Abstract
Background
Predisposing factors, long-term occurrence, and histopathological changes associated with recovery or progression to allograft failure from chronic rejection (CR) were studied in adult patients treated primarily with tacrolimus.
Methods
CR cases were identified using stringent criteria applied to a retrospective review of computerized clinicopathological data and slides.
Results
After 1973 days median follow-up, 35 (3.3%) of 1049 primary liver allograft recipients first developed CR between 16 and 2532 (median 242) days. The most significant risk factors for CR were the number (P<0.001) and histological severity (P<0.005) of acute rejection episodes and donor age >40 years (P<0.03). Other demographic and matching parameters were not associated with CR in this cohort. Ten patients died with, but not of, CR. Eight required retransplantation because of CR at a median of 268 days. Ten resolved either histologically or by normalization of liver injury tests over a median of 548 days. CR persisted for 340 to 2116 days in the remaining seven patients. More extensive bile duct loss (P<0.01), small arterial loss (P<0.03), foam cell clusters (P<0.01) and higher total bilirubin (P<0.02) and aspartate aminotransferase (P<0.03) were associated with allograft failure from CR.
Conclusions
Early chronic liver allograft rejection is potentially reversible and a combination of histological, clinical, and laboratory data can be used to stage CR. Unique immunological and regenerative properties of liver allografts, which lead to a low incidence and reversibility of early CR, can provide insights into transplantation biology.
Chronic rejection (CR) is the major obstacle to morbidity-free long-term survival of mostly all vascularized solid organ allograft recipients. Liver allografts are an exception in that the incidence of CR at 5 years after transplantation has steadily decreased from 20 to 40% in the early 1980s to less than 5% in current recipients (1, 2). The unique immunological properties of the liver as an allograft (3, 4), and better recognition and control of acute rejection and the early phases of CR with tacrolimus (5–8), are thought to be responsible for the recent decline in the number of cases (3, 5, 8–10). Furthermore, the liver is the only organ in humans in which CR is potentially reversible (5, 7, 8, 11–13), and a hepatic allograft can protect other organs from the same donor from CR (14).
Until recently, no general agreement existed regarding the histological definition of CR in the liver. Some groups recognized early and late stages of CR, the former being recognized by bile duct atrophy/pyknosis affecting a majority of the ducts, with or without <50% ductopenia (8, 11, 13, 15). In other centers, CR was diagnosed only when bile ducts were absent in more than 50% of portal tracts or obliterative arteriopathy was seen (16–18). Since obliterative arteriopathy usually affects medium-sized muscular arteries in the liver hilum, which are rarely sampled in needle biopsies, significant weight was placed on the 50% ductopenia (16–18).
A recent study of CR in a large multicenter primarily cyclosporine-treated patient cohort that included children, clarified the issue by showing that early CR was a distinct clinicopathological syndrome that could be reliably diagnosed using the above criteria (15). An in-depth study of that population showed that the early stage is more frequently reversible (19), consistent with previous studies (5, 7,8,11–13). This is likely due to less extensive bile duct loss, lack of significant fibrosis, and minimal vascular abnormalities in the early stage of CR, combined with the ability of hepatocytes and biliary epithelial cells to regenerate. In contrast, the late stage of CR, which is less commonly reversible, shows evidence of more extensive fibrosis, widespread bile duct loss, and obliterative arteriopathy, some of which may be irreversible (5, 8, 13, 20).
The current study was conducted to firstly investigate predisposing factors and the long-term occurrence of CR in an adult patient population receiving tacrolimus as baseline immunosuppression and secondly to further characterize histopathological changes associated with recovery from CR or progression to allograft failure.
MATERIALS AND METHODS
Case selection
Data retrieved from our in-house transplant clinical information system, the Electronic Data Interface for Transplantation (EDIT), showed that a total of 1049 adults received a first liver transplant between April 15, 1990 and June 30, 1994 at the Presbyterian University Hospital, University of Pittsburgh Medical Center (UPMC). Patients must have survived for more than 14 days after surgery and had follow-up information available. The period during which transplantations were conducted is the same as that of the Liver Transplant Database (LTD) study on CR (19, 21), but patients in our study were followed for 3 years longer than the LTD cohort. Clinical and histological data were obtained from April 15, 1990 to September 1998. Baseline information included age, sex, race, which was recorded as Caucasian, black and other, MHC and blood types, primary disease of the recipient, and cold ischemia time. Preservation injury was assessed histologically. Ninety-eight (97.5) percent of patients received tacrolimus as baseline immunosuppression, and the remainder were treated with a cyclosporine-based regimen. Side effects warranted conversion from tacrolimus to cyclosporine in less than 5% of patients, whereas more than 50% of the patients who started on cyclosporine were switched to tacrolimus. Liver biopsies, cholangiograms, angiograms, and liver injury tests were performed when clinically indicated. After hospital discharge, clinical follow-up was conducted on patients twice a week for the first 6 weeks, once a week for 3 months, once every 2 weeks for 6 months, then monthly up to 1 year. Thereafter, contact was made every 6 weeks for 2 years, and then every 2 months thereafter, unless otherwise medically indicated.
Of the 1049 patients, 112 had a primary or secondary histological diagnosis of CR on at least one biopsy specimen, using suboptimally defined criteria for CR. All biopsy specimens and explants with any diagnosis of CR, the biopsy specimen before the diagnosis of CR and, in case of resolution, the biopsy specimen upon which the resolution of CR was diagnosed, and the last available biopsy specimen, cholangiogram, ultrasound, and angiogram reports from these patients were reviewed. The objective was to determine whether the original diagnosis of CR was sustainable.
More than four portal tracts were required to render a biopsy adequate. Bile duct pyknosis/atrophy, ductular reaction, arterial loss, obliterative arteriopathy, bridging necrosis, infarcts, and foam cell clusters were recorded as present or absent. Bile duct loss was calculated as the percentage of portal tracts without bile ducts. Portal and lobular inflammation, portal and central fibrosis and central lobular dropout were scored as none, mild, moderate, and severe. CR was defined as “early” in cases of atrophy/pyknosis involving a majority of bile ducts but with less than 50% bile duct loss and as “late” when duct loss equaled or exceeded 50% or obliterative arteriopathy was seen. In addition, the presence of comorbid conditions such as recurrent primary disease, biliary disease, ischemia, and acute cellular rejection (ACH) was recorded.
After reviewing the histological evidence and clinical data, 77 patients/grafts were excluded as bona fide examples of CR. The largest group of patients (n=41) was excluded because of insufficient histological evidence of CR. In most of these cases, CR was a secondary diagnosis and detected only on one sample. These were added to the no-CR group (Group 1). Fourteen patients were excluded because they had conditions that can be histologically indistinguishable from CR, namely biliary tract strictures (n=8) and hepatic artery stenosis or occlusion (n=6). In 22 cases, biopsy specimens were unavailable for review. The remaining 35 patients (Group 2) were analyzed in the first part of the study, which aimed to identify risk factors for the development of CR. Eighteen patients were analyzed for the second part of the study, which compared patients with graft failure due to CR and patients in whom CR resolved. Group 2A (n=8) consisted of patients in whom CR was the sole cause of graft failure. In group 2 B (n=10), CR resolved either histologically or by normalization of liver injury tests. In seven patients, CR persisted, leading neither to resolution nor to graft failure by the end of the follow-up period (Group 2C). Ten patients with CR were excluded from the second part of the study because the graft failed or the patient died primarily due to reasons other than CR (Group 2D): sepsis (n = 6); gastrointestinal hemorrhage (n = l); ruptured aortic aneurysm (n = l); motor vehicle accident (n = 1); and unknown (n = 1).
Statistical analysis
Summary statistics are presented as the number and percentage for categorical data and as the median and range for continuous data. The Wilcoxon rank sum test was used for data that were ordinal or continuous, and chi-square tests were used for nominal data. Due to the small sample size, exact methods were used where appropriate. Proportional hazards models were fit to examine the relationship between age and CR after adjusting for preservation injury and number of acute rejection episodes. Time was defined as time to CR or to end of follow-up. P values less than 0.05 indicate statistical significance.
RESULTS
Comparison of CR and non-CR groups
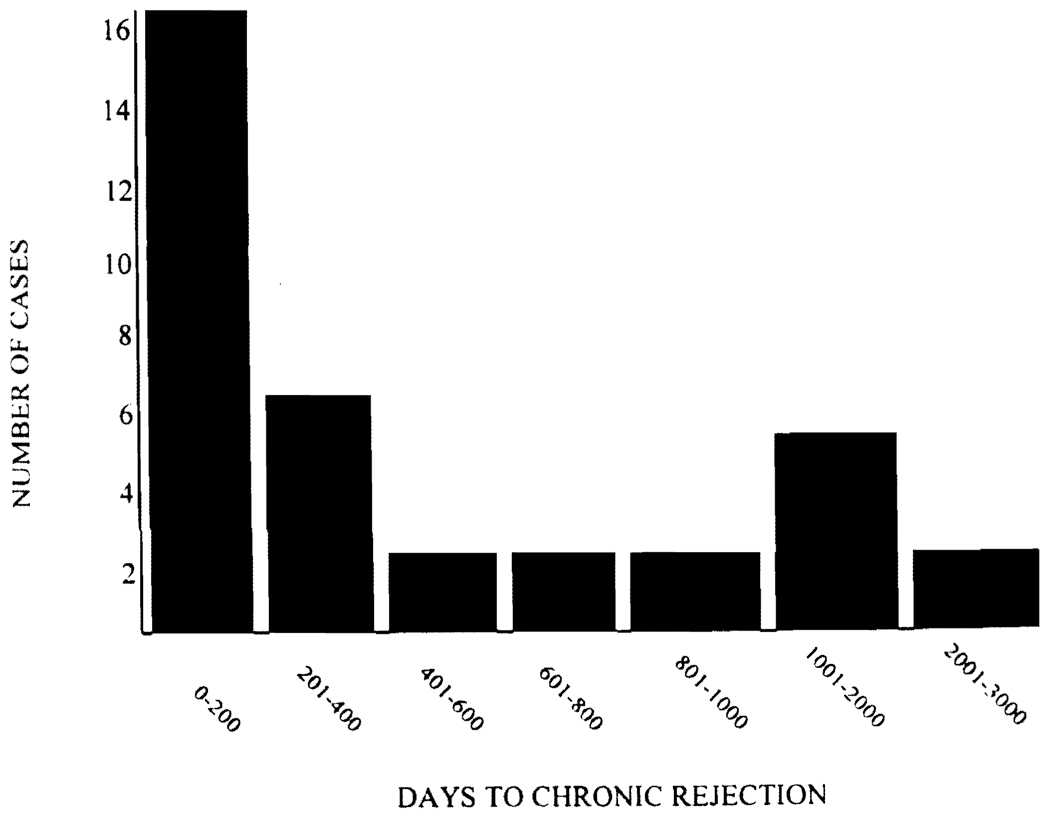
Thirty-five (group 2) of 1049 patients (3.3%) developed CR; the remaining 1014 patients comprised group 1. The median follow-up time was 1973 days with a range from 16 to 3072 days. CR was first diagnosed between 16 and 2532 (median 242) days after transplantation with the incidence peaking early after transplantation and decreasing with time (Fig. 1). Recipient age ranged from 18 to 77 years and did not differ between the two groups. No differences were found in recipient sex, race, and primary diagnoses, which were divided into nine groups (primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis, viral hepatitis, alcohol abuse, malignancy, cryptogenic, metabolic, and other) (Table 1). Sixty-three (6%) of the patients initially received cyclosporine-based therapy in this study; there was no significant difference in the rate at which these patients developed CR. However, there was a significant crossover of cyclosporine-treated patients to tacrolimus.
FIGURE 1.
Time to the first histopathological diagnosis of CR.
TABLE 1.
Demographic information and preservation injury in patients with and without CR
| Variable | Group 1 (no CR) n=1014 Number(%) |
Group 2 (CR) n=35 Number(%) |
P value |
|---|---|---|---|
| Primary diagnosis | |||
| PBC | 91 (9) | 4 (11) | NS |
| PSC | 78 (8) | 4 (11) | |
| Metabolic | 65 (6) | 1 (3) | |
| Viral hepatitis | 259 (26) | 9 (26) | |
| Neoplasm | 67 (7) | 1 (3) | |
| Cryptogenic | 133 (13) | 5 (14) | |
| Autoimmune hepatitis | 32 (3) | 3 (7) | |
| Alcohol abuse | 180 (18) | 6 (17) | |
| Miscellaneous | 98 (10) | 2 (6) | |
| Missing | 11 | 0 | |
| Donor age | |||
| <40 years | 614 (61) | 15 (43) | 0.035 |
| >40 years | 389 (39) | 20 (57) | |
| Missing | 11 | ||
| Preservation injury | |||
| Yes | 483 (48) | 17 (49) | NS |
| No | 531 (52) | 18 (51) |
Abbreviations used in table: NS, not significant; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis.
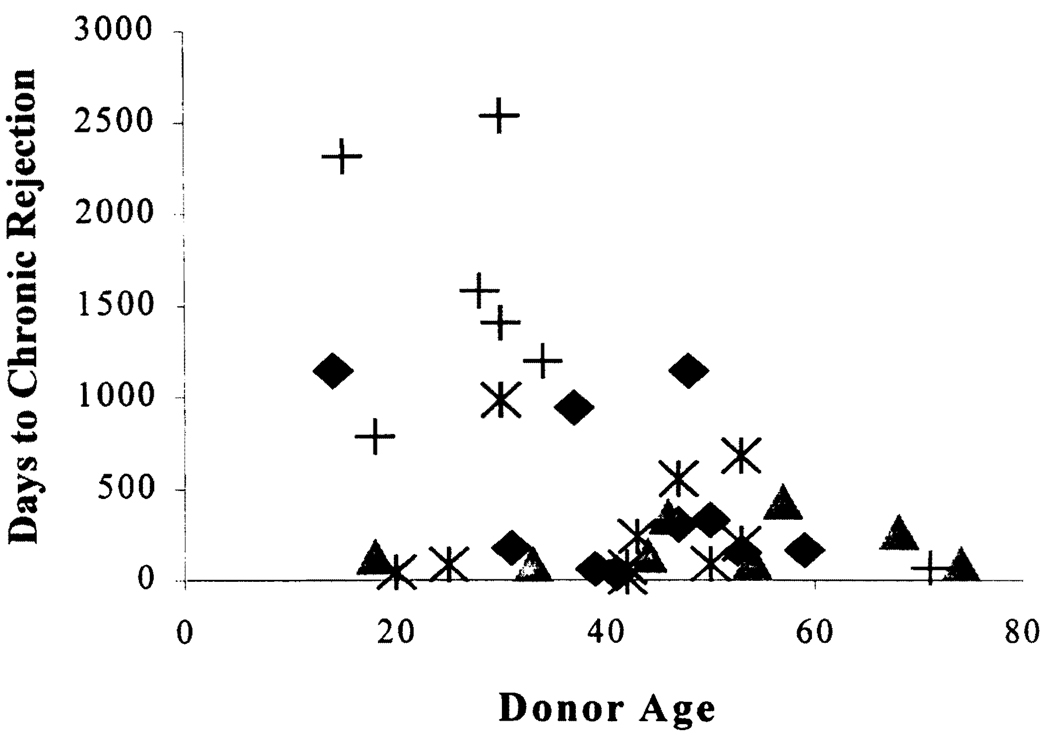
Donor age was significant when treated as a continuous variable. To further establish the approximate age at which this occurred, the data was plotted (Fig. 2), which resulted in a clustering of cases at 40–55 years. Thus, donor age was significantly higher in the group that developed CR (Table 1), and CR was associated with a donor age of more than 40 years (P<0.04). This relationship was independent of the length of the intensive care unit stay of the donor, which was a median of 2 days in both age groups. In a proportional hazards regression analysis, which included donor age, preservation injury, and number of ACR episodes, only donor age and number of ACR episodes remained independent risk factors for CR (data not shown). CR also occurred earlier in recipients of older organs (Fig. 2). There were no statistically significant differences in donor sex and race between the two groups. No differences could be detected in the length of cold ischemia time (analyzed continuously and as greater or less than 14 hr). Sex match and mismatch were analyzed according to all possible combinations, and in addition, the combination of female recipient/male donor was tested against all other pairings. No differences could be detected between the two groups (data not shown). Similarly, there was no difference between the different race matches, even when the combinations white donor/white recipient and black donor/white recipient and all donors/black recipients were tested separately. The differences in ABO match and the number of HLA-A, B, A + B, and DR mismatches did not reach statistical significance.
FIGURE 2.
Time to onset of CR by donor age. All of the symbols represent cases of CR, and the outcome of the various cases are represented by the different symbols. (♦, graft failed or patient died, but not primarily because of CR; ▲, allograft failed because of CR; *, CR resolved as determined by liver injury tests and/or liver biopsy; +, patients with CR that neither resolved nor required retransplantation over the period of study) (see text).
The number (P<0.001) and histopathological severity (P<0.005) of acute rejection episodes showed the strongest correlation with the development of CR (Table 2). However, whether the onset of acute rejection occurred within the first 14 days was not significant.
TABLE 2.
Timing, number of episodes, and maximum histopathologic severity of acute rejection episodes in patients with and without CR
| Variable | Group 1 (no CR) n=1014 Number(%) |
Group 2 (CR) n=35 Number(%) |
P value |
|---|---|---|---|
| First episode of ACR <14 days |
|||
| Yes | 303 (55) | 14 (45 | NS |
| No | 250 (45) | 17 (55) | |
| Number of episodes of ACR |
|||
| 0 | 461 (45) | 4 (11) | <0.0001 |
| 1 | 406 (40) | 11 (31) | |
| 2 | 111 (11) | 11 (31) | |
| 3 | 27 (3) | 7 (20) | |
| >4 | 9 (1) | 2 (6) | |
| Maximum grade of ACR | |||
| Mild | 369 (67) | 13 (42) | 0.004 |
| Moderate | 160 (29) | 15 (48) | |
| Severe | 24 (4) | 3 (10) |
Outcome of CR
Based on clinical, histopathological, and biochemical criteria, patients with CR experienced one of four possible outcomes and were placed into the following groups (Fig. 2). Group 2A consisted of 8 patients who lost their grafts because of CR. In this group, time to CR ranged from 84 to 432 days with a median of 131 days, and graft failure occurred between 102 and 454 with a median of 268 days. No patient in this group had recurrent primary disease.
Group 2B consisted of 10 patients who recovered from CR to normal histology and liver function tests or to normal liver function tests. Seven of these patients also had biopsy-proven recovery. CR in this group was first diagnosed between 16 and 981 (median 145) days after transplantation, and recovery took place after 22 to 1285 (median 548) days. Two patients in this group also had recurrent disease (hepatitis B = 1, hepatitis C = 1). It should be emphasized that even though these patients eventually recovered, they experienced significant liver injury test elevations and histopathological changes on biopsy that merit inclusion under the diagnosis of early CR. These include marked elevations of the gamma-glutamyl transpeptidase, alkaline phosphatase, and alanine aminotransferase (ALT), combined with bile duct atrophy affecting a majority of the bile ducts. Other stringent clinical and radiological selection criteria were also applied in an effort to exclude possible false-positive diagnoses of CR in patients with comorbid conditions that could produce a similar clinicopathological profile.
Group 2C consisted of seven patients with CR that neither led to graft failure nor to recovery until the end of the follow-up period. In this group, CR occurred 68 to 2532 (median 1406) days after transplantation and persisted for 340 to 2116 days with a median 814 days. One patient also had recurrent primary biliary cirrhosis as a secondary diagnosis. Of the patients in group C, five had the changes of early CR and two patients had 82% and 69% bile duct loss, respectively. Of the 10 other patients in group 2D who were excluded because of death (see Materials and Methods), only two had changes that could have contributed to significant allograft dysfunction, but it was determined not to be a significant contribution to the cause of death.
Comparison of graft failure and resolution groups
No statistical differences could be found in the time to CR, cold ischemia time, recipient and donor age, race and sex, ABO match, and the number of HLA-B and DR mismatches when comparing group 2A (failure) and group 2B (recovery) (data not shown). A higher number of HLA-A (P=0.08) and A + B mismatches (P=0.09) were of borderline significance for graft failure from CR. More patients in the recovery group had a cold ischemia time of more than 14 hr (P=0.09). There was an equal distribution of primary diagnoses between the two groups. Sex and race matches were analyzed in the same combinations as in the analysis comparing patients who developed CR with those who did not and showed no statistically significant differences (data not shown). However, the race match/mismatch analysis was limited by the fact that all donors in both groups were Caucasian.
No association with outcome was found in the number and severity of ACR episodes or whether the first episode occurred before or after day 14 after transplantation (data not shown). However, maximum total bilirubin (P<0.02) and aspartate aminotransferase (AST) (P<0.03) were significantly higher in the group that went on to graft failure (Table 3). There was no difference in ALT and gamma-glutamyl transpeptidase between the groups. Alkaline phosphatase was also higher in group A (P=0.08).
TABLE 3.
Liver injury tests (highest value) during CR in patients with CR leading to graft failure or recovery
| Variable | Group 2A (graft failure) n=8 Median [range] |
Group 2B (recovery) n=10 Median [range] |
P value |
|---|---|---|---|
| Total bilirubin | 42.8 [3.9–51.7] | 2.3 [0.5–7.4] | 0.016 |
| AST | 1574 [257–3598] | 189 [27–673] | 0.023 |
| ALT | 648 [199–2359] | 475 [38–990] | NS |
| Gamma-glutamyl transpeptidase |
2133 [312–4681] | 825 [26–6111] | NS |
| Alkaline phospbatase | 2402 [45–3024] | 251 [44–2209] | 0.083 |
The features of late CR were found significantly more often in patients whose grafts failed due to CR (Table 4). The maximum percentage of bile duct loss was higher in group A (P<0.01), and foam cell clusters were also more frequent in this group (P<0.01). However, only 38% of the patients who went on to graft failure showed bile duct loss involving more than 50% of portal tracts. The median percentage of bile duct loss in the graft failure group was 43%. No patient in group B had bile duct loss in more than 50% of portal tracts (P=0.07). Arterial loss occurred in 50% of patients in group 2A but in no patients from group 2B (P<0.02). Severity of central fibrosis, when severe fibrosis was compared with all other grades, did not differ between the two groups. Only one patient whose graft failed due to CR had severe central fibrosis. Only one allograft from the graft failure group did not have obliterative arteriopathy on the liver allograft removed at the time of retransplantation. However, this same failed allograft showed severe perivenular fibrosis, small arterial loss, and bile duct loss in >50% of the portal tracts.
TABLE 4.
Histological features of CR in patients going on to graft failure or recovery
| Variable | Group A (graft failure) n=8 Number(%) |
Group B recovery) n=10 Number (%) |
P value |
|---|---|---|---|
| Maximum % bile duct loss | |||
| Median [range] | 43 [0–100] | 0[0–16] | <0.01 |
| Maximum bile duct loss >50% |
|||
| Yes | 3 (38) | 0(0) | 0.07 |
| No | 5 (62) | 10 (100) | |
| Arterial loss | |||
| Yes | 4 (50) | 0 (0) | 0.02 |
| No | 4 (50) | 10 (100) | |
| Maximum severity of central fibrosis |
|||
| None, mild, moderate | 7 (88) | 10 (100) | NS |
| Severe | 1 (12) | 0 (0) | |
| Foam cell clusters | |||
| Yes | 7 (88) | 1 (10) | <0.01 |
| No | 1 (12) | 9 (90) |
DISCUSSION
Similar to other organs, “immunological” factors most significantly predisposed patients to the development of chronic liver allograft rejection. As in previous studies of liver allografts, the strongest correlation with the onset of CR was the frequency and severity of acute rejection episodes (12, 22, 23). However, in contrast to previous studies, there was no association between CR and MHC mismatches (24–26), male-to-female mismatch or non-Caucasian recipient race (12, 27), or late onset acute rejection (12, 22, 28). Nor was CR more common in younger recipients (12, 19) or those with a primary diagnosis of autoimmune hepatitis (17, 29, 30). This suggests that tacrolimus more effectively controls the contribution of mismatching and other immunological factors to CR and might also have a more potent early “tolerizing” effect in liver transplantation.
This study also shows for the first time that the apparently nonimmunological factor of donor age >40 years contributed to the development of CR in the liver. This is a well-described risk factor in kidney and heart allografts (31–33), but in liver allografts, older donor age has only been associated with delayed graft function (34) and a higher incidence of acute rejection (35). Although the mechanism is not known at this time, even the intrahepatic branches of the hepatic artery of older donors can be significantly affected by atherosclerosis (our unpublished observation) and, thus, may be more prone to the development of obliterative arteriopathy.
Inadequate immunosuppression because of complicating infections, neoplasms. or noncompliance clearly influenced the rate of CR in this study, but it was difficult to quantify because drug levels were not systematically analyzed in the entire study population. However, detailed examination of the clinical histories of the 35 patients with CR revealed that 18 (51%) of 35 either had infections or tumors or were noncompliant and, thus, “inadequately” immunosuppressed. We suspect, but do not have proof, that noncompliance might also account for the third group of patients that developed CR late after transplantation. They tend to have younger donors and most show the early histological changes of CR and live with CR for several years, neither failing nor recovering. Alternatively, the different timing of onset of CR in this group may reflect a different immunological process than the other groups. This particular population illustrates that the threat of CR persists, even in long-surviving liver allograft recipients.
Similar to the LTD study (19), serum AST, but not the ALT, and total bilirubin levels were significantly higher in those patients that ultimately required retransplantation. Since AST is the more liver-specific enzyme and mainly found in the mitochondria, it may be worthwhile to determine whether the mitochondrial fraction of the enzyme is elevated in the blood and whether mitochondria injury is an important cause of allograft dysfunction.
Compared to the LTD study (19), CR in this tacrolimus-treated population showed less bile duct injury/loss and less perivenular fibrosis. Of the patients requiring retransplantation in the LTD study, the median bile duct loss was 100% and only one patient of 13 had bile duct loss of less than 50%. In this study, although there was significantly more bile duct loss in patients who went on to graft failure, the median bile duct loss was only 43%. Perivenular fibrosis was more extensive in failed allografts from both studies, but although severe (central-to-central) bridging fibrosis was seen in most of the failed allografts in the LTD study, it was present in only one case from this study. Conversely, arterial loss was highly correlated with failure in the UPMC patients but only showed a trend in the LTD population (19). This finding further supports the theory that arterial damage in CR may be a stronger link to failure than bile duct loss, which may be reversible when the direct immunological attack is removed (12,20,36). The greater the potentially irreversible damage in the form of bile duct loss, obliterative arteriopathy, and perivenular fibrosis, the less likely the organ is to recover (Table 5).
TABLE 5.
Histopathological features associated with early and late CR
| CR |
||
|---|---|---|
| Early | Late | |
| Atrophy in >50% of (remaining) bile ducts | + | + |
| Bile duct loss in >50% of portal tracts | −/+ | +/− |
| Foam cell clusters | −/+ | + |
| Severe central fibrosis | − | +/− |
| Arterial loss | − | +/− |
| Severe arteriopathy | − | + |
The slightly different expression of CR in the two studies may be a result of the different immunosuppressive regimens administered. In tissue culture, cyclosporine, but not tacrolimus, has been found to induce fibrogenesis by increasing tumor growth factor-β production by small airway epithelial cells (37). Of the five different regimens in the LTD, three were cyclosporine based and only one contained tacrolimus, whereas UPMC patients were almost exclusively treated with tacrolimus.
There are two aspects of chronic liver allograft rejection that are clearly different from other organs: the relatively low incidence, even when patients are followed over a long period of time; and the reversibility of the early stages. In kidney transplantation, hypertension, drug toxicity, and donor-derived factors like atherosclerosis and accelerated senescence (31) all contribute to the relentless and progressive decline of allograft function and structure that eventually plagues most renal allograft recipients (31, 38–40). In the liver, the opposite pattern is observed: after an initial peak during the first year after transplantation, the incidence declines steadily, but CR can still occur after a follow-up of up to 8 years, which was the cutoff for this study. This difference might be explained by the following.
In contrast to clinical kidney or heart transplantation, experimental animal models of CR of these organs have been used to illustrate the point that the early stages of CR are “allo-antigen dependent” and reversible (41, 42). By retransplanting the kidney or heart allograft with the early stages of CR back into syngeneic recipients, the alloantigenic stimulus/injury is removed and the organ recovers. Extrapolation of this line of reasoning to human liver allograft recipients would suggest that resolution of CR very well might represent resolution of the immunological or alloantigen dependent factors and a greater tolerance of the allograft, as suggested nearly four decades ago (43). Theoretically, this could occur via activation-induced clonal deletion (4, 43, 44), augmentation of regulatory pathways (45), liver injury-induced augmentation of donor microchimerism (46), or most likely, a combination of the above.
In addition, architecturally correct repair of the damage already incurred would be much easier in the liver than in the kidney, because of the constraints imposed by the rigidity and complexity of organ structure, as well as the regenerative capacity of the epithelial cell populations. Even adult hepatocytes are thought to have an almost unlimited potential to regenerate (47), and injured or destroyed bile duct cells are able to reconstitute as well (11, 12, 19). Thus, it is not unreasonable to suggest that augmentation of epithelial regeneration during acute rejection damage in the kidney using growth factors might delay or prevent some of the architectural decline seen during the development of chronic allograft nephropathy.
In summary, better recognition and control of the acute and the early stage of CR has minimized the impact of CR on liver allograft recipients. However, the threat of CR still exists in long-term liver allograft recipients, and thus continues to be an important cause of allograft dysfunction (1, 17). Potentially irreversible damage to structures targeted in rejection, the bile ducts, arteries and veins, can be used along with clinical and laboratory data to stage CR and assist clinical management. The changing risk factors and the unique immunological and regenerative properties of a liver allograft, such as the low incidence and reversibility of early CR, will likely continue to provide insights into transplantation immunobiology.
Footnotes
This work was supported by NIH RO1AI38899-01A2 and 1R01 DK49615-01A1, and an unrestricted educational grant from Fujisawa.
REFERENCES
- 1.Demetris AJ, Murase N, Lee RG, et al. Chronic rejection: a general overview of histopathology and pathophysiology with emphasis on liver, heart and intestinal allografts. Ann Transplant. 1997;2(2):27. [PMC free article] [PubMed] [Google Scholar]
- 2.Pirsch JD, Kalayoglu M, Hafez GR, D’Alessandro AM, Sollinger HW, Belzer FD. Evidence that the vanishing bile duct syndrome is vanishing. Transplantation. 1990;49(5):1015. doi: 10.1097/00007890-199005000-00041. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance [see comments]. [Review] Lancet. 1992;339(8809):1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339(26):1905. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2(8670):1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Donner A, Eliasziw M, et al. Randomised trialomania? The multicentre liver transplant trials of tacrolimus [see comments] Lancet. 1995;346(8986):1346. doi: 10.1016/s0140-6736(95)92349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung JJ, Todo S, Tzakis A, et al. Conversion of liver allograft recipients from cyclosporine to FK 506-based immunosuppression: benefits and pitfalls. Transplant Proc. 1991;23((1 Pt 1)):14. [PMC free article] [PubMed] [Google Scholar]
- 8.Demetris AJ, Fung JJ, Todo S, et al. Pathologic observations in human allograft recipients treated with FK 506. Transplant Proc. 1990;22(1):25. [PMC free article] [PubMed] [Google Scholar]
- 9.Group EFMLS. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group [see comments] Lancet. 1994;344(8920):423. [PubMed] [Google Scholar]
- 10.Sher LS, Cosenza CA, Michel J, et al. Efficacy of tacrolimus as rescue therapy for chronic rejection in orthotopic liver transplantation: a report of the U.S. Multicenter Liver Study Group. Transplantation. 1997;64(2):258. doi: 10.1097/00007890-199707270-00014. [DOI] [PubMed] [Google Scholar]
- 11.Hubscher SG, Buckels JA, Elias E, McMaster P, Neuberger J. Vanishing bile-duct syndrome following liver transplantation: is it reversible? Transplantation. 1991;51(5):1004. doi: 10.1097/00007890-199105000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Freese DK, Snover DC, Sharp HL, Gross CR, Savick SK, Payne WD. Chronic rejection after liver transplantation: a study of clinical, histopathological and immunological features. Hepatology. 1991;13(5):882. [PubMed] [Google Scholar]
- 13.Demetris AJ, Fung JJ, Todo S, et al. Conversion of liver allograft recipients from cyclosporine to FK506 immunosuppressive therapy: a clinicopathologic study of 96 patients. Transplantation. 1992;53(5):1056. doi: 10.1097/00007890-199205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demetris AJ, Murase N, Ye Q, et al. Analysis of chronic rejection and obliterative arteriopathy: possible contributions of donor antigen-presenting cells and lymphatic disruption. Am J Pathol. 1997;150(2):563. [PMC free article] [PubMed] [Google Scholar]
- 15.Demetris AJ, Seaberg EC, Batts KP, et al. Chronic liver allograft rejection: a National Institute of Diabetes and Digestive and Kidney Diseases interinstitutional study analyzing the reliability of current criteria and proposal of an expanded definition. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Am J Surg Pathol. 1998;22(1):28. doi: 10.1097/00000478-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Anonymous. Terminology for hepatic allograft rejection. International Working Party. Hepatology. 1995;22(2):648. [PubMed] [Google Scholar]
- 17.Wiesner RH, Ludwig J, van Hoek B, Krom RA. Current concepts in cell-mediated hepatic allograft rejection leading to ductopenia and liver failure. Hepatology. 1991;14((4 Pt 1)):721. doi: 10.1016/0270-9139(91)90064-3. [DOI] [PubMed] [Google Scholar]
- 18.Wight DA. Chronic liver transplant rejection: definition and diagnosis. Transplant Proc. 1996;28(1):465. [PubMed] [Google Scholar]
- 19.Blakolmer K, Seaberg EC, Batts K, et al. Analysis of the reversibility of chronic liver allograft rejection implications for a staging schema. Am J Surg Pathol. 1999;23(11):1328. doi: 10.1097/00000478-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Oguma S, Belle S, Starzl TE, Demetris AJ. A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology. 1989;9(2):204. doi: 10.1002/hep.1840090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei YL, Detre KM, Everhart JE. The NIDDK liver transplantation database. Liver Transpl Surg. 1997;3(1):10. doi: 10.1002/lt.500030102. [DOI] [PubMed] [Google Scholar]
- 22.Candinas D, Gunson BK, Nightingale P, Hubscher S, McMaster P, Neuberger JM. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet. 1995;346(8983):1117. doi: 10.1016/s0140-6736(95)91797-7. [DOI] [PubMed] [Google Scholar]
- 23.Farges O, Nocci Kalil A, Sebagh M, Reynes M, Bismuth H. Low incidence of chronic rejection in patients experiencing histological acute rejection without simultaneous impairment in liver function tests. Transplant Proc. 1995;27(1):1142. [PubMed] [Google Scholar]
- 24.Batts KP, Moore SB, Perkins JD, Wiesner RH, Grambsch PM, Krom RA. Influence of positive lymphocyte crossmatch and HLA mismatching on vanishing bile duct syndrome in human liver allografts. Transplantation. 1988;45(2):376. doi: 10.1097/00007890-198802000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Donaldson PT, Alexander GJ, O’Grady J, et al. Evidence for an immune response to HLA class I antigens in the vanishing-bileduct syndrome after liver transplantation. Lancet. 1987;1(8539):945. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 26.Donaldson P, Underhill J, Doherty D, et al. Influence of human leukocyte antigen matching on liver allograft survival and rejection: “the dualistic effect.”. Hepatology. 1993;17(6):1008. [PubMed] [Google Scholar]
- 27.Devlin JJ, O’Grady JG, Tan KC, Calne RY, Williams R. Ethnic variations in patient and graft survival after liver transplantation: identification of a new risk factor for chronic allograft rejection. Transplantation. 1993;56(6):1381. doi: 10.1097/00007890-199312000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Anand AC, Hubscher SG, Gunson BK, McMaster P, Neuberger JM. Timing, significance, and prognosis of late acute liver allograft rejection. Transplantation. 1995;60(10):1098. doi: 10.1097/00007890-199511270-00007. [DOI] [PubMed] [Google Scholar]
- 29.Demetris AJ, Markus BH, Esquivel C, et al. Pathologic analysis of liver transplantation for primary biliary cirrhosis. Hepatology. 1988;8(4):939. doi: 10.1002/hep.1840080439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi M, Keeffe EB, Krams SM, et al. Allograft rejection after liver transplantation for autoimmune liver diseases. Liver Transpl Surg. 1998;4(3):208. doi: 10.1002/lt.500040313. [DOI] [PubMed] [Google Scholar]
- 31.Halloran PF, Melk A, Barth C. Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol. 1999;10(1):167. doi: 10.1681/ASN.V101167. [DOI] [PubMed] [Google Scholar]
- 32.Paul LC. Antibodies and chronic organ graft rejection. Ann Transplant. 1997;2(2):46. [PubMed] [Google Scholar]
- 33.Tullius SG, Nieminen M, Qun Y, et al. Synergistic mechanisms of alloantigen-dependent and independent events in chronic graft rejection. Transplant Proc. 1998;30(5):2411. doi: 10.1016/s0041-1345(98)00671-x. [DOI] [PubMed] [Google Scholar]
- 34.Yersiz H, Shaked A, Olthoff K, et al. Correlation between donor age and the pattern of liver graft recovery after transplantation [see comments] Transplantation. 1995;60(8):790. [PubMed] [Google Scholar]
- 35.Wiesner RH, Demetris AJ, Belle SH, et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998;28(3):638. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 36.Oguma S, Zerbe T, Banner B, Belle S, Starzl TE, Demetris AJ. Chronic liver allograft rejection and obliterative arteriopathy: possible pathogenic mechanisms. Transplant Proc. 1989;21((1 Pt 2)):2203. [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JG, Walmsley MW, Moy JV, et al. Differential effects of cyclosporin A and tacrolimus on the production of TGF-beta: implications for the development of obliterative bronchiolitis after lung transplantation. Transpl Int. 1998;11 (suppl 1):S325. doi: 10.1007/s001470050489. [DOI] [PubMed] [Google Scholar]
- 38.Paul LC. Functional and histologic characteristics of chronic renal allograft rejection. Clin Transplant. 1994;8((3 Pt 2)):319. [PubMed] [Google Scholar]
- 39.Kasiske BL, Kalil RS, Lee HS, Rao KV. Histopathologic findings associated with a chronic, progressive decline in renal allograft function. Kidney Int. 1991;40(3):514. doi: 10.1038/ki.1991.240. [DOI] [PubMed] [Google Scholar]
- 40.Kasiske BL. Clinical correlates to chronic renal allograft rejection. Kidney Int Suppl. 1997;63:S71. [PubMed] [Google Scholar]
- 41.Tullius SG, Hancock WW, Heemann U, Azuma H, Tilney NL. Reversibility of chronic renal allograft rejection: critical effect of time after transplantation suggests both host immune dependent and independent phases of progressive injury. Transplantation. 1994;58(1):93. [PubMed] [Google Scholar]
- 42.Forbes RD, Zheng SX, Gomersall M, Guttmann RD. Irreversible chronic vascular rejection occurs only after development of advanced allograft vasculopathy: a comparative study of a rat cardiac allograft model using a retransplantation protocol. Transplantation. 1997;63(5):743. doi: 10.1097/00007890-199703150-00022. [DOI] [PubMed] [Google Scholar]
- 43.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 44.Starzl TE. Experience in hepatic transplantation. Philadelphia: W.B. Saunders; 1969. [Google Scholar]
- 45.Demetris AJ, Murase N, Rao AS, Starzl TE. The role of passenger leukocytes in rejection and “tolerance” after solid organ transplantation: a potential explanation of a paradox. In: Touraine JL, et al., editors. Rejection and tolerance. Netherlands: Kluwer Academic Publishers; 1994. p. 325. [Google Scholar]
- 46.Sakamoto T, Ye Q, Lu L, Demetris AJ, Starzl TE, Murase N. Donor hematopoietic progenitor cells in nonmyeloablated rat recipients of allogeneic bone marrow and liver grafts. Transplantation. 1999;67(6):833. doi: 10.1097/00007890-199903270-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151(5):1273. [PMC free article] [PubMed] [Google Scholar]