Abstract
Ten cases of cryptococcosis have been identified in a 13 year experience with more than 650 renal transplants. Eight patients had meningitis, one patient had a cerebral granuloma, and in one patient the infection appeared to be limited to the lungs. The central nervous system infection often masqueraded as brain tumor and was not suspected initially. The most useful diagnostic test was cerebrospinal fluid examination including India ink preparation. Various therapeutic regimens with amphotericin B and 5-fluorocytosine were effective in suppressing the infection. A combination of low doses of amphotericin B, not affecting kidney function, with 5-fluorocytosine for at least 3 months was associated with remission of disease in five patients who still are alive, including three patients without recurrence for longer than one year. Five deaths 3 weeks to 4 years after the beginning of treatment were not due to cryptococcosis; death resulted from vascular disease and septicemia in three of the four patients with known causes of death. Central nervous system cryptococcosis, with the exception of the rare cerebral granuloma, is associated with little inflammation. If early death from increased intracranial pressure or cerebral edema is prevented, prolonged therapy with amphotericin B and 5-fluorocytosine may be expected to control the infection, even in immunosuppressed patients.
At the University of Colorado Medical Center and the Denver Veteran’s Administration Hospital, more than 650 kidney transplants have been done from 1962 through 1974. Prednisone and azathioprine have been the main drugs used for immunosuppression, although initially prednisone was reserved for rejection crises. Antilymphocytic globulin was added in 1966, and cyclophosphamide has been substituted for azathioprine in some patients since 1971. Rejection episodes have been treated by temporary increases of the daily prednisone dose, usually to 200 mg. followed by 10 to 20 mg. daily reductions and/or by intravenous infusions of 1 Gm. of methylprednisolone. In addition, some rejection episodes have been treated by graft irradiation (150 r × 3) or by actinomycin D.37
CASE MATERIAL
During the past 13 years, including the first 2 months of 1975, ten cryptococcal infections have been identified and treated in eight men and one woman (B. N.), ages 27 to 51 years, and in one 12-year-old girl (C. S.) (Table I). Three of these patients (E. T., R. K., C. S.) had received more than one renal transplant before the diagnosis of cryptococcosis.
Table I.
| Case No. |
Patient | Age (yr.) |
Sex | Donor | Interval between transplantation and diagnosis of infection |
|---|---|---|---|---|---|
| 1 | J.H. | 27 | M | Sister | 10 mo. |
| 2 | B.N. | 29 | F | Sister | 3½ mo. |
| 3 | R. K. | 48 | M | Son Cadaver Cadaver |
2 yr., 6 mo. 1 yr., 8 mo. 1 yr., 3 mo. |
| 4 | M. La. | 42 | M | Unrelated living |
6 yr., 9 mo. |
| 5 | L. O. | 35 | M | Brother | 4 yr. |
| 6 | W. S. | 34 | M | Cousin | 9 yr., 7 mo. |
| 7 | E. T. | 43 | M | Brother Cadaver |
5 yr. 8 mo. |
| 8 | C. S. | 12 | F | Mother Cadaver |
4 yr. 7 mo. |
| 9 | E. W. | 49 | M | Cadaver | 5 yr., 5 mo. |
| 10 | M. Li. | 51 | M | Cadaver | 3 yr., 7 mo. |
In eight patients the infection appeared after long-term immunosuppression during the third to tenth year after transplantation. In the other two patients the diagnosis was made during the fourth (B. N.) and tenth months (J. H.) after transplantation.
Immunosuppression
At the time of diagnosis of infection, all patients were receiving maintenance prednisone and azathioprine. One patient (B. N.) still was receiving antilymphocytic globulin injections. In three patients (E. T., W. S., C. S.), a temporary increase of the daily prednisone dose preceded exacerbation of symptoms. In another patient (L. O.), the onset of symptoms followed 3 months of weekly infusions of methylprednisolone (1 Gm.) in addition to maintenance immunosuppression.
Organ involvement
The central nervous system was affected in nine patients; there was meningitis in eight and a brain granuloma in one patient (Table II). The lungs were simultaneously involved in three patients (E. T., L. O., J. H.); Cryptococci were found in the urine of two of the three patients (M. La., C. S.) whose urines were cultured for fungi. The infection appeared to be confined to the lungs in one patient (M. Li.). Fever was detected in only three patients (B. N., W. S., C. S.).
Table II.
Organ involvement, duration of symptoms, outcome of treatment, and major complications in ten renal transplant recipients with cryptococcosis
| Case No. |
Patient | Organ involvement | Duration of symptoms before diagnosis and treatment |
Outcome of treatment | Cause of death or major complications |
|---|---|---|---|---|---|
| 1 | J.H. | Meninges, lungs | 6 wk. | Died after 2 yr. of treatment. |
Unknown. Renal and congestive heart failure |
| 2 | B.N. | Meninges | 7 wk. | Died 1 yr., 7 mo. after treatment |
Postoperative pulmonary emboli. Staphylococcal septicemia. |
| 3 | R. K. | Meninges | 4 wk. | Died after 3 wk. of treatment. |
Pulmonary emboli, pneumonia and Klebsiella septicemia. |
| 4 | M. La. | Meninges | 3 wk. | Died after 5 mo. of treatment. |
Mesenteric vein thrombosis, pulmonary emboli, pneumonia, peritonitis, bacteroides and klebsiella septicemia |
| 5 | L.O. | Meninges, mediastinal lymph nodes |
5 mo. | Died 3 yr., 9 mo. after treatment. |
Myocardial infarction |
| 6 | W. S. | Meninges | 2 mo. | Alive >1 yr. after treatment. |
Renal failure due to chronic rejection. Pneumonitis, pneumocystis carinii? |
| 7 | E.T. | Meninges, lungs | Several mo. | Alive >1 yr. after treatment. |
Recurrent pulmonary embolus. |
| 8 | C.S. | Meninges | Several mo. | Alive 2 mo. after treatment. |
Renal failure due to chronic rejection. Nocardia asteroides abscess. |
| 9 | E.W. | Cerebral granuloma |
6 wk. | Alive, on treatment for 2 mo. |
Cardiac arrhythmia. |
| 10 | M. Li. | Lungs | Several mo. | Alive >1 yr. after treatment. |
Recurrent pulmonary embolus. |
Pulmonary cryptococcosis
The pulmonary infection was recognized as indistinct nodular infiltrates in three patients (E. T., J. H., M. Li.), two of whom had positive sputum cultures (E. T., M. Li.). The infiltrates were detected on chest x-rays taken for suspected pulmonary embolus in one patient (M. Li.) and in the other two patients on routine films taken 2 months before and one month after the detection of cryptococcal meningitis.
Central nervous system cryptococcosis
The most common symptom of central nervous system infection was headache, which occurred in eight of the nine patients. The headaches antedated additional symptoms by weeks or months in four patients (E. T., W. S., C. S., J. H.). Other manifestations included memory loss, disorientation, confusion, dyscalculia, dysphasia, muscle weakness, unsteadiness, tremor, urinary incontinence, behavioral and emotional disturbances. Focal neurological signs included hemiparesis, truncal and limb ataxia, nystagmus, intermittent diplopia, dysarthria, and right-left disorientation. Four patients (B. N., L. O., W. S., J. H.) had seizures. Bizarre behavior, hallucinations, and disorientation were so prominent in one patient (R. K.) that he was thought to have a steroid-induced psychosis before the correct diagnosis was made about 4 weeks after the onset of symptoms. Another patient (L. O.) was evaluated for two episodes of transient hemiparesis or paresthesias and aphasia and had abnormal cerebrospinal fluid 5 months before the diagnosis was made.
Papilledema was noted in four patients (B. N., E. T., W. S., J. H.). Acute neurological deterioration and appearance of papilledema were observed in two patients with chronic headaches (E. T., W. S.) after temporary increases of corticosteroid for rejection episodes.
Two other patients (J. H., B. N.) developed progressively increased intracranial pressure; in one patient (B. N.) partial loss of vision was due to thrombosis of branches of the retinal veins, in the other patient (J. H.) ventriculoperitoneal shunt was required and was complicated by cardiorespiratory arrest.
Laboratory studies
Skull x-rays showed an erosion of the sella turcica of one patient (E. T.), a 7 mm. shift of the pineal gland to the right in another patient (E. W.), and calcifications in the area of the choroid plexus in a third patient (B. N.).
Eight patients had brain scans done with technitium pertechnetate. In one patient (E. W.), a space-occupying lesion with increased isotope uptake and cold center was found in the left frontal lobe. Brain scans of other patients showed a diffuse increase of isotope uptake over the brain surface (B. N.) and a localized increase of isotope uptake over the left occipital lobe (R. K.).
Electroencephalograms of all nine patients with central nervous system cryptococcosis showed some slowing and disorganization of electrical activity with focal predominance in four and paroxysmal activity in two patients.
Cerebral angiograms were done on six patients. The angiogram confirmed the one focal lesion detected by scan (E. W.). Angiographic abnormalities in other patients were delayed filling of the left rholandic vein and adjacent sagittal sinus (W. S.) and irregularities in arterial outlines (M. L.).
Two patients were subjected to pneumoencephalography. In one patient studied via lumbar puncture, a mild dilatation of the fourth ventricle was seen (B. N.). The other patient was studied by ventricular puncture with the finding of mild dilatation of the lateral ventricles and obstruction of the basilar cystern (J.H.).
Lumbar punctures were performed in all but one patient (E. W.) (Table III). The cerebrospinal fluid pressure usually was increased, and most patients with cryptococcal meningitis had a mild lymphocytic pleocytosis, elevated protein, and decreased glucose concentration. A borderline protein concentration of 42 mg. per 100 ml. (R. K.) or a decreased glucose concentration of 30 mg. per 100 ml. (B. N.) were the only abnormalities found in two patients with cryptococcal meningitis. Cryptococcus neoformans was isolated from cultures of the cerebrospinal fluid of eight patients with meningitis. India ink preparations demonstrated encapsulated yeasts in all six patients with meningitis in whom this cerebrospinal fluid examination was done. The infection was identified by needle biopsy in the patient with a granuloma of the left frontal lobe (E. W.) (Fig. 1), who was not subjected to lumbar puncture. Cerebrospinal fluid protein also was elevated in the patient with cryptococcosis limited to the lungs (M. Li.).
Table III.
Initial cerebrospinal fluid findings in renal transplant recipients with cryptococcosis
| Case No. |
Patient | Opening/closing pressure (mm. H2O) |
Red blood cells (per mm.3) |
White blood cells (per mm.3) |
PMN (per mm.3) |
Lymph (per mm.3) |
Protein (mg./dl) |
Glucose (mg./dl) |
India ink preparation |
Culture |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | J.H. | 150/— | 185 158* |
247 34* |
106 16* |
146 18* |
32 15* |
66 105* |
+ ND† |
+ +* |
| 2 | B.N. | 700/480 | 0 | 4 | 0 | 4 | 38 | 30 | + | + |
| 3 | R. K. | 300/200 | 0 | 2 | 0 | 2 | 42 | 56 | + | + |
| 4 | M. La. | —/— | 0 | 72 | 23 | 49 | 146 | 44 | ND | + |
| 5 | L. O. | 280/— | 14 | 29 | 0 | 29 | 74 | 20 | ND | + |
| 6 | W. S. | 400/340 | 3 | 80 | 10 | 70 | 83 | 6 | + | + |
| 7 | E. T. | 240/170 | 0 | 140 | 7 | 133 | 60 | 29 | + | + |
| 8 | C. S. | 190/— | 0 | 14 | 3 | 11 | 16 | 61 | + | + |
| 9 | E. W. | Not done, positive needle biopsy of brain | ||||||||
| 10 | M. Li. | —/— | 0 | 0 | 0 | 0 | 86 | 83 | Negative | Negative |
Ventricular fluid.
ND = not done.
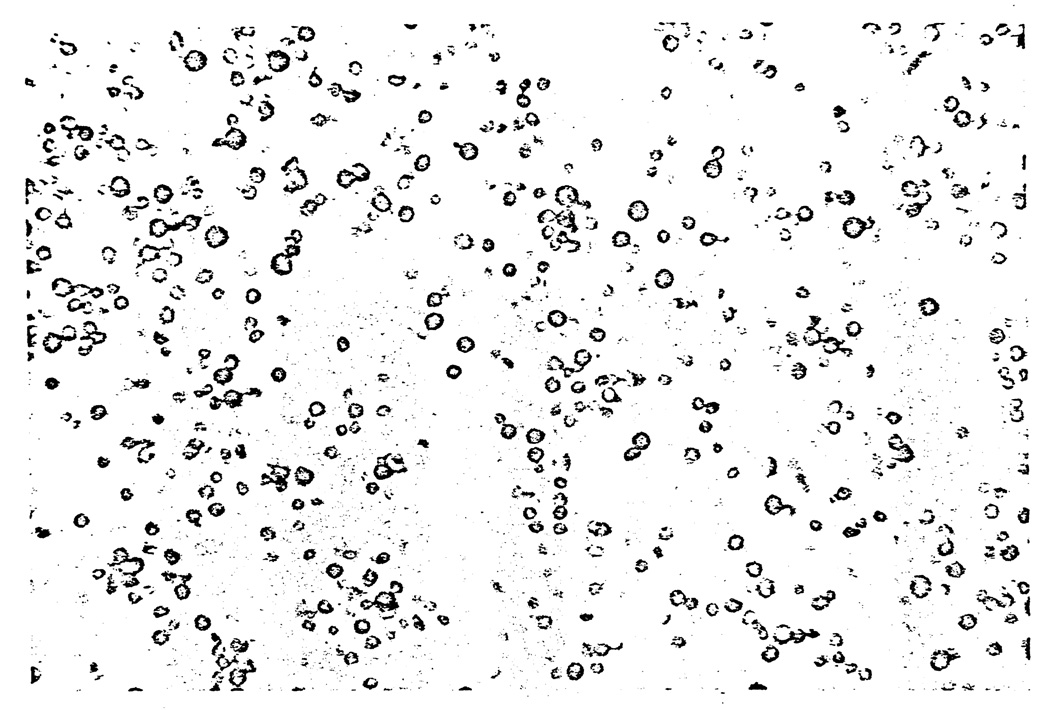
Fig. 1.
Needle biopsy of the cerebral granuloma of Patient 9 (E. W.) showing the yeasts stained by methenamine silver. (Original magnification, ×280.)
Results of therapy
The cryptococcal infections were treated with amphotericin B and 5-fluorocytosine alone or in simultaneous or sequential combination (Table IV). Immunosuppression was continued as considered necessary to avoid rejection of the graft. The duration of treatment was variable.
Table IV.
Treatment of cryptococcosis in ten transplant patients
| Amphotericin B | 5-fluorocytosine | ||||||
|---|---|---|---|---|---|---|---|
| Case No. |
Patient | Daily dose (mg.) |
Total dose (mg.) |
Duration of treatment |
Daily dose (Gm.) |
Duration of treatment |
Remarks |
| 1 | J.H. | 1 to 40 | 6,470 | 2 yr. | none | none | +5.25 mg. Amphotericin B intrathecally |
| 2 | B. N. | 1 to 40 | 1,000 | 7 wk. | 4 | 5 mo. | Amphotericin B replaced by 5-fluorocytosine, + 17.5 mg. Amphotericin B intrathecally |
| 3 | R. K. | 1 to 6 | 21 | 6 days | 12 | 3 wk. | Amphotericin B added 2 wk. after beginning of 5-fluorocytosine |
| 4 | M. La. | 1 to 15 | 195 | 4 wk. | 8 to 12 | 18 wk. | Amphotericin B added 2 mo. after beginning of 5-fluorocytosine |
| 5 | L. O. | 3 to 20 | 840 | 4 mo. | 0 | 0 | Amphotericin B alone |
| 6 | W. S. | 1 to 15 | 565 | 12 wk. | 4 to 8 | 12 wk. | Amphotericin B + 5- fluorocytosine simultaneously |
| 7 | E. T. | 1 to 20 | 810 | 12 wk. | 12 | 12 wk. | Amphotericin B + 5-fluorocytosine simultaneously |
| 8 | C. S. | 1 to 10 | 385 | 3 mo. | 1.5 | 1 mo. | Amphotericin B + 5-fluorocytosine simultaneously |
| 9 | E. W. | 1 to 20 | 428 | >2 mo. | 10-4-8 | >2 mo. | Amphotericin B + 5-fluorocytosine simultaneously |
| 10 | M. Li. | 1 to 20 | 450 | 12 wk. | 8–12 | 12 wk. | Amphotericin B + 5-fluorocytosine simultaneously |
The first two patients (J. H., B. N.) received standard doses of intravenous amphotericin B and additional amphotericin B intrathecally: J. H. received a total dose of 6.47 Gm. intravenously over a period of 2 years and 5.25 mg. intrathecally given during the first 2 months of treatment. The cerebrospinal fluid became normal during the fifth month of treatment. Cryptococci were found in India ink preparations and in cultures of cerebrospinal fluid only during the first week of treatment and on one isolated occasion one year and 7 months after the beginning of treatment, which was 3 months after temporary discontinuation of treatment. The major complication of the meningitis was increased intracranial pressure with signs of brain stem compression and diabetes insipidus. Twelve days after drainage of ventricular fluid through a Rickham reservoir, a ventriculoperitoneal shunt with a low pressure Holter valve was created, but it had to be removed 3½ months later because of Staphylococcus epidermidis infection. One month later a new ventriculoperitoneal shunt was implanted because of persistent increased intracranial pressure which had resulted in cardiorespiratory arrest. The new shunt appeared to function for the rest of the patient’s life. Severe low back pain and persistently high spinal fluid protein concentration in comparison to ventricular fluid suggested that the intrathecal administration of amphotericin B might have caused arachnoiditis. The high doses of intravenous amphotericin B were followed by renal failure 1½ years after the beginning of treatment, after 5 Gm. had been given. Hemodialysis became necessary one month later. The patient died 2 years after the detection of cryptococcosis and 2 years and 10 months after transplantation. An autopsy was not performed, but renal failure with congestive heart failure was the clinical cause of death. Additional problems before he died included unexplained fever, malnutrition, vomiting, diarrhea, and intermittent gastrointestinal hemorrhage.
B. N. received 1 Gm. of amphotericin B intravenously and 17.5 mg. intrathecally over a period of 7 weeks. The India ink preparations of cerebrospinal fluid consistently demonstrated Cryptococci for 2 months but the organism could be isolated in culture only during the first 3 weeks of treatment. Cerebrospinal fluid findings became normal 5 months after treatment. Initially her condition deteriorated, with signs of increased intracranial pressure, but she gradually improved after amphotericin B was discontinued and replaced by 5-fluorocytosine (4 Gm. per day), which she continued to take for 5 months. She remained asymptomatic until her death from staphylococcal septicemia shortly after total hip arthroplasty for steroid arthropathy of the right hip. At the time of death she also had chronic osteomyelitis, acute bronchopneumonia, acute purulent bacterial meningitis and cerebritis, and pulmonary emboli. She died 2 years and 4 months after transplantation. one year and 7 months after treatment for cryptococcal meningitis. Autopsy revealed residual cryptococcal organisms in the basal ganglia.
In eight other patients subsequently treated for cryptococcosis, amphotericin B was used intravenously in smaller doses, starting with one to 3 mg. on the first day followed by increases of one to 2 mg. per day until a maintenance dose of 10 to 20 mg. per day was reached. After evidence of improvement, usually at the time when the maintenance dose was reached, it was administered only every other day or less frequently. The total dose of amphotericin B was 385 to 840 mg. for five patients effectively treated with this regimen. No significant deterioration of renal function was seen with these low doses.
One patient (L. O.) was treated effectively with 840 mg. of amphotericin B alone during 4 months. He subsequently remained asymptomatic until death from recurrent myocardial infarction 7¾ years after transplantation or 3 years and 2 months after treatment. However, the protein concentration of his cerebrospinal fluid remained elevated and at autopsy residual cryptococcal meningitis and infected mediastinal lymph nodes were found.
Seven patients also received 5-fluorocytosine with the amphotericin B. The usual dose was 150 mg. per kilogram daily divided into four oral doses. Three patients (W. S., C. S., E. W.) also taking azathioprine, including two patients with impaired renal function (W. S., C. S.), developed reversible leukopenia necessitating reduction or discontinuation of the 5-fluorocytosine and the azathioprine. Two patients complained of loss of appetite and had diarrhea while taking full doses of 5-fluorocytosine.
The central nervous system infection of one patient (M. La.) cleared under treatment with 5-fluorocytosine alone, but 2 months after the beginning of treatment persistent urinary excretion of cryptococci, still sensitive to 5-fluorocytosine, subsided only after the addition of small doses (15 mg. per day) of amphotericin B. This patient died after 5 months of treatment, 7 years and 2 months after transplantation, from mesenteric vein thrombosis, bowel gangrene, septicemia, and pulmonary emboli. No residual cryptococcal infection was found at autopsy.
One patient (R. K.) died 3 weeks after the beginning of 5-fluorocytosine treatment and one week after addition of amphotericin B, one year and 4 months after his third transplant. His death was caused by massive pulmonary emboli and infection, but residual cryptococcal meningitis still was present at autopsy.
The five patients treated most recently—four with central nervous system infection (E. T., W. S., C. S., E. W.) and one with pulmonary cryptococcosis (M. Li.)—all were treated from the beginning with a combination of amphotericin B and 5-fluorocytosine in the doses outlined for a period of 12 weeks. In all patients the infection responded promptly to treatment. Three patients (E. T., W. S., M. Li.) have remained asymptomatic and without recurrence for over a year after treatment. One patient (C. S.) has just completed treatment. The outcome of continuous treatment, started 2 months ago in the patient (E. W.) with a large cerebral granuloma in the left frontal lobe, is undetermined at present.
Associated problems identified in these five surviving patients have included pulmonary emboli (M. Li., E. T.), renal failure due to chronic rejection (W. S., C. S.), multiple squamous cell carcinoma of the skin (W. S.), herpetic stomatitis (W. S.), possible pneumocystis carinii pneumonitis (treated with Pentamidine isethionate) (W. S.). abscess of the thigh due Nocardia asteroides (C. S.), unexplained liver dysfunction (C. S.), and cardiac arrhythmia with atrial fibrillation (E. W.). One patient (W. S.) also has residual ataxia following cryptococcal meningitis but also following gentamicin administration.
DISCUSSION
Cryptococci are yeastlike fungi widely distributed in nature. Cryptococcus neoformans is the only species known to cause disease in man. Cryptococci have been found in soil, especially in soil containing bird droppings: but they also have been identified in man as a saprophyte on the skin, in the respiratory tract, and in the gastrointestinal tract.27, 29 Cryptococcus neoformans has a capsule of polysaccharides which contributes to its pathogenicity by protecting the organism from being phagocytosed by leukocytes and macrophages.9, 10 This capsule may prevent an acute inflammatory response, usually absent with cryptococcal infections2 especially of the central nervous system,28 by covering other fungal antigens and preventing host sensitization to these more potent fungal antigens19.
The capacity to form this capsule is determined not only genetically but also by the environment.23 Capsule formation requires available free water and is an energy-consuming metabolic process. Under certain growth conditions, such as in soil with high mineral content or in culture medium with high osmolarity, the fungus is unable to produce a capsule.18 Because of the smaller size of nonencapsulated variants, these can be expected to have a greater chance of passing through the human upper respiratory tract and reaching the lungs after inhalation.8 Inhalation of the smaller nonencapsulated forms actually may be the most common mode of infection. In the lungs the organisms find an environment favorable for capsule formation; however, the high natural immunity of man against the nonencapsulated form, which stimulates an inflammatory response19 and is readily phagocytosed,9, 10 may account for the infrequent occurrence of cryptococcal disease despite the many opportunities for exposure. The incidental autopsy finding of primary pulmonary cryptococcal complexes, with subpleural pulmonary granuloma and hilar lymphnodes containing Cryptococcus neoformans, is consistent with the hypothesis that inhalation is a common route of infection.32 Inhalation of organisms can result in colonization or disease of the respiratory tract.23, 40, 43 Following dissemination, the infection has a tendency to localize in the central nervous system,11,14, 17 although central nervous system involvement may not become apparent until pulmonary involvement no longer is detectable. Pulmonary infection was evident in only two of our nine patients with central nervous system infection (E. T., J. H.); in one other patient (L. O.) infected mediastinal lymph-nodes were found at autopsy years later.
Compromise of host defense mechanisms due to immunosuppressive therapy or malignancy can be found in a significant number of patients with cryptococcal infections.13, 21,43 However, only a few cases of this complication after renal transplantation have been reported.35, 38, 39
In our experience cryptococcal infections usually occur late after transplantation and therefore this infection might not have been included in an earlier publication from this center concerning fungal infections after transplantation.31 The late appearance of this disease suggests acquisition of this infection under immunosuppression rather than exacerbation of a pre-existing, inapparent infection, which might be expected to reveal itself earlier after initial intensive immunosuppression. However, the time of acquisition remains uncertain because of the insidious nature of this infection. In three of our patients, an exacerbation of symptoms was observed after a temporary increase of corticosteroid therapy. In rats and guinea pigs, reactivation of experimental cryptococcal infection under the influence of corticosteroids has been observed,20 and the possibility of autogenous reinfection from an inapparent focus cannot be ruled out.
The clinical manifestations of cryptococcal infection of the central nervous system were mainly signs of disturbed brain function and increased intracranial pressure, frequently creating a suspicion of brain tumor and directing initial studies to search for a space-occupying lesions; however, such a lesion was detected in only one patient (E. W.). Fever and meningeal signs frequently were absent. Spinal fluid analyses indicated only mild inflammation.
In the majority of patients the central nervous system infection remains limited to the meninges. Cerebral granulomata were found in only one of our patients and are rare; only 26 cases have been described in the English language medical literature, compared to several hundred cases of cryptococcal meningitis.7, 12, 42 The majority of patients with cryptococcal granulomata of the brain or spinal cord have an associated meningitis. The development of granulomata can be considered a late complication of a meningeal infection with extension into the gray matter from an adjacent meningeal focus.
In mice it has been shown that the reaction of the host to the presence of encapsulated Cryptococcus neoformans is organ dependent.26 The encapsulated yeast or capsular polysaccharides evoke almost no inflammation and the organisms tend to persist if directly inoculated into the brain of the animal. On the other hand intracutaneous or intraperitoneal inoculation is followed by inflammation and elimination of the yeast by phagocytosis.
Lack of an exudative inflammation also is characteristic of human cryptococcosis of the central nervous system.2, 28 The inflammation found at autopsy after death from cryptococcal meningitis frequently is not extensive enough to explain death. Increased intracranial pressure due to cerebral edema, impaired cerebrospinal fluid circulation, and metabolic effects appears to play an important role in the morbidity and mortality rates of patients with cryptococcal meningitis. The effect of increased intracranial pressure is well illustrated by our patient who required ventriculoperitoneal shunting, as well as by reported cases benefiting from intermittent or continuous removal of cerebrospinal fluid.3, 24 Immunosuppressive therapy, including corticosteroids, can be expected to delay elimination of the fungus from the respiratory tract following inhalation, to activate a dormant focus, and to increase the risk of dissemination. The contribution of corticosteroids to the modification of an established central nervous system infection is less certain. Corticosteroids at least might be expected to alleviate temporarily the increased intracranial pressure and the papilledema which rapidly appeared in two of our patients after withdrawal of high corticosteroid doses.
Therapy with amphotericin B has improved the prognosis of cryptococcal meningitis.11, 14, 17, 33 Our approach to therapy with amphotericin B doses was based on the recommendation of Drutz and co-workers.16 Low doses permit prolonged therapy without irreversible renal damage, which is related to the total amount of amphotericin B received.44 The nature of cryptococcal infection and the tendency to relapse after treatment makes prolonged treatment advisable.
Amphotericin B attaches to the sterols of cell membranes and its activity against fungi is due to interruption of the integrity of cell membranes.15 Amphotericin B has unusual pharmacokinetics4 in that it disappears rapidly from the circulation after intravenous infusion because of either storage at unknown sites or rapid inactivation; only a small amount of the administered dose is detectable in the urine. The persistence of low serum levels and continuous excretion in the urine for weeks after therapy suggests early storage followed by slow return to the circulation, rather than rapid inactivation. Saturation of cellular binding sites, including those of fungi, may be responsible for the therapeutic effect of low doses of amphotericin B given over a prolonged period of time, following which therapeutic serum and cerebrospinal fluid concentrations cannot be expected. An immediate response to therapy cannot be expected with this low dose approach. Early in therapy, prevention of increased intracranial pressure by corticosteroid administration or by removal of spinal fluid may be important, as illustrated by a patient treated with a combination of these measures by Ikemoto and associates.24
Five-fluorocytosine is a newer antifungal agent used for the treatment of cryptococcal infections.22, 41 It interferes with nucleic acid synthesis in the yeast cell nucleus. It has many advantages over amphotericin B, including good absorption from the gastrointestinal tract after oral administration, good tissue penetration, and much less toxicity. It is not metabolized in the human body and 90 percent is excreted by the kidney, necessitating dose adjustment in the presence of renal failure.34 In spite of these pharmacological advantages, 5-fluorocytosine has not replaced amphotericin B for the treatment of cryptococcal meningitis. Only 50 percent of patients primarily treated have had a good therapeutic response and the relapse rate was high.1 These disappointing results may be explained by the tendency of Cryptococcus strains to become resistant to 5-fluorocytosine6 during treatment and by the need for prolonged treatment. We treated only one patient primarily with 5-fluorocytosine alone, and there was remission of his meningitis, although persistent funguria subsided only after the addition of small doses of amphotericin B. In our other patients 5-fluorocytosine was used in combination with amphotericin B. A synergistic effect has been demonstrated for these two agents,30 possibly based on the increased penetration of 5-fluorocytosine into yeast cells with membranes damaged by exposure to amphotericin B.36 Amphotericin B also has shown to delay development of 5-fluorocytosine resistance in experimental cryptococcal infection in mice.5 Better results with 5-fluorocytosine have been obtained in patients failing to respond to initial amphotericin B therapy.1 The available information about the pharmacokinetics of amphotericin B suggests that residual amphotericin B may enhance the 5-fluorocytosine effect.
The final outcome of the treated cryptococcal meningitis is known in five of the ten transplant patients who died after treatment (Table II). One patient died early in the course of treatment for cryptococcal meningitis and the other four patients died 5 months to four years after detection of the infection. No death could be attributed to the cryptococcal infection. Four patients died of vascular or septic complications or both. The cause of death of one patient is uncertain. Although therapy appeared clinically to have been effective in all five patients who died, residual organisms were found years after treatment in two patients. Three of the five living patients have been observed for over one year after treatment All five of these patients have no clinical signs of cryptococcosis.
Acknowledgments
Supported by research grants from the Veterans Administration; by grants Nos. AI-AM-08898 and AM-07772 from the National Institutes of Health: by grants Nos. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
Footnotes
Presented at the First Annual Meeting of the American Society of Transplant Surgeons, Chicago, III., May 23, 1975.
ADDENDUM
Since this paper was completed, an additional patient (C. S.) died from multiple organ failure, unrelated to the previous cryptococcal meningitis. At autopsy, 7 weeks after completion of therapy, slight fibrosis of the leptomeninges, with scattered macrophages, was observed, but no cryptococci were identified in the brain.
REFERENCES
- 1.Abernathy RS. Treatment of systemic mycosis. Medicine. 1973;52:385. doi: 10.1097/00005792-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Baker RD, Hugen RK. Tissue changes and tissue diagnosis in cryptococcosis. Am. J. Clin. Pathol. 1955;25:14. doi: 10.1093/ajcp/25.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Beeson PB. Cryptococcal meningitis of nearly sixteen years duration. Arch. Intern. Med. 1952;89:797. doi: 10.1001/archinte.1952.00240050111008. [DOI] [PubMed] [Google Scholar]
- 4.Bindschadler DD, Bennett JE. A pharmacologic guide to the clinical use of amphotericin. Br. J. Inf. Dis. 1969;120:427. doi: 10.1093/infdis/120.4.427. [DOI] [PubMed] [Google Scholar]
- 5.Block ER, Bennett JE. The combined effect of 5-fluorocytosine and amphotericin B in the therapy of murine Cryptococcus. Proc. Soc. Exp. Biol. Med. 1973;142:476. doi: 10.3181/00379727-142-37049. [DOI] [PubMed] [Google Scholar]
- 6.Block ER, Jennings AE, Bennett JE. 5-fluorocytosine resistance in Cryptococcus neoformans. Antimicrob. Agents Chemother. 1973;3:649. doi: 10.1128/aac.3.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisman R, Reid R, Harrington C. Intracranial cryptococcal granuloma. Amphotericin B and surgical excision. Surg. Neurol. 1973;1:43. [PubMed] [Google Scholar]
- 8.Brown JH, Cook KM, Ney FG, et al. Influence of particle size upon the retention of particulate matter in the human lung. Bacteriol. Rev. 1950;28:296. doi: 10.2105/ajph.40.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulmer GS, Sans MD. Cryptococcus neoformans. III. Inhibition of phagocytosis. J. Bacteriol. 1968;95:5. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulmer GS, Tacker JR. Phagocytosis of Cryptococcus neoformans by alveolar macrophages. Infect. Immun. 1975;17:73. doi: 10.1128/iai.11.1.73-79.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler WT, Ailing DW, Spickard A, et al. Diagnostic and prognostic values of clinical and laboratory findings in cryptococcal meningitis. N. Engl. J. Med. 1964;270:59. doi: 10.1056/NEJM196401092700201. [DOI] [PubMed] [Google Scholar]
- 12.Ching AD, Wolf SM, Ruskin J. Cryptococcosis: A cause of calcified intracranial mass lesions. Calif. Med. 1973;119:59. [PMC free article] [PubMed] [Google Scholar]
- 13.Collins VP, Gellhorn A, Trimble JR. Coincidence of cryptococcosis and disease of reticuloendothilial and lymphatic systems. Cancer. 1951;4:883. doi: 10.1002/1097-0142(195107)4:4<883::aid-cncr2820040426>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. Ann. Intern. Med. 1974;80:176. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 15.Drouhet E. Basic mechanisms of antifungal chemotherapy. Mod. Treat. 1970;7:539. [PubMed] [Google Scholar]
- 16.Drutz DJ, Spickard A, Rogers DE, et al. Treatment of disseminated mycotic infection. Am. J. Med. 1968;45:405. doi: 10.1016/0002-9343(68)90075-2. [DOI] [PubMed] [Google Scholar]
- 17.Edwards VE. Cryptococcosis of the central nervous system. Epidemiological, clinical and therapeutic features. J. Neurol. Neurosurg. Psychiatry. 1970;33:415. doi: 10.1136/jnnp.33.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farki F, Bulmer GS, Tacker JR. Cryptococcus neoformans. IV. The not so encapsulated yeast. Infect. Immun. 1970;1:526. doi: 10.1128/iai.1.6.526-531.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer SG, Komorowski RA. Histologic response to capsule-deficient Cryptococcus neoformans. Arch. Pathol. 1973;96:383. [PubMed] [Google Scholar]
- 20.Gadebusch HH, Gikas PW. The effect of cortisone upon experimental pulmonary cryptococcosis. Am. Rev. Respir. Dis. 1965;92:64. [Google Scholar]
- 21.Goldstein E, Rambo ON. Cryptococcal infection following steroid therapy. Ann. Intern. Med. 1962;56:114. doi: 10.7326/0003-4819-56-1-114. [DOI] [PubMed] [Google Scholar]
- 22.Grunberg E, Fitsworth E, Bennett M. Chemo-therapeutic activity of 5-fluorocytosine. Antimicrob. Agents Chemother. 1963:556. [PubMed] [Google Scholar]
- 23.Hammerman KJ, Powell KE, Christianson CS, et al. Pulmonary cryptococcosis: Clinical forms and treatment. Am. Rev. Resp. Dis. 1973;108:1116. doi: 10.1164/arrd.1973.108.5.1116. [DOI] [PubMed] [Google Scholar]
- 24.Ikemoto H, Yashiro M, Furugori T, et al. Unusual treatment of cryptococcal meningitis. J. Antibiot. (Series A) 1967;20:374. [PubMed] [Google Scholar]
- 25.Ishaq CH, Bulmer GS, Felton FG. An evaluation of various environmental factors affecting the propagation of Cryptococcus neoformans. Mycopathol. Mycol. Appl. 1968;35:81. doi: 10.1007/BF02049570. [DOI] [PubMed] [Google Scholar]
- 26.Levine S, Hirano A, Zimmerman HM. The reaction of the central nervous system to cryptococcal infection: An experimental study with light and electron microscopy. Res. Publ. Assoc. Nerv. Ment. Dis. 1968;44:393. [PubMed] [Google Scholar]
- 27.Littman ML, Zimmerman LE. Cryptococcosis (torulosis) New York: Grune & Stratton, Inc.; 1956. pp. 6–7. [Google Scholar]
- 28.Littman ML, Zimmerman LE. Cryptococcosis (torulosis) New York: Grune & Stratton, Inc.; 1956. pp. 71–83. [Google Scholar]
- 29.Littman ML, Walter JE. Cryptococcosis, current status. Am. J. Med. 1968;45:922. doi: 10.1016/0002-9343(68)90190-3. [DOI] [PubMed] [Google Scholar]
- 30.Medoff G, Comfort M, Kobayashi GS. Synergistic action of amphotericin B and 5-fluorocytosine against yeast-like organisms. Proc. Soc. Exp. Med. Biol. 1971;138:571. doi: 10.3181/00379727-138-35943. [DOI] [PubMed] [Google Scholar]
- 31.Rifkind D, Marchioro TL, Schneck SA, et al. Systemic fungal infections complicating renal transplantation and immunosuppresive therapy. Am. J. Med. 1967;43:28. doi: 10.1016/0002-9343(67)90146-5. [DOI] [PubMed] [Google Scholar]
- 32.Salyer WR, Salyer DC, Baker RD. Primary complex of cryptococcus and pulmonary lymphnodes. J. Infec. Dis. 1974;130:74. doi: 10.1093/infdis/130.1.74. [DOI] [PubMed] [Google Scholar]
- 33.Sarosi GA, Parker JD, Doto IL, et al. Amphotericin B in cryptococcal meningitis. Ann. Intern. Med. 1969;71:1079. doi: 10.7326/0003-4819-71-6-1079. [DOI] [PubMed] [Google Scholar]
- 34.Schönbeck J. Studies on 5-fluorocytosine and its relationship to Candida albicans. Scand. J. Urol. Nephrol. 1972;11 Suppl.:35. [Google Scholar]
- 35.Slapak M, Lee HM, Hume DM. Transplant lung and lung complications in renal transplantation. In: Daussett J, Hamburger J, Mathe J, editors. Advances in transplantation. Baltimore: The Williams and Wilkins Company; 1968. p. 769. [Google Scholar]
- 36.Smith JW. Synergism of amphotericin B with other antimicrobial agents. Ann. Intern. Med. 1973;78:450. doi: 10.7326/0003-4819-78-3-450. [DOI] [PubMed] [Google Scholar]
- 37.Starzl TE, Porter KA, Husberg BS, et al. Curr. Prob. Surg. Chicago: Year Book Medical Publishers, Inc.; 1974. Apr, Renal homotransplantations. Part I. pp. 6–35. [Google Scholar]
- 38.Steer PL, Marks ML, Klike PD, et al. 5-fluorocyostine: An oral antifungal compound. A report on clinical and laboratory experience. Ann. Intern. Med. 1972;76:15. doi: 10.7326/0003-4819-76-1-15. [DOI] [PubMed] [Google Scholar]
- 39.Swenson RS, Kountz SL, Blank N, et al. Successful renal allograft in a patient with pulmonary Cryptococcus. Arch. Intern. Med. 1969;124:502. [PubMed] [Google Scholar]
- 40.Tynes B, Mason KN, Jennings AE, et al. Variant forms of pulmonary cryptococcosis. Ann. Intern. Med. 1968;69:1117. doi: 10.7326/0003-4819-69-6-1117. [DOI] [PubMed] [Google Scholar]
- 41.Van de Velde AG, Mauceri AA, Johnson JE. 5-fluorocytosine in the treatment of mycotic infections. Ann. Intern. Med. 1974;77:43. doi: 10.7326/0003-4819-77-1-43. [DOI] [PubMed] [Google Scholar]
- 42.Vijayan N, Bhatt GP, Dreyfus PM. Intraventricular gryptococcal granuloma: A case report with review of the literature. Neurology. 1971;21:728. doi: 10.1212/wnl.21.7.728. [DOI] [PubMed] [Google Scholar]
- 43.Warr W, Bates JH, Stone A. The spectrum of pulmonary cryptococcosis. Ann. Intern. Med. 1968;69:1109. doi: 10.7326/0003-4819-69-6-1109. [DOI] [PubMed] [Google Scholar]
- 44.Winn WA. Coccidioidomycosis and amphotericin. B. Med. Clin. North Am. 1963;47:1131. doi: 10.1016/s0025-7125(16)33522-2. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman LE, Rappaport H. Occurrence of cryptococcosis in patients with malignant disease of reticuloendothelial system. Am. J. Clin. Pathol. 1954;24:1050. doi: 10.1093/ajcp/24.9.1050. [DOI] [PubMed] [Google Scholar]