Abstract
Our objectives were to develop and test a new system for the potential for stable, real-time cancellation of residual stimulation artefacts (RSA) using surface electrode autogenic electromyography-controlled functional electrical stimulator (aEMGcFES). This type of closed-loop FES could be used to provide more natural, continuous control of lower extremity paretic muscles. We built upon work that has been done in the field of FES with one major technological innovation, an adaptive Gram-Schmidt filtering algorithm, which allowed us to digitally cancel RSA in real-time. This filtering algorithm resulted in a stable real-time estimation of the volitional intent of the stimulated muscle, which then acted as the direct signal for continuously controlling homonymous muscle stimulation. As a first step toward clinical application, we tested the viability of our aEMGcFES system to continuously control ankle dorsiflexion in a healthy subject. Our results indicate positively that an aEMGcFES device with adaptive filtering can respond proportionally to voluntary EMG and activate forceful movements to assist dorsiflexion during controlled isometric activation at the ankle. We also verified that normal ankle joint range of movement could be maintained while using the aEMGcFES system. We suggest that real-time cancellation of both primary and RSA is possible with surface electrode aEMGcFES in healthy subjects and shows promising potential for future clinical application to gait pathologies such as drop foot related to hemiparetic stroke.
Keywords: Gram-Schmidt adaptive filter, drop foot, stimulation artefact, M-wave, rehabilitation, hemiparetic gait, locomotion
Introduction
Stroke is a leading cause of serious, long-term disability in many countries. The inability to appropriately control ankle dorsiflexion during gait, or drop foot, remains a long-term disability in up to 20% of stroke survivors (Lyons et al., 2002). By far the most common clinical treatment for drop foot today is a passive ankle-foot orthosis (AFO) due to its simplicity, low cost and reliability. Yet, the passive AFO has numerous disadvantages the most significant being that it has no therapeutic value and can actually promote muscle atrophy from disuse. Other disadvantages can include a lack of ventilation in hot weather and poor fit after significant weight gain or loss. In contrast electrical stimulation has been shown to strengthen muscles (Kralj and Bajd 1989; Belanger et al. 2000) and corticospinal connections (Khaslavskaia et al., 2002; Knash et al., 2003) indicating the potential therapeutic value of functional electrical stimulation (FES) for treating drop foot (Burridge, 2001; Kottink et al., 2007; Kottink et al., 2004). For FES systems to benefit gait rehabilitation, however, the timing and intensity of stimulation must be controlled accurately to allow flexibility for adapting to different walking speeds, stairs, and obstacles during gait and to a variety of movements unrelated to locomotion. This requires sensing technologies that can reliably distinguish the volitional intent of movement.
The most common methods for sensing when to begin FES of the ankle dorsiflexor muscles rely on mechanical on/off switches that sense a mechanical state in the gait cycle (e.g., heel strike). These methods trigger a predetermined stimulation sequence using sensors within hand switches (Vodovnik L, 1965), foot switches (Brandell, 1982; Burridge et al., 1997; Liberson, 1961; Waters et al., 1975), goniometers (Prochazka and Wiles, 1983), or accelerometers (Willemsen et al., 1990). The predetermined stimulation sequences may consist of simple square waveforms or as in the Odstock Dropped Foot Stimulator (Burridge et al., 1997) may employ a ramp up and ramp down trapezoidal waveform, to more closely mimic physiologically relevant activation (Liberson, 1961; Waters et al., 1985). These produce an all-or-none triggering of FES and are not adaptable for making on-line adjustments. Recently, O’Keefe and Lyons (O’Keeffe and Lyons, 2002) used a scaled linear envelope of voluntary EMG (vEMG) recorded in able-bodied gait of healthy individuals as the waveform to control FES amplitude, which underscores the general desire to progress toward more physiological control of FES. All of these methods, however, rely on discrete periods of feedback with preset FES waveforms based on assumptions about the volitional intent of the individual. Although these methods provide real-time continuous control, they do not utilize a natural, physiological feedback signal to continually respond to and enhance volitional control of the affected muscle.
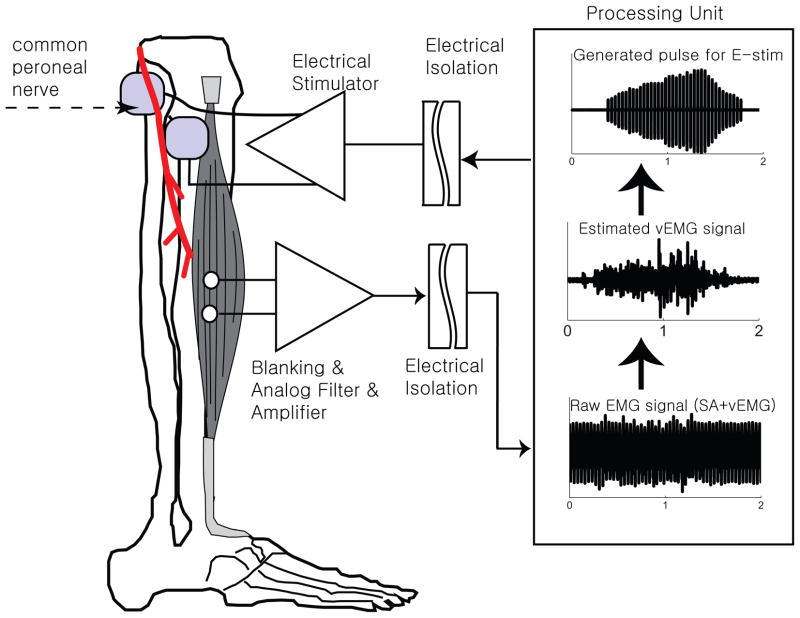
The most physiologically appropriate neural pathway to reinforce when retraining the tibialis anterior (TA) muscle would be to use homonymous vEMG signals to provide direct and immediate autogenic feedback for dorsiflexion control, which we refer to as Autogenic EMG-controlled FES, or aEMGcFES (Fig 1). We use the term autogenic to indicate that the control signal is coming from the homonymous muscle EMG—to discriminate from current systems like the Freehand (Smith et al. 1987) or others (e.g., Keller et al., 1998) that use muscles far away from the stimulation site.
Figure 1.
Schematic of aEMGcFES device for ankle dorsiflexion control. We placed recording surface EMG electrodes at the belly of the tibialis anterior muscle. An analog blanking filter and amplifier removed the primary stimulus artefact from the measured raw signal and amplified the EMG signal. A digital processing unit then removed the remaining residual stimulation artefacts (RSA) from the EMG signal and estimated the volitional EMG signal. A biphasic stimulation signal scaled to the integrated volitional EMG envelope was then sent to a pair of stimulating surface electrodes positioned over the common fibular (peroneal) nerve and verified through submaximal stimulation to activate the tibialis anterior muscle. Note images are not to scale and electrode positions are estimated.
A few research groups (Muraoka, 2002; Futami 2005) have attempted to use the amplified vEMG signal from the TA muscle to provide continuous FES control back to the TA muscle. Such methods may become unstable, however, because they rely on fixed comb filters (Frigo et al., 2000), which assume the M-wave generated by the stimulation to be stationary (Peasgood et al., 2000). The M-wave, however, is a non-stationary signal due to its temporal variation, which depends on many factors such as stimulation intensity, fatigue, and muscle activation level (Merletti et al., 1992).
The goal of our study was to introduce and validate the operation of an adaptive filtering technique for providing appropriate real-time elimination of the non-stationary M-wave. This could provide a stable solution to the residual stimulus artefact (RSA) problem when using homonymous vEMG signals to continuously control FES. The RSA during the previous stimulation period is strongly correlated with that of the current stimulation period because they are quasi-deterministic and periodic. In contrast, the volitional muscle activity data (vEMG) can be characterized as a band-limited Gaussian signal, which is a reasonable approximation when the number of active motor units exceeds 15 (Merletti et al., 1992). Thus, proper identification of these RSA within the recorded signal would allow for its isolation and removal from the volitional muscle EMG.
We previously showed that an adaptive Gram-Schmidt Prediction-Error-Filter (GS-PEF) was an effective noise canceller off-line (Yeom et al., 2007). This filtering algorithm was shown to have the potential for real-time artefact cancellation while using far less hardware or equivalently, requiring fewer numerical operations than other adaptive algorithms that would prohibit real-time application (Fig. 2). In this work, we provide the theoretical framework, methodology and results from a preliminary laboratory implementation of our real-time aEMGcFES device using a GS-PEF. Our goal was to positively demonstrate functional assistance of force enhancements during a closed chain, isometric activation and motion assistance during an open chain concentric activation.
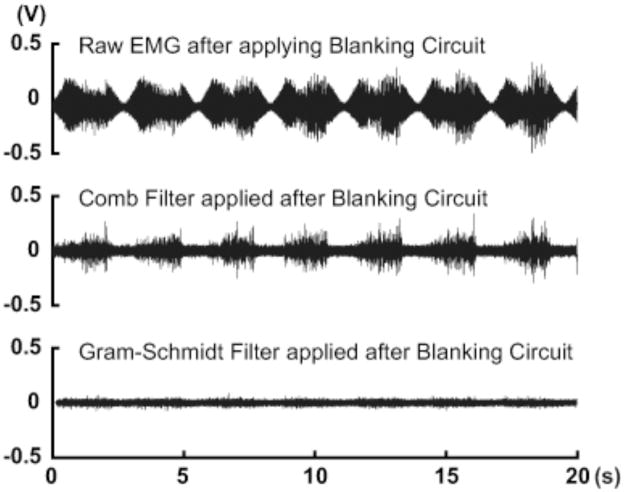
Figure 2.
Sample electromyography (EMG) data comparing offline-filtering techniques recorded from quiescent muscle with an applied (open-loop) sinusoidal envelope (0.3 Hz) of electrical stimulation pulses. Raw EMG data with a blanking circuit (top) shows the residual stimulation artefacts (RSA) from the stimulation signal. The addition of a comb filter (middle, after Frigo et al. 2000) was not able to satisfactorily filter out the RSA and resulted in false positive vEMG signals. In contrast, the Gram-Schmidt Prediction Error Filter (bottom) was able to identify and remove nearly all of the RSA signal leaving no detectable vEMG activity, which is expected of quiescent muscle.
Materials and Methods
Raw EMG signal blanking stage to filter primary stimulation artefact
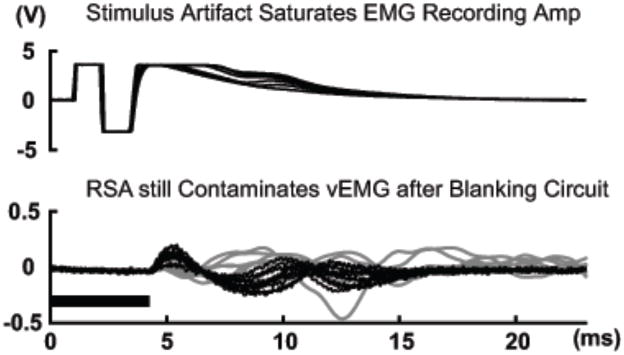
The primary, main stimulation artefact must be removed prior to amplifying the EMG signal, because the high magnitude of surface stimulation completely engulfs the amplified vEMG signal and saturates the EMG recording amplifier (Fig 3). Thus, our first stage of filtering removed this primary stimulation artefact using a hardware blanking circuit (i.e., sample and hold strategy) to briefly turn off EMG recording. This can significantly reduce the energy recorded in the EMG signal due to the primary stimulation artefact. The electrical stimulation pulse was synchronized to the blanking control signal (10 ms blanking window). Additional digital blanking was performed during these blanking intervals to minimize the effects of any transients when the analog switch was turned on and off (Fig 4). Stimulation frequencies lower than about 20 Hz will cause a “tremor”, or fasciculation contraction of the muscle, as it leads to an incomplete tetanic contraction. Therefore, greater stimulation frequencies would be physiologically advantageous to avoid this condition. In contrast to this, however, the elimination of the stimulation artefact with a blanking circuit ideally requires stimulation frequencies as low as possible to allow adequate time to avoid recording the artefact. For example, Muraoka (Muraoka, 2002) and Sennels (Sennels S, 1997) used 20 Hz. Additionally, since stimulation frequencies greater than 35 Hz are more likely to produce muscle fatigue due to the increased rate of motor unit recruitment. Benton (Benton, 1981) suggested that smooth muscle contractions could be achieved by stimulating between 20 to 35 pulses per second (30–40 ms cycle period), which is what we used in our study.
Figure 3.
Raw EMG (dark lines) from electrically stimulated, but quiescent tibialis anterior muscle without (top) and with (bottom) a period of hardware and software blanking indicated by the dark horizontal band. 5 consecutive cycles are shown overlapped for comparison. The amplitude of volitional EMG signals (gray lines, bottom) from several cycles recorded earlier with no stimulation was on par with the amplitude of the remaining residual stimulation artefacts (RSA).
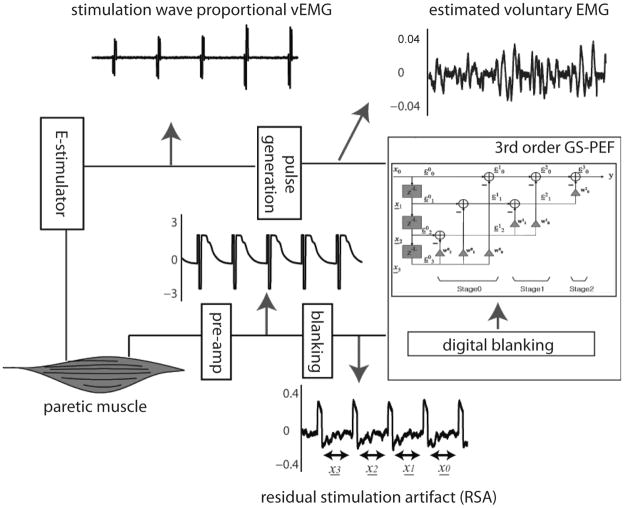
Figure 4.
Block diagram describing the steps for real-time EMG data acquisition, signal processing, and proportional stimulation of same muscle.
Amplification and analog filtering stage
We used a low noise instrumentation amplifier with a high common mode rejection ratio (120 dB) for pre-amplification of the EMG signal. After this stage, but before the main amplifier stage, a high pass filter with a cut off frequency of 30 Hz was implemented to remove motion artefact from the recorded signal and a low-pass filter with a cut-off of 1000 Hz implemented anti-aliasing. We used a commercially available operational amplifier (TL064, Texas Instruments) as the main amplifier.
Adaptive filter for cancelling residual stimulation artefacts
An off-line aEMGcFES technique was previously developed for RSA cancellation based on a GS-PEF (Yeom et al., 2005). The GS-PEF performs the Gram-Schmidt orthogonalization as follows:
where M is the order of the filter, superscript m is the stage index and subscript i is the element index of the corresponding stage. The resulting GS-PEF output is given by . The GS-PEF is able to self-adjust its parameters (i.e., transfer function) to better match and cancel an unpredictable noise signal compared to non-adaptive filters like the comb filter. The GS-PEF mathematically predicts non-stationary waveforms like RSA (consisting primarily of the M-wave) in real-time through digital signal processing. Despite how common adaptive filters are in other technological fields (e.g., mobile phone and digital camera industries), there has been no application of adaptive filters in the clinical field of FES to treat drop foot.
GS-PEF implementation and stimulation waveform generation
We coded a GS-PEF onto a Texas Instruments TMS320C6713 Digital Signal Processor Starter Kit (DSK) using algorithms previously tested off-line (Yeom et al. 2005) to provide real-time cancellation of RSA at the digital signal processing (DSP) stage. We used the audio codec (AIC23) built into the DSK for A/D conversion and we set the sampling rate at 8000 Hz, from which the DSK automatically sets the anti-aliasing filter at 4000 Hz. The DSK also generated a real-time biphasic stimulation waveform in proportion to the measured vEMG envelope. The vEMG envelope was obtained with a 1 pole Infinite Impulse Response (IIR) filter, which rectified the vEMG signal according to the following equation:
where, y is the GS-PEF filter output (vEMG envelope), x is the GS-PEF filter input (raw EMG),
and, θ = 2fsfc where sampling frequency fs is 8000Hz, and cut-off frequency fc is 3Hz (as recommended by Winter 1990). This DSK output signal was sent to the stimulation circuit (maximally, ±90V, 30mA) consisting of a custom-built isolated amplifier that stimulated the homonymous muscle via the common fibular (peroneal) nerve through a pair of stimulating surface electrodes with a gain of 80 times the maximum voluntary contraction (MVC) recorded signal, which was set to 1V.
Electrode placement
A healthy male subject (36 yrs. old, 83.9 kg) gave informed consent to participate in this study approved by the Georgia Institute of Technology IRB (protocol #H10018). This subject had no history of neuromuscular or skeletal injury to the tested leg. The area on the anterolateral aspect of the lower leg along the upper two-thirds of the tibialis anterior muscle was first shaven with a disposable razor and then vigorously cleaned with an alcohol swab to reduce input impedance at the EMG recording and electrical stimulation sites. Once the alcohol had dried, pre-gelled disposable bipolar pellet surface electrodes (Ag-AgCl, 10 mm diameter, Noraxon, OH) were placed with an interelectrode distance of 2 cm along the midline of the belly of the TA muscle parallel to the direction of the muscle fibres approximated by the anterior crest of the tibia. We verified accurate placement of the recording electrodes with a qualitative check of the recorded EMG signal during voluntary ankle dorsiflexion movements (with no electrical stimulation). We placed self-adhesive stimulating surface electrodes (5×5 cm square, Medical Supplies Shop, NY) with an interelectrode distance of 10cm at the level of the fibular head in the region of the common fibular (peroneal) nerve. We applied a series of open-loop submaximal sinusoidal stimulations (~60 V, 1 ms pulse width, 25 Hz) and manually adjusted this position until we could visually verify activation of the tibialis anterior muscle before beginning our closed-loop trials.
Data measurement and analysis
We used a second parallel data acquisition system for testing the inputs and outputs of our aEMGcFES system. A commercial data acquisition card (National Instruments, Texas USA, PCI-6289 DAQ card) and custom software (Mr. Kick, Sensory Motor Institute, Aalborg University) independently samples the electrical signals going into our device (raw EMG data), out of our device (filtered EMG data) and other signals (stimulation pulse and force transducer data) associated with, but separate from, our real-time aEMGcFES system. These signals were collected for the data analyses shown in this study. Raw EMG data going into the aEMGcFES system were sampled at 16,000 Hz, which contained the volitional EMG signal, stimulation artefacts, movement artefacts and any ambient electrical noise. A custom-built isolation unit provided electrical isolation between the subject and our data acquisition hardware and software.
Isometric ankle torque measurements
To test our aEMGcFES system performance under submaximal isometric muscle activation, we analyzed consecutive cycles of ankle dorsiflexion torque generated with and without electrical stimulation (E-stim) from the aEMGcFEs system. The subject sat in an upright, seated position with knee extended and the leg supported under the thigh and upper shank area without affecting the lower shank. The subject wore a custom ankle foot orthotic (AFO) that restrained ankle movement and allowed measurement of the isometric dorsiflexion torque generated by the tibialis anterior muscle. We modified standard metal bracing components (Becker Orthopedic, Troy, MI) and straps to create the major framing elements of the AFO (see Chang et al., 2008). The AFO was sufficiently secured to the leg such that there was little relative movement of the leg. Adjustable heel and ankle straps further restricted movement within the AFO. The foot was positioned to align the apex of the medial malleolus with the AFO’s only axis of rotation, which approximated the ankle dorsiflexion/plantarflexion axis. A uniaxial load transducer (Omegadyne, Sunbury, OH) was positioned posterior to the ankle joint and connected to eyehooks at known distances from the AFO axis of rotation with the ankle in a neutral standing position of approximately 110° (see Fig 5). The ankle dorsiflexor torque was calculated from measured forces from a linear transducer attached to the AFO at a moment arm of 12.08 cm.
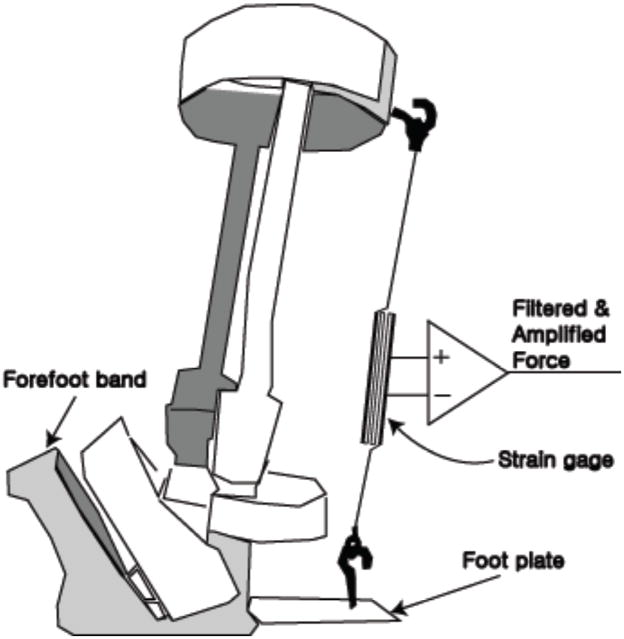
Figure 5.
Ankle-foot orthosis (AFO) with a uniaxial strain gage force transducer added posterior to the ankle joint to measure the force proportional to isometric dorsiflexion torque. The foot and shoe would slide into the AFO with the toes pointing to the left of the figure and the shank of the leg fit snugly within the calf band at the top of the device. A freely rotating hinge joint allowed relative motion at the ankle with only the strain gage force transducer impeding dorsiflexion.
We asked the subject to isometrically dorsiflex his ankle against the resistance load for 20 seconds while matching his real-time torque measurements against a sinusoidal reference signal (24 Nm peak amplitude) displayed on a monitor. As these were submaximal activations, increases in stimulation gain would likely lead to increased ankle torque output, however, the exact relationship between gain and ankle torque is an important area for future research. The recorded raw EMG signals included the tibialis anterior muscle vEMG, stimulus artefacts, M-waves, and movement artefacts. In real-time, the aEMGcFES system filtered, rectified, and integrated the raw EMG signal, then used the rectified, integrated EMG to control the biphasic stimulation (±1V, 33 Hz) outputted to a D/A amplifier where the signal was amplified (±90V) and sent back to the same muscle using a conventional fibular (peroneal) nerve stimulation protocol.
Open chain ankle kinematic measurements
To test aEMGcFES performance under conditions simulating the open chain ankle kinematics of swing phase, we analyzed consecutive ankle flexion and extension movements of approximately the same range of motion with and without E-stim. The subject sat in an upright, seated position within the capture volume of a six-camera motion analysis system (Vicon Motion Systems; Los Angeles, CA). The instrumented leg was supported beneath the thigh such that the heel barely contacted the ground with little weight bearing. The subject timed his ankle flexion and extension movements to coincide with a sinusoidal kinematic target oscillating at 0.5Hz. We collected 3-D kinematics of the instrumented leg (120 Hz) using retro-reflective markers placed according to a Vicon Plug-in-Gait marker set: distal 2nd metatarsal ray, lateral malleolus, calcaneous, mid-shank, lateral femoral epicondyle, mid-thigh, femoral greater trochanteric notch, anterior superior iliac spine, and posterior superior iliac spine. Ankle joint data were exported and low-pass filtered at 10 Hz (zero-lag, 4th-order Butterworth) in Matlab (Natick, MA) and synchronized to the EMG data. We asked the subject to maintain the same range of ankle joint movement with the aEMGcFES turned on and then turned off after ten seconds.
Results
Isometric ankle torque generated with electrical stimulation
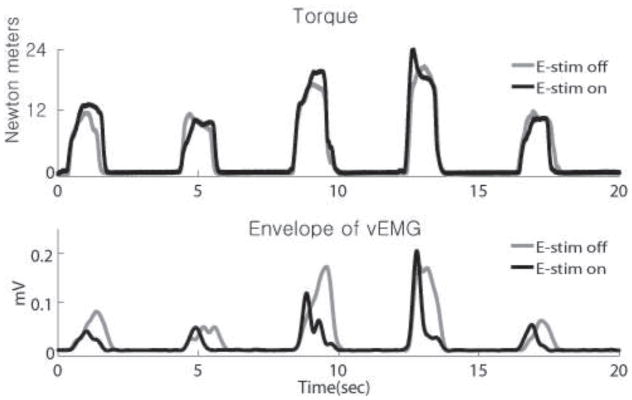
EMG envelopes associated with the corresponding isometric ankle torques generated by the TA muscle demonstrate that the subject could complete the torque-matching task equally well with and without E-stim (Fig 6, top). With the E-stim on, however, he was able to generate the same dorsiflexor torques with less voluntary muscle activation as indicated by the area under the EMG envelope (Fig 6, bottom).
Figure 6.
Tibialis Anterior (TA) muscle vEMG during force tracking with E-stim from the aEMGcFES system turned ON (dark thick lines) vs. OFF (light thin lines). Ankle torques measured (top) and the corresponding vEMG (bottom) during repeated torque tracking trials. Greater volitional muscle activity was required when the E-stim was turned OFF to match the same torques reached when the E-stim was turned ON. The 4th and 5th cycles in particular were well matched for ankle torque magnitude and timing, but indicated a clear difference in vEMG activity.
Performance of adaptive filter for real-time artefact removal
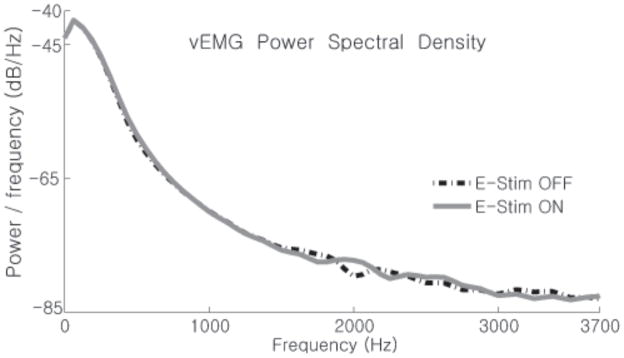
We analyzed the power spectrum densities (Fig 7) of the vEMG signals from the isometric activations shown in Figure 6. Regardless of whether the E-stim was on or off, the frequency spectra of both vEMG signals were nearly identical indicating that the aEMGcFES filtering method had adequately estimated the natural voluntary muscle activity, or volitional intent, of the subject during both activations.
Figure 7.
Comparison of TA vEMG power spectrum density with E-stim ON (gray line) vs. OFF (black line). The logarithmic power spectrum densities of TA vEMG data from Figure 4using Welch’s method shows that the power spectrum of the volitional EMG signal is hardly changed by the real-time Gram-Schmidt prediction error filter. vEMG with no E-stim is nearly identical to the vEMG estimated with the E-stim turned on.
Open chain ankle flexion and extension movements
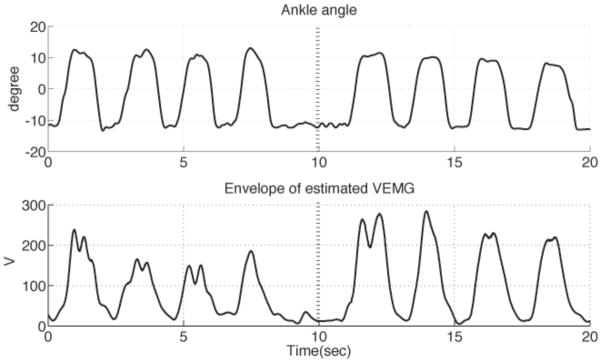
The same range of motion and velocities could be reached with and without E-stim during a set of open chain, isokinetic ankle flexion and extension movements. The subject successfully completed four consecutive ankle flexion-extension movement cycles with E-stim ON followed by four similar kinematic movements with the E-stim OFF (Fig 8, top). Muscle activity of the TA increased for the same kinematic movements when E-stim was turned off (Fig 8, bottom). The integrated vEMG signal over the second set of four successive muscle activations with the E-stim OFF (4.2798×106μV-s) was 36.7% greater compared to the four previous cycles with the E-stim ON (3.1313×106μV-s). This represents a 26.8% reduction in the vEMG when the E-stim was turned ON.
Figure 8.
Matched open-chain dorsiflexion movements with E-stim turned ON vs. OFF. Ankle dorsiflexion angle (top) and linear envelope of rectified vEMG from TA muscle (bottom) during an open-chain ankle movement task. The E-stim was turned off after 10 seconds (dashed vertical line). Note that for the same range and timing of ankle kinematics, the vEMG was lower with assistance from the aEMGcFES.
Discussion
The aEMGcFES device responded proportionally to volitional muscle activity of the tibialis anterior muscle to activate forceful movements assisting dorsiflexion during voluntary movements of the ankle joint (Fig 6 and 8). Extensive research has been done in the past using other forms of FES to improve the lives of persons with weak or paralyzed muscles following neurological insult. Previous use of natural electromyography (EMG) signals to control FES has been attempted in at least three ways: (1) EMG-triggered FES detects a threshold EMG signal to trigger onset of a predetermined stimulation sequence with no further EMG measurements made during stimulation; (2) EMG-controlled FES continuously measures EMG from another muscle to proportionally stimulate the affected muscle; and more recently (3) aEMGcFES specifically measures and uses the volitional EMG from the stimulated muscle as the control signal. As described earlier in the introduction, this latter method remains the most promising for allowing more physiologically appropriate (continuous, real-time) closed-loop control. Yet, this same method also requires the most technological development for overcoming a considerable artefact filtration problem for surface electrode recording. This method also has some potential limitations, which can include the possibility for antidromic annihilation of the efferent signal and increased recurrent inhibition with increased stimulation intensity via the Renshaw cells (Taylor & Chappell 2004). Also, it is likely that in the pathological case, patients will not be able to generate adequate EMG amplitude or activation patterns for controlled movement. Both of these issues, however, point to the importance of further investigation into different control strategies, which may solve these problems through non-linear, non-proportional control and individualized control parameters for each patient. In fact, it is likely that translation to practice will require the device to be set specifically for each individual’s specific needs and abilities. As with any rehabilitative treatment plan, however, a major goal of such a system should be to increase the patient’s volitional function through the periodic adjustment of the FES control parameter settings. Furthermore, our main goal of this work was only to show potential for this new method of FES filtering and control. As such, quantitative comparisons are less important than the general results that show functional enhancement with this technique. Lastly, patients may find daily use of surface electrodes to lead to skin irritation, however, these procedures would be no different than those used by currently available electrical surface stimulation systems. Furthermore, the filtering algorithm used in this device in particular could also have beneficial application to the more invasive percutaneous fine wire or surgically implanted electrodes systems.
We provide in this study positive evidence for a new method to provide stable, non-invasive aEMGcFES to allow healthy individuals to continuously control the level of FES using the target TA muscle as the sensor. Although further testing in more healthy and impaired subjects is necessary to fully validate the efficacy of this new method for delivering FES, as a first step this study demonstrates the ability of the aEMGcFES system to assist the voluntary intent of a person in controlling ankle dorsiflexion. This new approach for aEMGcFES suggests the following potential added benefits: (1) it uses surface electrodes, which may appeal to many potential users that want a reversible, non-invasive alternative to percutaneous needle electrodes and surgically implanted systems; (2) by effectively filtering out nearly all of the RSA, it allows higher stimulation frequencies (25–33Hz) that would result in smoother muscle activations and control (blanking circuits alone allow the RSA to grow in amplitude and duration with the stimulation signal, thus, limiting stimulation ~15 Hz); (3) it provides the individual with continuous, real-time control of force production from the affected muscle; and, (4)it provides a physiologically appropriate control loop and reinforces the natural autogenic control of muscle activation since the volitional EMG from the affected stimulation target serves as the control signal for the FES. This work has the potential to expand into many other areas of rehabilitation that may involve other muscle groups of the lower and upper extremities, in fine motor tasks such as grasping, and combating the debilitative effects of other neurological trauma such as spinal cord injury. Furthermore, we hope this work will lead to future studies to validate this technology in both healthy and impaired subjects to provide better understanding of the benefits of using natural sensors to control FES and the importance of immediate biofeedback to the targeted muscles in FES therapy.
RESEARCH HIGHLIGHTS.
Autogenic EMG-controlled FES uses volitional electromyography (EMG) signals from the stimulated muscle to continuously drive ongoing Functional Electrical Stimulation (FES) in real-time to the same, recorded muscle in a closed-loop controller.
A major barrier to effective Autogenic EMG-controlled FES is the inability to distinguish true voluntary muscle activity amidst the large stimulation artifacts contaminating the EMG signal.
A Gram-Schmidt adaptive filter can accurately distinguish and extract volitional muscle activity in real-time from electromyography (EMG) signals contaminated with residual stimulation artifacts and M-waves.
Autogenic EMG-controlled FES with a Gram-Schmidt adaptive filtering algorithm provides stable real-time estimation of volitional intent from the tibialis anterior muscle to assist in controlled ankle dorsiflexion movements and shows promise for future clinical applications.
Acknowledgments
Many thanks to Knud Larsen, Michael Grey, and Thomas Sinkjaer of the Sensory Motor Institute in Aalborg University for access to and assistance with the Mr. Kick EMG software. We also appreciate helpful suggestions and comments on this study from the members of the Georgia Tech Comparative Neuromechanics Lab. This study was supported in part by NIH AR054760 to Y.H.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- Benton LA, Baker LL, Bowman BR, Waters RL. Functional electrical stimulation: a practical clinical guide. 2. Downey: Rancho Los Amigos Medical Center; 1981. [Google Scholar]
- Brandell BR. Development of a universal control unit for functional electrical-stimulation (FES) Am J Phys Med Rehabil. 1982;61:279–301. [PubMed] [Google Scholar]
- Burridge J, Taylor P, Hagan S, Swain I. Experience of clinical use of the Odstock dropped foot stimulator. Artif Organs. 1997;21:254–60. doi: 10.1111/j.1525-1594.1997.tb04662.x. [DOI] [PubMed] [Google Scholar]
- Burridge JH. Does the drop-foot stimulator improve walking in hemiplegia? Neuromod. 2001;4:77–83. doi: 10.1046/j.1525-1403.2001.00077.x. [DOI] [PubMed] [Google Scholar]
- Chang YH, Roiz RA, Auyang AG. Intralimb compensation strategy depends on the nature of joint perturbation in human hopping. J Biomech. 2008;41:1832–9. doi: 10.1016/j.jbiomech.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Frigo C, Ferrarin M, Frasson W, Pavan E, Thorsen R. EMG signals detection and processing for on-line control of functional electrical stimulation. J Electromyography & Kinesiology. 2000;10:351–60. doi: 10.1016/s1050-6411(00)00026-2. [DOI] [PubMed] [Google Scholar]
- Futami KS, Kawanishi T, Sugiyama T, Cikajlo I, Handa Y. Application of Local EMG-Driven FES to Incompletely Paralyzed Lower Extremities. International FES Society, 2005; 10th Ann. Conference. [Google Scholar]
- Keller T, Curt A, Popovic MR, Dietz V, Signer A. Grasping in high lesioned tetraplegic subjects using the EMG controlled neuroprosthesis. J Neurorehab. 1998:251–255. [Google Scholar]
- Khaslavskaia S, Ladouceur M, Sinkjaer T. Increase in tibialis anterior motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve. Exp Brain Res. 2002;145:309–15. doi: 10.1007/s00221-002-1094-9. [DOI] [PubMed] [Google Scholar]
- Knash ME, Kido A, Gorassini M, Chan KM, Stein RB. Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res. 2003;153:366–77. doi: 10.1007/s00221-003-1628-9. [DOI] [PubMed] [Google Scholar]
- Kottink AI, Hermens HJ, Nene AV, Tenniglo MJ, van der Aa HE, Buschman HP, Ijzerman MJ. A randomized controlled trial of an implantable 2-channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch Phys Med Rehabil. 2007;88(8):971–8. doi: 10.1016/j.apmr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Kottink AIR, Oostendorp LJM, Buurke JH, Nene AV, Hermens HJ, Ijzerman MJ. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: A systematic review. Artif Organs. 2004;28:577–86. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- Kralj AR, Bajd T. Functional electrical stimulation: standing and walking after spinal cord injury. CRC Press, Inc; Boca Raton: 1989. [Google Scholar]
- Liberson WT, Holmquest HJ, Scott D, Dow M. Functional electrotherapy in stimulation of the peroneal nerve synchronized with the swing phase of gait in hemiparetic patients. Arch Phys Med Rehabil. 1961;42:101–5. [PubMed] [Google Scholar]
- Lyons GM, Sinkjaer T, Burridge JH, Wilcox DJ. A review of portable FES-based neural orthoses for the correction of drop foot. IEEE Trans Neural Syst Rehabil Eng. 2002;10(4):260–79. doi: 10.1109/TNSRE.2002.806832. [DOI] [PubMed] [Google Scholar]
- Merletti R, Knaflitz M, Deluca CJ. Electrically evoked myoelectric signals. Critical Reviews in Biomedical Engineering. 1992;19:293–340. [PubMed] [Google Scholar]
- Muraoka Y. Development of an EMG recording device from stimulation electrodes for functional electrical stimulation. Frontiers of Medical and Biological Engineering. 2002;11:323–33. doi: 10.1163/156855701321138969. [DOI] [PubMed] [Google Scholar]
- O’Keeffe DT, Lyons GM. A versatile drop foot stimulator for research applications. Jan. 2002;24:237–42. doi: 10.1016/s1350-4533(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Peasgood W, Whitlock T, Bateman A, Fry ME, Jones RS, Davis-Smith A. EMG-controlled closed loop electrical stimulation using a digital signal processor. Electronics Letters. 2000;36:1832–3. [Google Scholar]
- Prochazka A, Wiles CM. Electrical-stimulation of paretic leg muscles in man, allowing feedback-controlled movements to be generated from the wrist. J Physiol-London. 1983;343:20–1. [Google Scholar]
- Sennels S, Biering-Sorensen F, Andersen OT, Hansen SD. Functional neuromuscular stimulation controlled by surface electromyographic signals produced by volitional activation of the same muscle: adaptive removal of the muscle response from the recorded EMG signal. Ieee Trans on Biomedical Engineering. 1997;5:12. doi: 10.1109/86.593293. [DOI] [PubMed] [Google Scholar]
- Smith BT, Peckham PH, Keith MW, Roscoe DD. An externally powered, multichannel, implantable stimulator for versatile control of paralyzed muscle. IEEE Trans Biomed Eng. 1987;34:499–508. doi: 10.1109/tbme.1987.325979. [DOI] [PubMed] [Google Scholar]
- Taylor P, Chappell P. Variation in system gain when using voluntary EMG to control electrical stimulation of the same muscle. 9th Ann. Conference.International FES Society; 2005. [Google Scholar]
- Vodovnik L, Dimitrijevic MR, Prevec T, Logar M. Electronic walking aids for patients with peroneal palsy. Proc European Symp Medical Electronics. 1965:58–61. [Google Scholar]
- Waters RL, McNeal D, Perry J. Experimental correction of footdrop by electrical-stimulation of peroneal nerve. J Bone Joint Surg-Am Vol. 1975;57:1047–54. [PubMed] [Google Scholar]
- Waters RL, McNeal DR, Faloon W, Clifford B. Functional electrical-stimulation of the peroneal nerve for hemiplegia - long-term clinical follow-up. J Bone Joint Surg-Am Vol. 1985;67A:792–3. [PubMed] [Google Scholar]
- Willemsen ATM, Vanalste JA, Boom HBK. Real-time gait assessment utilizing a new way of accelerometry. J Biomech. 1990;23:859–63. doi: 10.1016/0021-9290(90)90033-y. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. 2. New York: John Wiley & Sons; 1990. [Google Scholar]
- Yeom H, Park Y, Yoon H. Gram-Schmidt M-wave canceller for the EMG controlled FES. Ieice Transactions on Information and Systems. 2005;E88D:2213–7. [Google Scholar]
- Yeom HJ, Park YC, Chang YH. Eigen filter to detect volitional EMG signals in autogenic EMG-controlled FES. Electronics Letters. 2007;43:1410–1. [Google Scholar]