggesting a greater excitability of the sacral dermatomes to surface stimulation than of the lumbar dermatomes. Despite the limited parameter variation in the present study, the results demonstrate functional improvement using a non-invasive stimulation modality.
Clinical Relevance
A non-surgical and effective means of suppressing DSD could address a major unmet clinical need. Urinary neuroprostheses can improve voiding (Gaunt and Prochazka, 2006) and reduce the costs of post-SCI bladder care (Creasey and Dahlberg, 2001), but often require irreversible dorsal rhizotomy that causes undesirable side effects (Creasey et al., 2001). Modification of these existing devices to add sacral dermatome stimulation could improve effectiveness and clinical acceptance. For example, a rhizotomy-free Brindley approach using surface stimulation to reduce aberrant EUS reflexes and afferent inhibitory stimulation to provide continence could increase the number of individuals choosing this management approach.
Conclusions
Urethral reflexes were generated in an acute spinal feline preparation. Non-invasive electrical stimulation of the sacral dermatomes could suppress these aberrant reflexes. Although further work is needed to optimize the suppression effect in chronic spinalized animals, the results of the present study have clear clinical implications. Enhanced neuroprostheses incorporating this non-invasive approach could alleviate DSD in humans with voiding dysfunction from injury, stroke, or disease with a reduced surgical burden, increasing patient acceptance and vastly improving quality of life.
Acknowledgements
The authors thank Adam Boger, Tim Bruns, Tina Emancipator, Yanina Grinberg, Obinna Nwanna, and Petar Bajic for technical assistance.
Funding
This work was supported by NIH DK077089, Department of Veterans Affairs RR&D B3675R, and Department of Education GAANN P200A040207. The first author received prior partial support from NIH T32 GM007250 (CWRU MSTP).
References
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. Journal of neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Bajd T, Munih M, Savrin R, Benko H, Cikajlo I. Dermatome electrical stimulation as a therapeutic ambulatory aid for incomplete spinal cord injured patients. Artificial organs. 2002;26(3):260–262. doi: 10.1046/j.1525-1594.2002.06947.x. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Grunewald V, Creasey G, Mortimer JT. Selective suppression of sphincter activation during sacral anterior nerve root stimulation. Neurourology and urodynamics. 2002;21(1):55–64. doi: 10.1002/nau.2068. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Grunewald V, Creasey GH, Mortimer JT. Selective activation of the sacral anterior roots for induction of bladder voiding. Neurourology and urodynamics. 2006a;25(2):185–193. doi: 10.1002/nau.20184. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Mortimer JT, Grunewald V. Combined stimulation of ventral and dorsal sacral roots for control of bladder function. 7,142,925. United States patent. 2006b
- Boger A, Bhadra N, Gustafson KJ. Bladder voiding by combined high frequency electrical pudendal nerve block and sacral root stimulation. Neurourology and urodynamics. 2007 doi: 10.1002/nau.20538. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. Journal of neurophysiology. 2005;93(5):2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. Journal of neural engineering. 2006a;3(1):43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. The Journal of physiology. 2006b;577(Pt 1):115–126. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PB, Fuchs JL. Somatotopic representation of hindlimb skin in cat dorsal horn. Journal of neurophysiology. 1975;38(1):1–9. doi: 10.1152/jn.1975.38.1.1. [DOI] [PubMed] [Google Scholar]
- Craggs MD, Balasubramaniam AV, Chung EA, Emmanuel AV. Aberrant reflexes and function of the pelvic organs following spinal cord injury in man. Auton Neurosci. 2006:126–127. doi: 10.1016/j.autneu.2006.03.010. 355–370. [DOI] [PubMed] [Google Scholar]
- Creasey GH, Dahlberg JE. Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Archives of physical medicine and rehabilitation. 2001;82(11):1520–1525. doi: 10.1053/apmr.2001.25912. [DOI] [PubMed] [Google Scholar]
- Creasey GH, Grill JH, Korsten M, U HS, Betz R, Anderson R, Walter J. An implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: a multicenter trial. Archives of physical medicine and rehabilitation. 2001;82(11):1512–1519. doi: 10.1053/apmr.2001.25911. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature reviews. 2008;9(6):453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeano C, Jubelin B, Germain L, Guenette L. Micturitional reflexes in chronic spinalized cats: the underactive detrusor and detrusor-sphincter dyssynergia. Neurourol and Urodyn. 1986;5:45–63. [Google Scholar]
- Gaunt RA, Prochazka A. Control of urinary bladder function with devices: successes and failures. Progress in brain research. 2006;152:163–194. doi: 10.1016/S0079-6123(05)52011-9. [DOI] [PubMed] [Google Scholar]
- Gustafson KJ, Creasey GH, Grill WM. A catheter based method to activate urethral sensory nerve fibers. The Journal of urology. 2003;170(1):126–129. doi: 10.1097/01.ju.0000070821.87785.14. [DOI] [PubMed] [Google Scholar]
- Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neuroscience letters. 2004;360(1–2):9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kirkham AP, Knight SL, Craggs MD, Casey AT, Shah PJ. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord. 2002;40(6):272–281. doi: 10.1038/sj.sc.3101278. [DOI] [PubMed] [Google Scholar]
- Kuhn RA. Organization of tactile dermatomes in cat and monkey. Journal of neurophysiology. 1953;16(2):169–182. doi: 10.1152/jn.1953.16.2.169. [DOI] [PubMed] [Google Scholar]
- Mariano TY, Boger AS, Gustafson KJ. The feline dorsal nerve of the penis arises from the deep perineal nerve and not the sensory afferent branch. Anatomia, histologia, embryologia. 2008;37(3):166–168. doi: 10.1111/j.1439-0264.2007.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCouch GP, Snape WJ, Stewart WB. NOTE ON REFLEX THRESHOLDS IN THE CAT DURING SPINAL SHOCK. The American journal of physiology. 1935;111(2):263–271. [Google Scholar]
- Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neuroscience letters. 1998;244(3):137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- Sheth NV, Dell'Osso LF, Leigh RJ, Van Doren CL, Peckham HP. The effects of afferent stimulation on congenital nystagmus foveation periods. Vision research. 1995;35(16):2371–2382. doi: 10.1016/0042-6989(94)00321-c. [DOI] [PubMed] [Google Scholar]
- Tai C, Shen B, Wang J, Chancellor MB, Roppolo JR, de Groat WC. Inhibitory and excitatory perigenital-to-bladder spinal reflexes in the cat. American journal of physiology. 2008;294(3):F591–F602. doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Rivas DA, Chancellor MB. Urodynamics of spinal cord injury. The Urologic clinics of North America. 1996;23(3):459–473. doi: 10.1016/s0094-0143(05)70325-6. [DOI] [PubMed] [Google Scholar]