Abstract
Rationale
While it is well documented that substance users exhibit attentional bias toward addiction-related stimuli, the exact mechanism remains unclear.
Objectives
To differentiate between distinct aspects of attentional allocation in the smoking-cue attentional bias observed in smokers.
Methods
Active smokers (AS) and non-smoking controls completed spatial cueing tasks with pairs of smoking and neutral pictorial cues to measure attentional capture, and an attentional blink task with either a smoking or neutral image appearing behind the first target (T1) to measure aspects of attention separate from capture. In addition, we tested groups of sports enthusiasts, and non-enthusiasts in corresponding tasks replacing smoking images with sports-related images to address the possibility that effects found in the smoking study were due simply to greater stimulus familiarity.
Results
Smoking cues reflexively capture smokers' attention, as AS showed a greater bias toward smoking cues in short stimulus-onset asynchrony (SOA; the time between the onset of two stimuli) trials, but not in trials with a longer SOA. These effects represent a facilitation of responding to smoking- versus neutral-cued targets, and were absent in the sports control task. The attentional blink effects were similar in the smoking- and sports-cue experiments: the special T1 resulted in better detection of the second target for the smokers and sports enthusiasts.
Conclusions
Stimulus familiarity may contribute to some aspects of attentional bias in regular nicotine users, but selective quick capture of attention by smoking cues may be nicotine-habit specific.
Keywords: Addiction, Attention, Attentional blink, Attentional capture, Inhibition of return, Nicotine, Spatial cueing, Substance abuse, Visual
Introduction
Addictive disorders involve powerful urges to use a substance despite likely unfavorable outcomes. Such urges may be triggered by exposure to addiction-related sensory cues. For example, smoking urges may be triggered by the sight of cigarettes, smoking paraphernalia, or others smoking. Laboratory-based data suggest that such cue-triggered urges result in part from abnormal allocation of attention to drug cues (Franken et al. 2000; Mogg et al. 2003; Field et al. 2005a, 2009; Attwood et al. 2008). This effect occurs across a wide variety of addictive disorders including those involving nicotine (Waters and Feyerabend 2000; Ehrman et al. 2002; Mogg et al. 2003), alcohol (Stetter et al. 1995; Townshend and Duka 2001; Field et al. 2004, 2005b), cannabis (Jones et al. 2002, 2003; Field et al. 2006), cocaine (Copersino et al. 2004; Hester et al. 2006), opiates (Franken et al. 2000; Lubman et al. 2000), caffeine (Yeomans et al. 2005), and even gambling (McCusker and Gettings 1997; Boyer and Dickerson 2003). The frequency with which attentional bias (AB) toward addiction-related stimuli is observed, together with mounting evidence suggesting that the strength of this bias predicts relapse risk (Cox et al. 2002, 2007; Marissen et al. 2006; Streeter et al. 2008), craving levels (Franken et al. 2000; Mogg et al. 2003; Field et al. 2005a, 2009; Attwood et al. 2008), and drug of choice consumption behavior (Waters and Feyerabend 2000; Field and Eastwood 2005) highlights the importance of further investigating such AB.
AB towards drug cues has been demonstrated using a variety of testing paradigms, such as modified Stroop tasks (Stetter et al. 1995; Sharma et al. 2001; Hester et al. 2006) and change blindness paradigms (Jones et al. 2002, 2003, 2006). However, perhaps the most commonly employed paradigms are spatial cueing tasks (Posner et al. 1984), in which simultaneously presented visual cues (one addiction-related and one neutral) are followed by a target at one of the locations. Studies of addiction-related AB with spatial cuing tasks have typically employed a variant known as the “dot-probe” task, in which the targets consist of dots or asterisks (Lubman et al. 2000; Townshend and Duka 2001; Ehrman et al. 2002; Bradley et al. 2003; Mogg et al. 2003, 2005; Duka and Townshend 2004; Yeomans et al. 2005; Noel et al. 2006; but c.f. Bradley et al. 2003, 2004; Field et al. 2004, 2005b, 2006). Most of these tasks have required participants to report target locations (Lubman et al. 2000; Townshend and Duka 2001; Ehrman et al. 2002; Duka and Townshend 2004; Yeomans et al. 2005; Noel et al. 2006), while others have required participants to report some target attribute (Mogg et al. 2003, 2005; Bradley et al. 2004; Field et al. 2004, 2005b, 2006). These spatial cueing-based studies have generally found that substance dependent individuals respond more quickly to targets appearing in the previous location of a drug-related stimulus than to those appearing at the previous location of a neutral stimulus. However, the nature of this AB is not yet clear.
As initially explored by Mogg and Bradley (2002), different tasks can measure distinct aspects of attentional allocation toward drug cues; drug cue bias may reflect (1) selective attentional capture, in which attention is automatically directed to a drug cue's location, (2) extended hold of attention, in which attention fails to rapidly move on after landing on a drug cue, or (3) generalized heightened vigilance and arousal of attention, in which drug cues induce an overall efficiency in stimulus processing (as in the “alerting” network discussed by Fan et al. 2002). In the present study, we investigated several aspects of attentional allocation by using complementary attentional task types within the same participants. The first task type is a spatial cueing task, which provides a measure of quick attentional capture when using a short (≤300 ms) time between the onset of the cue and the onset of the target (cue–target stimulus onset asynchrony; SOA). Our first two experiments compared responding to cue-congruent and –incongruent targets using one short (200 ms) and one long (550 ms) SOA. Experiment 3 used a single SOA (200 ms) but included trials with two neutral cues to distinguish between two possible explanations for faster reaction time (RTs) in smoking-congruent trials: facilitation of attentional capture by smoking cues on congruent trials or prolonged attentional hold by the smoking cues on incongruent trials (Koster et al. 2004). The second task type is a modified attentional blink paradigm, which can provide a measure of attentional hold or a general arousal of attention. The phenomenon of decreased detection of a second target (T2) when it follows a first target (T1) in close temporal proximity in a rapid visual stimulus stream is termed an attentional blink (Broadbent and Broadbent 1987; Raymond et al. 1992; Chun and Potter 1995). The attentional blink effect is diminished in substance users when T2 is drug-related (Liu et al. 2008; Tibboel et al. 2009), but the effects of manipulations of T1 are unknown. If a drug-related T1 increases or prolongs the hold of attentional resources, one would predict a larger or longer attentional blink. Alternatively, if a drug-related T1 induces hyper-vigilance of attention, one would predict attenuation of the attentional blink. Here, we tested these possibilities by comparing smoking and neutral image T1s. Our strategy of testing the same participants with both the spatial cueing and attentional blink tasks allows assessment of the relationship between attentional capture and other attentional effects in nicotine users.
Stimulus familiarity can affect attentional allocation (Chanon and Hopfinger 2008; Parks and Hopfinger 2008), and an unsettled issue is whether addiction-related AB is specifically associated with addictive processes or rather reflects a more general stimulus salience due to extensive familiarity with drug-related stimuli. Here, we addressed the contribution of stimulus familiarity in a control experiment in which sports enthusiasts and non-enthusiasts completed similar tasks using sports-related images as the salient cues.
Experimental procedures
Experiment 1
Participants
Active smokers (AS; n=23) and non-smokers (NS; n=23), were recruited from the University of North Carolina at Chapel Hill (UNC) Introductory Psychology subject pool and from the campus and surrounding community (see Table 1 for demographic data). AS participants were recruited based on self-report of smoking an average of ≥5 cigarettes/ day (mean=13.9; range: 5–22.5). These participants arrived for testing an average of 96 min after smoking (range: 1– 840 min). No explicit instructions were given regarding smoking leading up to study prior to participation. Occasional and ex-smokers were excluded from the NS group. NS participants reported having never regularly smoked in the past. All subjects were between 18 and 40 years of age, free of neurological and psychiatric disease and psychoactive drugs, excepting moderate caffeine or alcohol intake. Subjects were compensated for their participation. All participants gave informed consent and all procedures were approved by the UNC Office of Human Research Ethics.
Table 1.
Demographic data
| Experiment 1: | ||||
| AS (n=23) | NS (n=23) | t(44) | p | |
| Age | 26.0 | 22.9 | 1.887 | NS |
| Sex (% male) | 65.2 | 39.1 | NS* | |
| Ethnicity (% white) | 82.6 | 56.5 | NS* | |
| Experiment 2: | ||||
| SE (n=17) | NE (n=17) | t(32) | p | |
| Age | 21.5 | 22.9 | 0.934 | NS |
| Sex (% male) | 94.1 | 41.2 | 0.001* | |
| Ethnicity (% white) | 82.4 | 64.7 | NS* | |
| Experiment 3: | ||||
| AS (n=20) | NS (n=21) | t(39) | p | |
| Age | 26.0 | 23.8 | 1.218 | NS |
| Sex (% male) | 60.0 | 38.1 | NS* | |
| Ethnicity (% white) | 75.0 | 33.3 | 0.012* |
Reported p values represent the results of unpaired two-tailed comparisons between groups.
AS active smokers, NS non-smokers, SE sports enthusiasts, NE non-enthusiasts
p value represents results of a χ2 test
Materials and procedures
Prior to completing the tasks, participants completed questionnaires regarding smoking and sports watching habits including questions from the smoking section of the World Health Organization's MONICA project (Pekkanen et al. 1992); additionally, participants rated current cigarette craving on a scale of 1 (no craving) to 10 (most intense craving ever experienced). This paperwork took ~20 min to complete, thus each participant had been without nicotine for ≥20 min prior to completing the behavioral tasks. Behavioral tasks were implemented in E-Prime 1.2 (PST Inc., Pittsburgh, PA) and presented on a color LCD screen in a darkened room. Subjects used a manual keypad for response selection and were given a brief practice session immediately prior to beginning each task. Task order was counter-balanced across participants.
Spatial cueing task
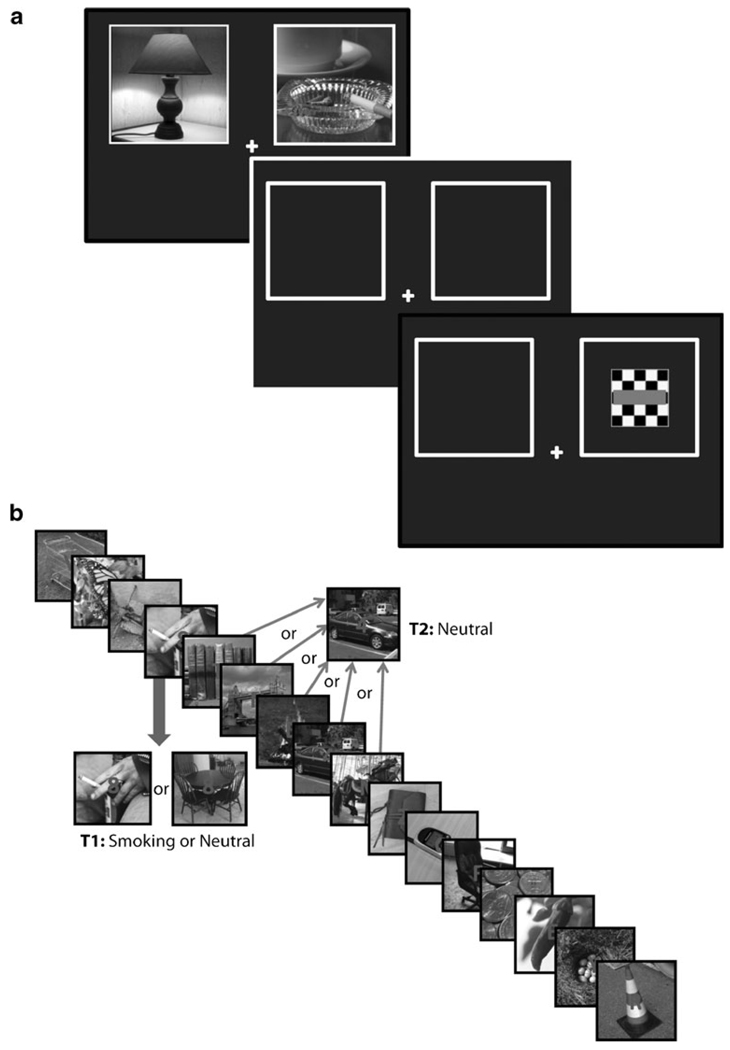
Participants completed four blocks of 120 trials each. In each trial, two grayscale images (11.1°× 9.0°) appeared, one on each side of a fixation cross (Fig. 1a). Grayscale images were used in order to avoid a significant confound to our results due to the high salience of color in visual processing and the potential difficulty in equating the color content of smoking versus neutral cues. Although grayscale images may reduce our tasks' ecological validity, direct comparisons of target detection with grayscale versus color images report equivalent performance (Fabre-Thorpe et al. 1998; Delorme et al. 2000). Moreover, as participants were instructed to maintain fixation on the cross throughout the experiment, foveal (i.e., color) vision was relatively less engaged by the stimuli. While our use of grayscale images differs from previous addiction-related AB studies, this experimental design is in keeping with the broader field of visual attention (e.g., O'Craven et al. 1999; Corbetta et al. 2005; Bar et al. 2006), in which color images are more rarely employed unless the effects of color are specifically under investigation (e.g., Bonnel and Prinzmetal 1998; Reeves et al. 2005). The maintenance of central fixation means that we were exploring covert attentional orienting, although covert effects are comparable to overt attention effects (Bradley et al. 2000).
Fig. 1.
Schematic depictions of experimental trials in experiment 1. For experiment 2, smoking images were replaced by sports-related images. a One spatial cueing trial. A smoking and neutral cue pair appears for either 150 or 500 ms, followed by an ISI of 50 ms, and then a checkerboard target for 200 ms. The time between trials varied randomly between 1, 2, and 3 s. In this trial, the target appears in the location of the smoking cue. The bar within the checkerboard appears red in each trial. b One attentional blink trial. T1 occurred in position 4, 5, 6, 7, or 8, and T2 occurred at a lag of 1, 2, 3, 4, or 5 stimuli after T1. The figure depicts a trial in which T1 occurred as the 4th stimulus in the sequence. Numbers and letters in the middle of each image appeared in blue
Cues were presented for either 150 or 500 ms (ratio 1:1); stimulus duration was pseudo-randomly ordered. Following a 50-ms inter-stimulus interval (ISI), a target appeared for 200 ms. The target was a black and white checkerboard pattern with a red line through the middle (3.6°×3.6°). Participants reported left or right target location with a left or right button press. The inter-trial interval varied randomly between 1, 2, and 3 s. See Fig. 1a for schematic trial depiction. The long and short SOA trials were intermixed within blocks to reduce the likelihood of participants forming temporal expectancies for the targets, leading to anticipatory responses that could differently affect orienting toward each cue type (Hopfinger and Mangun 1998). Including both short and long display durations was a critical manipulation based on classic studies of reflexive attentional capture, which suggest that reflexive capture occurs with short cue–target SOAs, but that with long SOAs, attention has moved away from the reflexively attended cue by the time the target appears (Posner and Cohen 1984). With 50 ms between cue offset and target onset, a 150-ms cue duration gives a 200-ms SOA, which is well below the SOA where capture effects start to diminish (~300 ms). A pattern of results in which faster RTs for smoking-congruent targets are seen in short but not long SOA trials would suggest reflexive capture of attention by smoking stimuli. In each trial, one image depicted smoking-related content, while the other depicted neutral content. Left or right position of the smoking images was pseudorandomly ordered across trials (ratio 1:1). Each image was randomly drawn from a set of 20 smoking or 45 neutral images (with more neutral stimuli due to more frequent neutral images in the attention blink task described below); selection of each image was independent for each trial. Stimuli were analyzed with respect to their spatial frequency content to ensure the sets did not differ in terms of basic visual properties. On measures of both the spectral peak (neutral: 0.0204, smoking: 0.0205, t(63)=–0.067, p=0.95) and spectral width (neutral: 54.81, smoking: 54.01, t(63)=0.183,p=0.86), the two stimulus sets did not differ. These are objective, quantitative measures of image complexity and provide an indirect measure of brightness within the images. While we did not measure the mean brightness of images directly, differences in brightness should not affect attentional capture (Jonides and Yantis 1988). Additionally, stimulus sets were matched on percentage of images containing human faces and hands.
One key difference between our task and those used in previous drug-cue spatial cueing studies is the fixation point that remains on the screen throughout each trial. This is to eliminate confounding contributions of automatic, reflexive attention unrelated to the image cues. In particular, both sudden onsets and offsets capture attention (Theeuwes 1991; Pratt and McAuliffe 2001; Hopfinger and Maxwell 2005; Hopfinger and West 2006). Thus, sudden fixation cross offset could mask spatial shifts in attention to the smoking cues, especially in short SOA trials. More specifically, if the fixation cross disappeared at target onset, attention would likely be drawn to the screen's center, competing with attentional shifts to the smoking cues.
Attentional blink task
Participants completed three blocks of 50 trials each. Each trial consisted of a rapid serial central presentation of 16 grayscale images (11.1°×9.0°) for 80 ms each, with 0-ms ISI (same sets used in the cueing task). Superimposed on each image was a blue, capitalized letter or numeral. Participants were instructed to ignore the letters and detect the numerals in each trial (Fig. 1b). The numerals 0, 1, and 5, and the letters O, I, and S were omitted due to similar appearance. There were two target numerals in each trial (T1 and T2). At trial end, participants reported the numerals seen via a 10-key pad. T1 was superimposed on either a smoking-related or a neutral image (1:1 ratio); T2 always appeared on a neutral image. The lag between T1 and T2 varied between one (T2 immediately following T1) and five.
Data analysis
Spatial cueing
RT values were calculated for 200- and 550-ms cue-target SOA trials. We quantified bias for smoking images versus neutral images according to this equation:
| (1) |
where RTN and RTS are the mean RT to targets appearing in the location of neutral and smoking images, respectively. Data reported for correct trials only; average accuracy was 98.6±2.1%.
Attentional blink
We calculated the accuracy of T2 responses on trials in which the T1 response was correct (T2|T1), separated by T2 lag, and by T1 type (neutral or smoking-related). We calculated AB for smoking images versus neutral images according to this equation:
| (2) |
where ACCS and ACCN are T2|T1 for smoking and neutral T1's, respectively. A positive number would support the hypothesis that smoking images promote attentional vigilance, while a negative number would suggest that smoking images draw more sustained processing by attention systems.
General
Single factor comparisons between subject groups employed unpaired two-tailed t tests for continuous variables and χ2 tests for categorical variables. Multifactorial comparisons employed mixed repeated measures analyses of variance (ANOVA; SPSS), with group as a between subjects factor. Where sphericity assumptions were violated, a Greenhouse–Geisser correction was applied. Post hoc paired comparisons were performed where indicated using two-tailed t tests. To ensure the validity of parametric statistical tests, when data were not normally distributed, an arcsine-root transformation was applied prior to statistical tests. To estimate which demographic and psychometric variables had the greatest predictive value for the smoking-cue effects on AB, we carried out linear multiple regression analyses in SPSS. For each multiple regression analysis, we entered variables stepwise, divided into two blocks. The blocks were as follows: block 1—age, sex; block 2—average number of cigarettes/day, time since last cigarette, craving rating (1–10), estimated time to next cigarette, and years since smoking onset.
Experiment 2
Participants
Recruiting was as for EXPERIMENT 1, except that rather than AS and NS, EXPERIMENT 2 included sports enthusiasts (SE; n = 17) and non-enthusiasts (NE; n=17; see Table 1 for demographic data). Group assignment was based on scores from a lab-developed questionnaire about sports viewing habits (included in the online Supplementary material); football and basketball scores were weighted more heavily, as the experimental stimuli featured those sports. The top third of scorers on the scale (range: 146– 239) was recruited to the SE group, and the bottom third (range: 24–75) was recruited to the NE group. On average, the SE group rated their sports watching enjoyment at 8.9 (on a scale of 1–10), versus 3.4 for the NE group (t(32)= 10.75, p<0.001); the SE group reported watching an average of 12.6 h of sports per week, versus 1.7 h for the NE group (t(32)=9.82, p<0.001). Compensation was as for EXPERIMENT 1.
Materials and procedures
Materials and procedures were identical to those for EXPERIMENT 1, except that salient cues depicted football and basketball content rather than smoking content. The sports stimulus set was also analyzed with respect to spatial frequency content to ensure that it did not differ from the neutral set in terms of basic visual properties. The neutral and sports stimulus sets did not differ in terms of spectral peak (Neutral: 0.0204, Sports: 0.0212, t(63)=0.598, p=0.55) or spectral width (Neutral: 54.81, Sports: 52.26, t(63)=0.478, p=0.63).
Data analysis
Approach was as for EXPERIMENT 1, with the following modifications. The blocks for multiple regression analyses were as follows: block 1—age, sex, and group (SE or NE); block 2—self-reported ratings (scale: 1–10) of: (1) athleticism, (2) live sports watching enjoyment, (3) TV sports watching enjoyment, (4) hours of sports watched on TV by season, (5) hours sports watched live by season, and (6–12) ratings of how closely the participants follow different sports including: professional football, college football, professional basketball, college basketball, professional hockey, Major League Baseball, and college baseball (scale:1–10). RT calculations included correct trials only; average accuracy was 98.8±0.2%.
Experiment 3
Participants
Participants were recruited to participate in a functional magnetic resonance imaging (fMRI) study (AS, n=12; NS, n=12; fMRI results reported elsewhere) or to complete the same task outside of the fMRI scanner (AS, n=8; NS, n=9; see Table 1 for demographic information on all participants). Recruitment criteria were as for EXPERIMENT 1, with the exception that participants recruited for fMRI scanning had no contraindications to fMRI. The AS group smoked an average of 11.6 cigarettes/day (range: 5–20).
Materials and procedures
Participants completed a spatial cueing task as in EXPERIMENT 1 with three modifications: (1) in addition to smoking-congruent and smoking-incongruent trials, trials with two neutral cues preceding the target were included, (2) the target screen included one dot in one image location, and two dots in the other location; participants reported the location of the two dots, and (3) only the short (200 ms) SOA was included.
Data analysis
Approach was the same as for EXPERIMENT 1, omitting multiple regression analysis.
Results
Experiment 1 (smokers versus non-smokers)
Spatial cueing
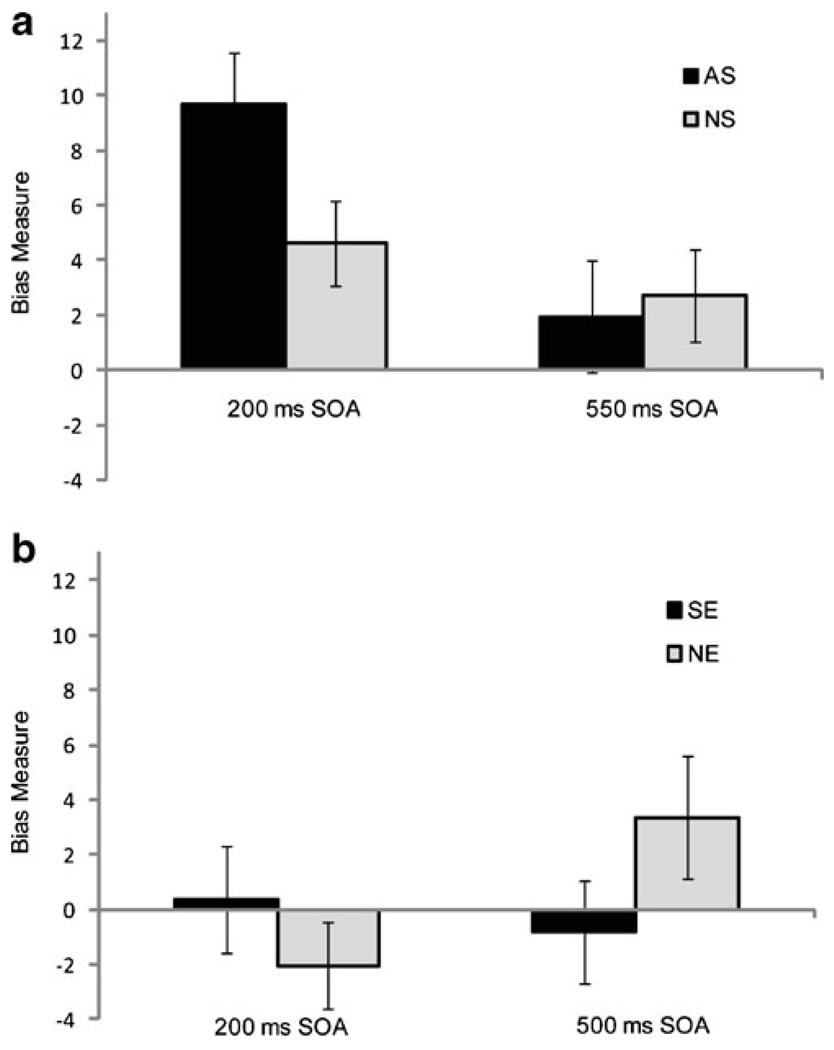
A mixed model ANOVA taking RT as the dependent measure indicated no significant main effect of subject group (F(1,44)=1.24, p=0.27), suggesting that both groups performed the task equally well. We did detect a significant main effect of cue–target SOA (F(1,44)=17.18, p<0.001), with faster RTs to targets following short (358±45 ms) versus long (367±53 ms) SOAs. We also found a significant main effect of cue type (F(1, 44)=18.27, p< 0.001), such that RTs were longer for smoking-incongruent trials (365±50 ms) versus smoking-congruent trials (360± 48 ms). Most importantly, we found a significant three-way interaction between cue type, SOA, and group (F(1,44)= 44.00, p=0.018). To determine the source of this interaction, we calculated a cue type bias measure (RT Bias Index; Eq. 1), and evaluated the effects of SOA and subject group on cue bias using a mixed model ANOVA. This analysis revealed a significant main effect of SOA (F(1,44)=16.41, p<0.001; Fig. 2a) due to a significantly greater smoking cue bias with a 200 ms cue–target SOA than with a 550-ms SOA (7.15 ms versus 2.34 ms; Fig. 2a), and a significant SOA×group interaction (F(1,44)=6.08, p=0.018). Post hoc t tests for each SOA suggest that this interaction is based on greater AB toward the smoking cues in the AS group at the 200-ms SOA (AS: 9.69 ms, NS: 4.61 ms; t(45)=2.11, p= 0.040; Fig. 2a), but not at the 550-ms SOA (AS: 1.95 ms, NS: 2.73 ms; (t(45)=0.30, p=0.77); Fig. 2a). These findings provide evidence that smoking cues selectively capture attention at a pre-volitional level in active smokers.
Fig. 2.
Reaction time bias measures from the spatial cueing paradigm. a Experiment 1. Non-smokers (NS) showed a small bias toward smoking cues for both stimulus onset asynchronies (SOAs), while smokers (AS) showed a stronger bias to smoking cues in the 200-ms SOA condition, and a small bias in the 550-ms SOA condition. A mixed model repeated measures ANOVA demonstrated a significant SOA × subject group interaction (F(1,44) =6.08, p=0.018). b Experiment 2. Familiarity with sports-related images had no effect on target reaction times in either sports enthusiasts (SE) or non-enthusiasts (NE). A mixed model repeated measures ANOVA found no significant main effects or interactions
Multiple linear regression analysis of the RT Bias Index (Eq. 1) tested whether demographic or smoking habit severity factors significantly predicted the cue type effect on RT in AS. The sole factor predicting AB magnitude for the 200-ms SOA was average number of cigarettes smoked daily, with heavier smokers showing a larger bias toward smoking cues (t(22)=2.25, p=0.038; Table 2). Magnitude of the 500-ms SOA bias was predicted by the time since last cigarette, with longer abstinence predicting smaller bias (t(22)=4.35, p<0.001; Table 2).
Table 2.
Stepwise regression results
| B | SE B | β | ||
|---|---|---|---|---|
| Experiment 1: | ||||
| 200-ms SOA RT bias | ||||
| Model 1: | Constant | 1.71 | 7.09 | |
| Cigarettes/day | 0.88 | 0.39 | 0.49* | |
| 550-ms SOA RT bias | ||||
| Model 1: | Constant | 0.79 | 6.60 | |
| TSL cigarette | −0.04 | 0.01 | −0.77*** | |
| AB lag 2 bias | ||||
| Model 1: | Constant | 0.218 | 0.168 | |
| TTN cigarette | −0.001 | 0.000 | −0.510* | |
Results from multiple linear regression analysis of predictors of smoking cue effect on attentional bias
AB attentional blink, B beta value, β standardized beta, RT reaction time, SE B beta value standard error, SOA stimulus onset asynchrony, TSL time since last, TTN time to next
p<0.05
p<0.001
Attentional blink
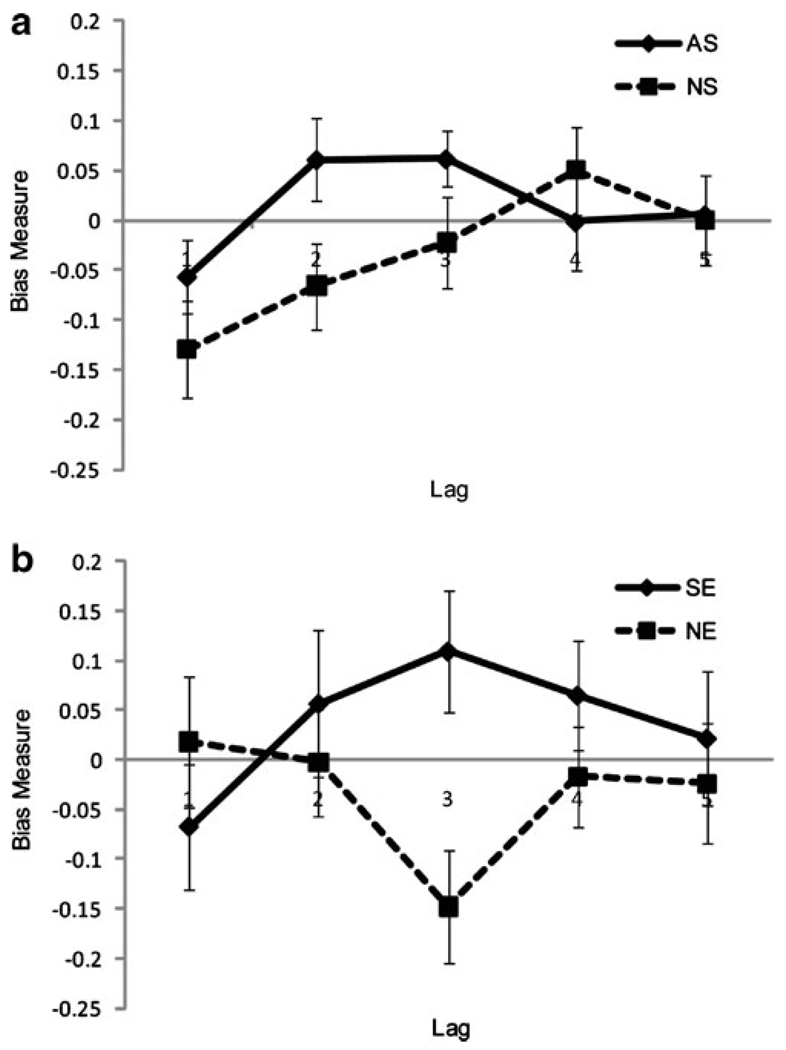
On average, participants responded correctly to 79.4±1.9% of T1s. The dependent measure of interest was T2|T1 for each of five T1–T2 lags. A mixed model ANOVA indicated no significant main effect of subject group (F(1,44)=3.36, p=0.07) or cue type (F(1,44)=0.59, p=0.47) on T2 detection. However, as expected for an attentional blink paradigm, we observed a significant effect of T2 lag (F(4,41)=11.98 p<0.001); T2 detection decreased from lag 1 to lag 2, then increased over the remaining lags (lag 1: 91.4% lag 2: 80.2%, lag 3: 81.9%, lag 4: 83.0%, lag 5: 85.6%). These data suggest that the normal attentional blink pattern was obtained, including lag-1-sparing (Raymond et al. 1992). Taking the cue type bias (Blink Bias Index; Eq. 2) as our dependent measure, an ANOVA revealed a significant main effect of lag (F(4,41)=7.13 p=0.011). There was no significant effect of group (F(4,41)=3.10 p=0.09), and a trend towards a group×lag interaction (F(4,41)=3.37 p= 0.06). At the attentional blink peak (lag 2) there was a significant difference between groups (t(44)=2.11, p=0.043; Fig. 3a). Bias effects in the attentional blink task did not significantly correlate with those in the spatial cueing task (minimum p=0.14).
Fig. 3.
T2|T1 accuracy bias measures from the attentional blink paradigms. a Experiment 1. Non-smokers (NS) showed a slight bias toward extended attentional processing of T1's superimposed on smoking-related images, while smokers (AS) showed the opposite result. b Experiment 2. Non-enthusiasts (NE) showed a slight bias toward extended attentional processing of T1's with sports-related images in the background, while sports enthusiasts (SE) showed the opposite result, showing a pattern of responding very similar to that of smokers in Experiment 1 (a)
Multiple linear regression analysis of the AS bias data was used to determine whether any of the demographic or self-reported smoking habit severity factors had significant predictive value in terms of the cue type effect on the attentional blink. The only factor predicting AB magnitude at lag 2 was the estimated time to smoke the next cigarette, where those planning to smoke sooner exhibited a larger bias measure (that is, more correct T2 responses following smoking T1s versus neutral T1s; t(22)=2.22, p=0.040; Table 2).
Experiment 2 (sport enthusiasts versus non-enthusiasts)
Spatial cueing
Unlike EXPERIMENT 1, a mixed model ANOVA for RT found no significant main effects of cue type, cue–target SOA, or subject group (minimum p=0.14). Calculated AB toward sports images for each group and cue–target SOA are shown in Fig. 2b. These results suggest that the effects observed in EXPERIMENT 1 are not likely to simply reflect stimulus familiarity in the AS group. Given the unequal sex ratio between SE and NE groups (Table 1), we separately investigated sex as a factor, finding that females responded more slowly to targets following the 550 ms SOA versus the 200 ms SOA resulting in a significant sex× SOA interaction (female difference: 10.88 ms, male difference: −2.38 ms; F(1,32)=6.48, p=0.016). However, there was no significant main effect of sex on RT, and no sex×cue type interaction (minimum p=0.13).
Attentional blink
On the average, participants responded correctly to 76.9± 1.8% of T1 targets. A mixed model ANOVA indicated no significant main effect of group (F(1,32)=1.65, p=0.21) or cue type (F(1,32)=0.003, p=0.96) on T2 detection. However, as for EXPERIMENT 1, we did observe a significant effect of T2 lag on T2 detection accuracy (F(4,29)=15.34, p< 0.001; lag 1: 94.4%, lag 2: 82.5%, lag 3: 84.6%, lag 4: 87.2%, lag 5: 88.8%). An ANOVA taking the blink bias index (Eq. 2) as the dependent measure revealed a significant lag×group interaction (F(4,29)=2.53, p=0.044).
Independent t tests with the bias measures revealed a significant group difference (t(32)=3.09, p=0.004; Fig. 3b) at lag 3 in which the SE group's attention was more available for T2's following sports-related T1s compared to neutral images; the NE group showed the opposite pattern. These results are similar to the difference found between smokers and non-smokers in EXPERIMENT 1 (Fig. 3a). Multiple Regression analysis revealed that group was a significant predictor of lag 3 bias, an effect that was independent of the sex differences between groups.
Experiment 3 (spatial cueing effect of smoking cues: facilitation versus distraction)
Spatial cueing task: smoking-neutral versus neutral-neutral trials
A mixed model ANOVA found a significant cue type×group interaction (F(2,38)=6.952, p=0.003). Post hoc t tests revealed that AS responded significantly faster to smoking-congruent targets than to smoking-incongruent targets (t(19)=4.398, p< 0.001) or to targets in trials with two neutral cues (t(19)= 2.180, p=0.042); this effect was not observed in the NS group (congruent versus incongruent: t(20)=0.956, p=0.35; congruent versus two neutral: t(20)=0.595, p=0.56). These results indicate that the effects found in the cueing task in EXPERIMENT 1 are likely due to facilitated attentional capture rather than to attentional hold by the smoking-related images. Given the unequal ethnicity ratio between the AS and NS groups, we separately investigated ethnicity as a factor. There was no significant main effect of ethnicity and no ethnicity×cue type interaction (minimum p=0.28).
Discussion
Although AB toward addiction-related stimuli is commonly reported, how the brain's attentional systems are affected to render such bias has remained unclear. For example, it is not clear whether this bias reflects a substance users' attention being selectively drawn towards drug-related items, or being held for longer periods and/or more extensive processing. Here, we investigated this issue using multiple attention tasks within subjects. Our data indicate that active nicotine use is associated with selective capture of attention by smoking cues, an effect that is positively correlated with smoking habit severity.
Classic studies of attentional capture by a reflexive cue found that short cue–target SOAs (up to ~300 ms) produce facilitation of processing at the cued location, while longer SOAs do not, and may produce an inhibition of return (IOR), in which processing is enhanced for cue-incongruent targets (Posner and Cohen 1984; Klein 2000). Here, we found that smokers' responses to smoking-cued targets were like those in classic reflexive cueing studies, supporting the interpretation that involuntary attentional capture by cigarette cues is enhanced in smokers, as the 200-ms SOA is not sufficiently long to allow voluntarily direction of attention toward smoking cues. The positive correlation between the AB at the 200-ms SOAs and the number of cigarettes smoked per day suggests that this attentional effect provides an objective index of smoking addiction severity. This absence of reflexive attentional capture in the control experiment with sports enthusiasts, suggests that the attentional capture effect in smokers is not due simply to the familiarity of the smoking cues. We speculate that this enhanced attentional capture reflects alteration of the brain's object detection system as a result of addictive processes. The incentive-sensitization theory of addiction posits that repeated use of an addictive substance heightens the “incentive salience” of stimuli associated with using that drug, leading to increased attention toward, and processing of such stimuli (Robinson and Berridge 1993). A similar theory posits that as drug-related stimuli become associated with substance use, exposure to such stimuli increases dopamine release in corticostriatal circuitry, drawing attention to these stimuli; this heightened attention increases perception and processing of drug cues, helping to perpetuate the cycle of drug use (Franken 2003). It is important to note that smoking habit duration varied among our AS group, with a large portion of relatively novice smokers; we would expect larger group differences with more experienced smokers in the AS group. Additionally, based on the wide range of “time since last cigarette” in this study, the dependence and/or withdrawal levels within the AS group was quite variable. While this variation allowed us to use regression analysis to assess individual differences, it potentially diluted the attentional effects in our AS group, which should be considered in future studies.
The current finding of quick attentional capture by smoking cues in smokers is consistent with results from previous studies of smoking cue bias using spatial cuing tasks. While few studies have used such short cue presentations, Bradley et al. (2004) also found a greater bias towards smoking cues in smokers relative to non-smokers using a 200-ms cue. That study also found AB towards smoking cues with a 2000-ms SOA, a finding confirmed in other studies (Bradley et al. 2003; Mogg et al. 2005), which would seem to contradict our finding of no bias at a longer SOA (550 ms). However, studies of the time course of reflexive attention indicate that capture, and subsequent IOR, decays over a period of seconds and that by 1,500–2,000-ms IOR may be extinguished (Coward et al. 2004; Zhou 2008), allowing voluntary attentional processes to take over. Thus, the targets presented at the long SOA in Bradley and colleagues' study may have appeared outside the temporal window for reflexive attention and IOR. The fact that Bradley et al. (2004) observed a bias towards smoking cues using a 2,000-ms SOA suggests that additional attention factors, such as voluntary deployment of attention, may also play a role in addiction-related bias. Another spatial-cueing study providing support for quick capture of smokers' attention by smoking cues is that of Mogg et al. (2005), which found that most mildly dependent smokers more often made their first fixation to a smoking cue than did nonsmokers. Importantly, our inclusion of trials with two neutral stimuli in EXPERIMENT 3 allows us to definitively conclude that smoking cues are facilitating responses to smoking-congruent stimuli in active smokers. This latter result strongly supports the interpretation that smoking cues reflexively capture the attention of smokers.
While studies using very short or very long cue presentations provide fairly consistent evidence of smoking-cue bias in smokers, results from studies using a middle range of timings are more variable. Here, we found no AB with a cue-target SOA of 550 ms, but two studies using 500-ms cue durations found conflicting results (Bradley et al. 2003; Ehrman et al. 2002). This discrepancy likely reflects the proportion of smokers in those studies who had made repeated quit attempts. Although we did not collect information as to quit attempts in our participants, the fact that they were a largely novice group of smokers would suggest that few had made repeated quit attempts. Interest in quitting and repeated quit attempts could produce bias effects at the longer cue presentation based a greater contribution of volitional control of attention. Individuals trying to quit smoking may actively attend to smoking cues in an effort to avoid cigarettes and smoking-related situations. In addition, the other studies using a mid-range SOA used color images and a small dot or asterisk target, each of which might have contributed to differences in our results.
A potential caveat for this study is the possibility of group differences in nicotine levels during testing, given the attention-enhancing effects of nicotine (Wesnes and Warburton 1984; Koelega 1993; Stolerman et al. 1995; Foulds et al. 1996; Mancuso et al. 1999; Newhouse et al. 2004). However, the possibility that the observed group differences are due solely to differences in nicotine levels can be refuted by several arguments. First, plasma nicotine falls back to baseline levels within 10 min after smoking a cigarette (Sakurai and Kanazawa 2002); our subjects arrived for testing an average of 96 min after smoking, and testing began ~20 min after arrival. Second, our regression analyses did not identify any significant correlations between bias effects and reported time from last cigarette. Despite this evidence, it remains possible that acute nicotine played a role in the observed effects; future tests of whether acute nicotine administration, or short- or long-term abstinence modulates these attentional effects will prove informative. A related concern is the effect of caffeine consumption on attention, as cigarette smokers are reportedly more likely to consume caffeine (Istvan and Matarazzo 1984), which also affects attentional processing (Lorist et al. 1994). While caffeine would not be expected to selectively affect attention toward smoking-related cues, future studies would benefit from quantifying and controlling for caffeine use.
A few attentional blink studies have shown that a drug-related T2 can preferentially capture the attention of addicts (Liu et al. 2008; Tibboel et al. 2009); however our novel test of the effects of T1 manipulation in an attentional blink paradigm found that detection of T2 was enhanced by a smoking-related T1 for smokers or a by sports-related T1 for sports enthusiasts. While our paradigm is novel, some published studies shed light on our results. First, Most et al. (2005, 2007) have described an “attentional rubbernecking” phenomenon caused by emotional stimuli in attentional blink tasks. Specifically, processing of a target was interrupted when it appeared shortly after an emotionally charged stimulus. Similarly, Munafo et al. (2005) found that cigarette-related words presented within a rapid stream can interrupt processing of a subsequent target in some smokers. A difference between these and more typical attentional blink studies (including our own) is that the critical stimuli that interrupt target processing are irrelevant to the task (i.e., they are not targets). This difference may be functionally important, as Most and Junge (2008) recently showed that the interruption of T2 processing by emotionally charged stimuli disrupts lag-1-sparing, in which T2 detection is not impaired when it immediately follows T1. Here, in EXPERIMENT 1, in addition to lag-1-sparing, smokers more accurately detected T2s at lag 2 following a smoking T1, which is when the attentional blink usually peaks. These data would appear to support the idea that when smokers view a smoking-related T1, instead of experiencing a “rubbernecking” effect, in which their attention is preferentially held by that T1, they experience a general increase in attentional vigilance temporally extending to the detection of T2 beyond lag 1.
An alternative explanation to the increased vigilance theory is that, in our task design, the images appearing in the background are interrupting the foreground task of numeral detection, including processing of the T1 (numeral) stimulus. Supporting this interpretation is a recent study showing that visual processing resources were withdrawn from relevant foreground stimuli by an emotionally arousing image presented in the background (Muller et al. 2008). If arousal caused by the smoking images interfered with smokers' processing of the T1 numerals, then T2 processing would be expected to be enhanced following a T1 superimposed on a smoking cue, as we observed here. While the preceding interpretation of our attentional blink results could be consistent with heightened attentional capture to the background task by smoking cues, two pieces of evidence argue against that interpretation. First, the bias effects measured at each lag of the attentional blink task were not correlated with any of the bias effects on the cueing task. This suggests that our attentional blink task is measuring an aspect of attentional allocation independent of reflexive attentional capture. Second, the effects of smoking cues on smokers' attention observed in the attentional blink task were similar to those of sports cues on sports enthusiasts' attention. Specifically, at lag 3, when the attentional blink should be near its peak, sports enthusiasts showed a larger blink for T2s following neutral T1s than for those following sports-related T1s. Another possibility is that stimulus familiarity effects are playing a role in the AB measured by our attentional blink paradigm. Indeed, recent evidence suggests that cue familiarity can alter T1 processing in attentional blink paradigms (Parks and Hopfinger 2008). Thus, while the specific attentional blink paradigm used here may not provide a reliable measure of attentional hold, it seems to index some other experience-dependent measure of attentional allocation. The results of our multiple regression analysis, suggest that this aspect of attentional allocation may be related to addictive processes, as participants' reported time to next cigarette predicted the size of the observed bias effect.
In summary, the current study indicates that smokers' attention can be reflexively captured by smoking-related stimuli; however, much remains to be learned about addiction-related AB. For example, further investigation of attentional hold by smoking cues in nicotine users is needed. Moreover, the neural mechanisms of addiction-related AB remain virtually unknown. Elucidating these mechanisms via neuroimaging and behavioral pharmacology studies is particularly warranted. An open and critical question is whether medications that reduce drug craving do so in part via effects on attentional processing. The present spatial cueing paradigm may prove useful in this regard. Ultimately, laboratory-based studies testing the diminishment of the smoking-related AB in response to therapeutic interventions could prove critical for identifying novel effective treatments for addiction.
Supplementary Material
Acknowledgements
The authors have no conflict of interest to report. Each participant gave signed informed consent before any sessions were begun, and APA human subjects research guidelines were followed. The experiments were approved by the Institutional Review Board at the University of North Carolina at Chapel Hill, and comply with the current laws of the United States. This work was supported by Award Number KL2RR025746 from the National Center for Research Resources (CAB) and F32DA025442 (VWC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-010-1953-1) contains supplementary material, which is available to authorized users.
Contributor Information
Vicki W. Chanon, Email: vmwest@email.unc.edu, Department of Psychology, University of North Carolina at Chapel Hill, CB 3270, Davie Hall, Chapel Hill, NC 27599-3270, USA.
Chandler R. Sours, Department of Psychology, University of North Carolina at Chapel Hill, CB 3270, Davie Hall, Chapel Hill, NC 27599-3270, USA
Charlotte A. Boettiger, Department of Psychology, University of North Carolina at Chapel Hill, CB 3270, Davie Hall, Chapel Hill, NC 27599-3270, USA Biomedical Research Imaging Center, Bowels Center for Alcohol Studies, University of North Carolina at Chapel Hill, CB 3270 Davie Hall, Chapel Hill, NC 27599-3270, USA; Neurobiology Curriculum, University of North Carolina at Chapel Hill, CB 3270, Davie Hall, Chapel Hill, NC 27599-3270, USA.
References
- Attwood AS, O'Sullivan H, Leonards U, Mackintosh B, Munafó MR. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103:1875–1882. doi: 10.1111/j.1360-0443.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hämäläinen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E. Top-down facilitation of visual recognition. P Natl Acad Sci. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnel A-M, Prinzmetal W. Dividing attention between the color and the shape of objects. Percept Psychophys. 1998;60:113–124. doi: 10.3758/bf03211922. [DOI] [PubMed] [Google Scholar]
- Boyer M, Dickerson M. Attentional bias and addictive behaviour: automaticity in a gambling-specific modified Stroop task. Addiction. 2003;98:61–70. doi: 10.1046/j.1360-0443.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Millar NH. Covert and overt orienting of attention to emotional faces in anxiety. Cogn Emotion. 2000;14:789–808. [Google Scholar]
- Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: vigilance for cigarette-related cues in smokers. Psychol Addict Behav. 2003;17:66–72. doi: 10.1037/0893-164x.17.1.66. [DOI] [PubMed] [Google Scholar]
- Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav Pharmacol. 2004;15:29–36. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Broadbent MH. From detection to identification: response to multiple targets in rapid serial visual presentation. Percept Psychophys. 1987;42:105–113. doi: 10.3758/bf03210498. [DOI] [PubMed] [Google Scholar]
- Chanon VW, Hopfinger JB. Memory's grip on attention: the influence of item memory on the allocation of attention. Vis Cogn. 2008;16:325–340. [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. J Exp Psychol Hum Percept Perform. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Serper MR, Vadhan N, Goldberg BR, Richarme D, Chou JC, Stitzer M, Cancro R. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Res. 2004;128:209–218. doi: 10.1016/j.psychres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Tansy AP, Stanley CM, Astafiev SV, Snyder AZ, Shulman GL. A functionalMRI study of preparatory signals for spatial location and objects. Neuropsychologia. 2005;43:2041–2056. doi: 10.1016/j.neuropsychologia.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Coward RS, Poliakoff E, O'Boyle DJ, Lowe C. The contribution of non-ocular response inhibition to visual inhibition of return. Exp Brain Res. 2004;155:124–128. doi: 10.1007/s00221-003-1803-z. [DOI] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers' treatment outcome. Drug Alcohol Depend. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Cox WM, Pothos EM, Hosier SG. Cognitive-motivational predictors of excessive drinkers' success in changing. Psycho-pharmacology (Berl) 2007;192:499–510. doi: 10.1007/s00213-007-0736-9. [DOI] [PubMed] [Google Scholar]
- Delorme A, Richard G, Fabre-Thorpe M. Ultra-rapid categorisation of natural scenes does not rely on colour cues: a study in monkeys and humans. Vis Res. 2000;40:2187–2200. doi: 10.1016/s0042-6989(00)00083-3. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology (Berl) 2004;176:353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O'Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depend. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Fabre-Thorpe M, Richard G, Thorpe SJ. Rapid categorization of natural images by rhesus monkeys. NeuroReport. 1998;9:303–308. doi: 10.1097/00001756-199801260-00023. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology (Berl) 2005;183:350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol Alcohol. 2005a;40:504–510. doi: 10.1093/alcalc/agh213. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Alcohol increases cognitive biases for smoking cues in smokers. Psychopharmacology (Berl) 2005b;180:63–72. doi: 10.1007/s00213-005-2251-1. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology (Berl) 2004;176:88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Field M, Munafó MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franken IH, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroin dependence. J Psychopharmacol. 2000;14:395–400. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Mangun GR. Reflexive attention modulates processing of visual stimuli in human extrastriate cortex. Psychol Sci. 1998;9:441–447. doi: 10.1111/1467-9280.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Maxwell JS. Appearing and disappearing stimuli trigger a reflexive modulation of visual cortical activity. Brain Res Cogn Brain Res. 2005;25:48–56. doi: 10.1016/j.cogbrainres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, West VM. Interactions between endogenous and exogenous attention on cortical visual processing. Neuroimage. 2006;31:774–789. doi: 10.1016/j.neuroimage.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use—a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- Jones BC, Jones BT, Blundell L, Bruce G. Social users of alcohol and cannabis who detect substance-related changes in a change blindness paradigm report higher levels of use than those detecting substance-neutral changes. Psychopharmacology (Berl) 2002;165:93–96. doi: 10.1007/s00213-002-1264-2. [DOI] [PubMed] [Google Scholar]
- Jones BT, Jones BC, Smith H, Copley N. A flicker paradigm for inducing change blindness reveals alcohol and cannabis information processing biases in social users. Addiction. 2003;98:235–244. doi: 10.1046/j.1360-0443.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- Jones BT, Bruce G, Livingstone S, Reed E. Alcohol-related attentional bias in problem drinkers with the flicker change blindness paradigm. Psychol Addict Behav. 2006;20:171–177. doi: 10.1037/0893-164X.20.2.171. [DOI] [PubMed] [Google Scholar]
- Jonides J, Yantis S. Uniqueness of abrupt visual onset in capturing attention. Percept Psychophys. 1988;43:346–354. doi: 10.3758/bf03208805. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends Cogn Sci. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Koelega HS. Stimulant drugs and vigilance performance: a review. Psychopharmacology (Berl) 1993;111:1–16. doi: 10.1007/BF02257400. [DOI] [PubMed] [Google Scholar]
- Koster EH, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav Res Ther. 2004;42:1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Liu N, Li B, Sun N, Ma Y. Effects of addiction-associated and affective stimuli on the attentional blink in a sample of abstinent opiate dependent patients. J Psychopharmacol. 2008;22:64–70. doi: 10.1177/0269881107077804. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Snel J, Kok A, Mulder G. Influence of caffeine on selective attention in well-rested and fatigued subjects. Psychophysiology. 1994;31:525–534. doi: 10.1111/j.1469-8986.1994.tb02345.x. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, Deakin JF. Attentional bias for drug cues in opiate dependence. Psychol Med. 2000;30:169–175. doi: 10.1017/s0033291799001269. [DOI] [PubMed] [Google Scholar]
- Mancuso G, Warburton DM, Melen M, Sherwood N, Tirelli E. Selective effects of nicotine on attentional processes. Psychopharmacology (Berl) 1999;146:199–204. doi: 10.1007/s002130051107. [DOI] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- McCusker CG, Gettings B. Automaticity of cognitive biases in addictive behaviours: further evidence with gamblers. Br J Clin Psychol. 1997;36(Pt 4):543–554. doi: 10.1111/j.2044-8260.1997.tb01259.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. J Psychopharmacol. 2002;16:385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98:825–836. doi: 10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Field M, Bradley BP. Attentional and approach biases for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology (Berl) 2005;180:333–341. doi: 10.1007/s00213-005-2158-x. [DOI] [PubMed] [Google Scholar]
- Most SB, Junge JA. Don't look back: retroactive, dynamic costs and benefits of emotional capture. Vis Cogn. 2008;16:262–278. [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: cognitive control and personality in emotioninduced blindness. Psychonomic Soc Inc; 2005. pp. 654–661. [DOI] [PubMed] [Google Scholar]
- Most SB, Smith SD, Cooter AB, Levy BN, Zald DH. The naked truth: positive, arousing distractors impair rapid target perception. Cogn Emotion. 2007;21:964–981. [Google Scholar]
- Muller MM, Andersen SK, Keil A. Time course of competition for visual processing resources between emotional pictures and foreground task. Cereb Cortex. 2008;18:1892–1899. doi: 10.1093/cercor/bhm215. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC, Mackintosh B. Association of serotonin transporter genotype with selective processing of smoking-related stimuli in current smokers and ex-smokers. Nicotine Tob Res. 2005;7:773–778. doi: 10.1080/14622200500259861. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Noel X, Colmant M, Van Der Linden M, Bechara A, Bullens Q, Hanak C, Verbanck P. Time course of attention for alcohol cues in abstinent alcoholic patients: the role of initial orienting. Alcohol Clin Exp Res. 2006;30:1871–1877. doi: 10.1111/j.1530-0277.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Downing PE, Kanwisher N. fRMI evidence for objects as the units of attentional selection. Nature. 1999;401:584–587. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- Parks EL, Hopfinger JB. Hold it! Memory affects attentional dwell time. Psychon Bull Rev. 2008;15:1128–1134. doi: 10.3758/PBR.15.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkanen J, Kuulasmaa K, Evans A, Zhang M for the WHO MONICA Project. Quality assessment of smoking data in the first surveys of the WHO MONICA Project. MONICA Memo 209A. 1992 January [Google Scholar]
- Posner M, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhis D, editors. Attent perform. vol. X. Erlbaum, Hillsdale: 1984. pp. 531–556. [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J, McAuliffe J. The effects of onsets and offsets on visual attention. Psychological Research. 2001;65:185–191. doi: 10.1007/s004260100058. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Reeves A, Fuller H, Fine EM. The role of attention in binding shape to color. Vis Res. 2005;45:3343–3355. doi: 10.1016/j.visres.2005.07.041. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Kanazawa I. Acute effects of cigarettes in nondeprived smokers on memory, calculation and executive functions. Hum Psychopharmacol. 2002;17:369–373. doi: 10.1002/hup.424. [DOI] [PubMed] [Google Scholar]
- Sharma D, Albery IP, Cook C. Selective attentional bias to alcohol related stimuli in problem drinkers and non-problem drinkers. Addiction. 2001;96:285–295. doi: 10.1046/j.1360-0443.2001.96228512.x. [DOI] [PubMed] [Google Scholar]
- Stetter F, Ackermann K, Bizer A, Straube ER, Mann K. Effects of disease-related cues in alcoholic inpatients: results of a controlled “Alcohol Stroop” study. Alcohol Clin Exp Res. 1995;19:593–599. doi: 10.1111/j.1530-0277.1995.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Shoaib M. Nicotine psychopharmacology: addiction, cognition and neuroadaptation. Med Res Rev. 1995;15:47–72. doi: 10.1002/med.2610150105. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Exogenous and endogenous control of attention—the effect of visual onsets and offsets. Percept Psychophys. 1991;49:83–90. doi: 10.3758/bf03211619. [DOI] [PubMed] [Google Scholar]
- Tibboel H, De Houwer J, Field M. Reduced attentional blink for alcohol-related stimuli in heavy social drinkers. J Psychopharmacol. 2009 doi: 10.1177/0269881109106977. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology (Berl) 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychol Addict Behav. 2000;14:111–120. doi: 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. The effects of cigarettes of varying yield on rapid information processing performance. Psychopharmacology (Berl) 1984;82:338–342. doi: 10.1007/BF00427682. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Javaherian S, Tovey HM, Stafford LD. Attentional bias for caffeine-related stimuli in high but not moderate or non-caffeine consumers. Psychopharmacology (Berl) 2005;181:477–485. doi: 10.1007/s00213-005-0004-9. [DOI] [PubMed] [Google Scholar]
- Zhou B. Disentangling perceptual and motor components in inhibition of return. Cogn Process. 2008;9:175–187. doi: 10.1007/s10339-008-0207-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.