Abstract
This study examined how children use word order and animacy cues to determine the agent of the action in an on-line sentence-comprehension task. The subject group included 15 children, 5–12years old, with brain injury incurred prior to the age of 2months; 12had left hemisphere (LH) damage and 3 had right hemisphere (RH) damage. The comparison group included 141 children, 5–10 years old, who were at the appropriate grade for age. The task required children to listen to sentences composed of two noun phrases (N) that varied in terms of animacy and a verb phrase (V) and then to indicate the agent of the action. Three word orders were presented: NVN, VNN, and NNV. Measures included the proportion of trials in which the first noun was selected (choice) and reaction time. Word order and animacy significantly influenced choice. The effect of subject group approached significance for choice. Word order and age influenced reaction time. The children with LH injury and two children with RH injury showed a developmental delay in choosing the appropriate N as agent; one child with RH injury had mature responses. The overlapping performance of children with LH and RH injury suggests that delays in the development of sentence comprehension strategies are more likely related to reliance on a smaller than usual neural network rather than to congenital specialization of the LH.
Keywords: Left hemisphere, Focal injury, Brain damage, Neurological disorders, Children, Language, Language development, Sentence comprehension
1. Introduction
Aphasia, the significant loss of language abilities following acquired brain injury, is reliably associated with damage to the left hemisphere (LH). This observation forms the foundation of the classical theory that the LH is essential for adult language processing (Goodglass, 1993). Modern imaging studies have demonstrated that many brain regions, including areas of the RH, become activated during certain language tasks, but these studies have not fundamentally challenged the concept of a privileged role for the LH in adult language function (Gaillard et al., 2000; Just, Carpenter, Keller, Eddy, & Thulborn, 1996; Tzourio, Crivello, Mellet, Nkanga-Ngila, & Mazoyer, 1998).
The key developmental issues have been when, how, and why LH specialization for language occurs. The study of children with early focal brain injury provides an opportunity to address these issues; the lesions serve as a naturalistic experimental manipulation and the performance of affected children indicates the contribution of that structure to the abilities under investigation. If children with LH injury are delayed or deficient in language skills, then LH specialization presumably predated their injury. Children whose injury occurred prior to language development (from prenatal or perinatal complications or strokes acquired in the early infancy era, which we will group together as “prelinguistic injury”) provide indications about whether LH specialization was congenital and presumably genetic. If such children are extremely delayed in language development compared to normal learners and children with RH damage, the results suggest that LH specialization is congenital. If, on the other hand, children with prelinguistic LH injury have normal or near normal language skills, or are not significantly delayed compared to children with RH damage, then the implication is that specialization evolves over time and that alternative organizations can serve language abilities effectively under certain unusual conditions.
A large body of evidence shows that children with early LH lesions impinging on classical language areas of the cortex have a remarkably favorable prognosis for the development of basic communicative skills (Banker & Larrouche, 1962; Bates et al., 1997; Bates, Vicari, & Trauner, 1999; DallOglio, Bates, Volterra, Di Capua, & Pezzini, 1994; Feldman, Holland, Kemp, & Janosky, 1992; Lenneberg, 1967; Thal et al., 1991; Vargha-Khadem et al., 1997; Woods & Teuber, 1977). Their language resembles the language of other children of the same age to a greater degree than the language of adults with aphasia resembles the language of adult controls (Bates & Roe, 2001). These findings have been attributed to the plasticity of human brain structures. Evidence from various sources shows that RH participation in language functioning increases after LH injury (Booth et al., 1999, 2000; Muller et al., 1998, 1999; Rasmussen & Milner, 1977). These data suggest that language reorganized to use RH regions is adequate for functional communication, at least under many circumstances.
Many studies have described mild to moderate deficits in some language abilities in children with unilateral lesions to either hemisphere. For example, children with injury to either hemisphere may show problems in lexical comprehension and production (Aram, Ekelman, Rose, & Whitaker, 1985) and fluency (Aram, Meyers, & Ekelman, 1990). In children with prelinguistic injuries, delays in communicative development have been documented in several subdomains: prelinguistic babbling and gesturing (Marchman, Miller, & Bates, 1991; Thal et al., 1991), the growth of early vocabulary and word combinations (Bates et al., 1997; Feldman, 1994; Feldman et al., 1992; Thal et al., 1991), and development of narrative discourse skills (Reilly, Bates, & Marchman, 1998). These findings suggest that the integrity of the whole brain may be important for launching language development and serving high-level language skills such as narrative discourse.
A number of studies have asserted that verbal intelligence and language impairments are more likely and/or more severe after LH than RH damage (Aram et al., 1985, 1990; Aram & Ekelman, 1987; Aram, Ekelman, & Whitaker, 1986; Eisele & Aram, 1994; Kiessling, Denckla, & Carlton, 1983; Rankin, Aram, & Horwitz, 1981; Vargha-Khadem, Walters, & OGorman, 1985; Woods & Carey, 1978; Woods & Teuber, 1977). In the domain of intelligence, some studies find that LH damage is associated with greater deficits in verbal intelligence and RH damage with greater deficits in performance intelligence (Glass et al., 1998). However, many studies have not demonstrated selective impairments as a function of lesion side (Bates & Roe, 2001), particularly when the influence of other variables, such as the presence of seizures and use of anticonvulsant medications, is controlled (Vargha-Khadem, Isaacs, & Muter, 1994; Vargha-Khadem & Polkey, 1992). Thal and colleagues found that during the main period of vocabulary and syntactic development (18 months to 4 years), children with prelinguistic damage to posterior regions of the LH showed more severe delays in the rate of development of language production than did children with damage to other regions (Bates et al., 1997; Thal et al., 1991). However, this differential effect lasted only until the end of the preschool period (Reilly et al., 1998).
The development of syntax in the aftermath of LH damage in childhood has been a particular focus of research based on the centrality of syntax in Chomsky's prominent theories of language (Chomsky, 1965, 1972) and on the further claims of an innate and genetically determined language acquisition device through which syntax is acquired (Pinker, 1994). In support of this view of innate LH specialization, landmark and recent studies of child hemispherectomy reported that those with an LH hemispherectomy, who therefore functioned with only an RH, were more likely to experience problems interpreting passive and non-canonical word forms than were those with right hemispherectomy who therefore functioned with only an LH (Dennis, 1980; Dennis & Kohn, 1975; Dennis, Lovett, & Wiegel-Crump, 1981; Dennis & Whitaker, 1976; Stark & McGregor, 1997). All these studies have been plagued by small sample sizes and subtle differences between groups, leaving them open to alternative interpretations (Bishop, 1983). Studies of children with acquired strokes throughout childhood have also reported that compared to children with RH damage, the children with LH lesions showed poorer performance than their controls on several measures of syntactic skills, including the syntax of spoken language (Aram et al., 1985; Aram et al., 1986), performance on the Revised Token Test, a measure of comprehension of complex syntax (Aram & Ekelman, 1987; Aram et al., 1986; Woods & Carey, 1978), and imitation of sentences with complex syntactic structures (Eisele & Aram, 1994). In these studies, the subjects with RH damage also showed some syntactic problems, though the differences between them and their controls were smaller than those between the children with LH damage and their controls. However, this experimental design has left open the strong possibility that larger differences between LH subjects and their controls might be attributable to the stronger performance of the LH controls over the RH controls rather than to differences between the subject groups. In addition, in many of these studies, the use of long sentences with non-canonical word orders may have taxed verbal working memory as well as syntactic processing to account for group differences.
Children with right hemiplegia and presumed or documented prelinguistic LH injury have been reported to show selective language and syntactic deficits in some (Kiessling et al., 1983; Rankin et al., 1981) but not all studies (Woods & Carey, 1978). However, these children have the ability to create structured and meaningful sentences that obey the rules of grammar by late preschool age (Feldman, 1994; Feldman et al., 1992), even in languages with complex morphological structures, such as Hebrew (Levy, Amir, & Shalev, 1994). Thus, at a basic performance level, they respect syntactic rules. Some recent studies have documented that school aged children with early brain injuries have poorer performance than children developing typically on measures of syntactic comprehension and production, but have found no differences between children with LH and RH damage (Bates & Elman, 2000; Bates & Roe, 2001; Bates et al., 1999; Dick et al., 1999). In our previous study, formal testing of language abilities in children with prelinguistic injuries revealed that school aged children with LH damage did well on formal measures that demanded good control of the lexicon, both of open and closed class words, but performed more poorly than children with other lesions on measures of the abilities to compose complex sentences with constrained vocabulary and to store and accurately execute complex oral directions (MacWhinney, Feldman, Sacco, & Valdes-Perez, 2000). However, these measures rely not only on syntactic skills but also on semantic and pragmatic skills. Thus, the issue of congenital LH specialization for syntax remains unsettled.
The purpose of the present study was to assess the syntactic abilities used for sentence comprehension in a group of children with prelinguistic LH and RH damage compared to a large group of children developing typically. We focused on functional aspects of syntactic skills rather than on the syntactic knowledge used to make grammatical judgments in an effort to evaluate those abilities likely to be involved in processing and interpreting sentences quickly, efficiently, and accurately under a wide range of conditions. We wanted to use tasks that would put minimal constraints on associated functions that may also be abnormal, particularly verbal working memory and phonological execution (MacWhinney et al., 2000) and thereby to focus on pure syntactic processing.
The task used in this study is based on the Competition Model, developed by Bates, MacWhinney and colleagues (Bates et al., 1984; Bates, McDonald, MacWhinney, & Appelbaum, 1991; MacWhinney & Bates, 1989; MacWhinney, Osman-Sagi, & Slobin, 1991). The Competition Model is an interactive activation model of sentence processing, in which language is characterized not as a set of rules but rather as a complex network of weighted form-function mappings. This theoretical model accounts for qualitative and quantitative differences in sentence comprehension in children and adults within and across languages and thereby holds promise for explaining qualitative and quantitative differences between children with brain injuries and normal peers. Language provides cues—lexical, syntactic, morphologic, or prosodic—to signal basic meanings, such as who is the agent of the action. The cues may be complementary, favoring the same interpretation of the sentence. For example, in English, the initial noun of the sentence is typically the agent of the action and animate nouns are also more likely to be agents. If a sentence uses the canonical noun-verb-noun word order and the first noun animate, word order and animacy cues converge to support the interpretation that the first noun is the agent. Cues may also be competing, pulling in different directions, such as an inanimate first noun and animate second noun. Cues vary in terms of how consistently and reliably they relate to an interpretation (cue validity), how important that cue is for interpretation (cue strength), and how much processing effort or time is associated with it (cue cost). The degree to which listeners trust these cues determines their response. Sentence comprehension is a process of constraint satisfaction and conflict resolution. The Competition Model allows for a full range of probabilistic values that characterize the performance of adults and children in tasks, such as assigning a noun the agent role, as the result of weighting many different sources of information.
The original procedure for testing the Competition Model was a “whodunit” task in which the listener had to indicate the agent of the action. For example, children were asked to act out “The cow is kicking the pencil” as well as “The pencil is kicking the cow.” By constructing stimuli in which the cues converge or compete, the experimenters assessed the hierarchy of importance of various cues. This task has been transformed into a real time, on-line processing task to approximate the rapid speed of language processing in functional settings (Evans & MacWhinney, 1999; Von Berger, Wulfeck, Bates, & Fink, 1996). To evaluate the importance of cues in different groups and across ages, the sentences have been pared down to simple noun phrases (N) and short verb phrases (V). In the canonical form of English sentences, NVN, the first N assumes the role of the agent of the action.
Previous studies using the Competition Model paradigm have documented developmental changes in sentence processing strategies (Von Berger et al., 1996). Adult performance favors first noun choice in NVN phrases and second noun choice for both VNN and NNV sentences. Children aged 7 years are as likely as adults are to choose the first N as the agent in canonical form, NVN phrases. However, they are less likely than adults to choose the second N as the agent in VNN phrases and are at chance in choosing the first noun as agent in NNV phrases. Adult performance in the VNN and NNV phrases occurs at 9–10 years of age (Von Berger et al., 1996). Children with a selective expressive specific language impairment are remarkably similar to chronological age-matched controls on these tasks, but children with combined expressive and receptive specific language impairment are inconsistent in their choices and more sensitive to animacy than word order cues (Evans & MacWhinney, 1999).
The current study compares the sentence-processing strategies of children with prelinguistic LH and RH damage and those developing typically using the on-line sentence processing methodology. We were interested in determining whether children with brain injuries would use the same strategies as children of the same age, particularly in regard to interpreting sentences with unusual word orders, such as VNN and NNV. If children with LH or RH damage are delayed or deficient in sentence comprehension, we would predict that they would rely more on animacy than would the control group or use a first noun strategy, despite the word order of sentences. However, if the subjects performed like the comparison group, it would suggest that they track and interpret syntactic cues similarly and that some of their problems in other tasks may be related to performance demands rather than sentence comprehension strategies per se. In addition, we were interested in whether the children with brain injuries could accomplish the sentence processing tasks in the same reaction time as children developing normally. We predicted slower reaction times given their consistently slower reaction times in a previous study of information processing in children with prelinguistic unilateral injuries (MacWhinney et al., 2000).
2. Methods
2.1. Subjects
Children with LH damage
A group of children with brain injury incurred before 2 months of age, ages 5–12years, were recruited through referrals from local hospitals, rehabilitation centers, and previous research studies. A total of 12with prelinguistic LH injury, all of whom had CT or MRI scans documenting their injuries, served as the subject group (S). The sample included 6 boys and 2 African Americans. The same group formed the study sample for a previous study on information processing (MacWhinney et al., 2000). Table 1 describes the age, sex, neurological lesion, non-verbal intelligence quotient (Leiter, 1979), and receptive language standard score (Dunn & Dunn, 1981) of these children.
Table 1.
Description of subjects
| Code name | Age | Sex | MRI findings | Non-verbal intelligence | Receptive vocabulary |
|---|---|---|---|---|---|
| Subject group with left hemisphere injuries | |||||
| BRAS | 6 | M | Enlargement of the L ventricle with minor reduction of L white matter | 114 | 82 |
| MAG | 12 | M | Enlargement of the L ventricle including damage to the interior part of the motor strip, somatosensory strip, and the adjacent parietal area | 66 | 125 |
| DES | 10 | F | Enlargement of L ventricle. White matter loss underneath the entire L cortex, with some retrograde white matter loss | 62 | 75 |
| DUP | 7 | M | Enlargement of both lateral ventricles L > R. Reduction in white matter in L periventricular region, in the areas anterior and posterior to the ventricle | 117 | 108 |
| TID | 10 | F | Enlargement of the L lateral ventricle into posterior cortical areas | 94 | 110 |
| ELS | 6 | F | Small lesion in the left parietal white matter | 110 | 101 |
| JOR | 6 | M | Damage to L dorsolateral prefrontal cortex (DLPFC) and nearby areas, including Broca's area; enlarged L ventricle | 93 | 91 |
| JUS | 9 | M | Near complete loss L parietal lobe; some insular loss; 50% loss of occipital, mostly anterior; significant frontal loss with only some anterior frontal preserved | 103 | 100 |
| KAM | 9 | F | L lateral/posterior/inferior frontal loss, adjacent to insula, sparing motor strip; L lateral/anterior parietal loss and some loss of the left insula | 107 | 97 |
| MAM | 7 | F | Enlargement of the L ventricle centrally with thinning of left white matter (corona radiata, centrum semiovale, corpus callosum) | 121 | 95 |
| RYB | 7 | M | L lateral inferior anterior frontal loss, affecting Broca's area and DLPFC; Enlargement of the L lateral ventricle, probable compensation for volume loss | 92 | 98 |
| STEW | 5 | F | Loss along the L central sulcus involving the posterior L frontal cortex and anterior L parietal area | 96 | 77 |
| Children with right hemisphere injuries | |||||
| EMF | 7 | F | R ventricular enlargement, particularly centrally and in parietal area | 111 | 125 |
| JOD | 8 | M | R frontal encephalomalacia involving prefrontal cortex | 79 | 92 |
| KAD | 7 | F | R fronto-parietal encephalomalacia | 107 | 98 |
Children with RH damage
Three children with focal RH injury were also evaluated in this study. Because the sample was small, we chose not to create a separate group. Instead, we compared their performance as individuals to that of the children in the S group and to comparison children. Details about these children are also included in Table 1.
Comparison group
The first step in this work involved the construction of a processing profile for children who were developing normally against which we could compare the performance of the experimental group. To do this, we recruited 150 children ranging in ages from 5 to 10 years to serve as a comparison (C) group. The C group was composed of 25 children at each age of the six age levels. All were functioning at grade level. They were recruited from parochial and private schools in the Greater Pittsburgh area and tested at their schools, in many cases on two occasions. The sample was composed of 86 males and 64 females of varying socioeconomic levels. Eighty-nine percent of the children were Euro-Americans, 9% were African American, and 2% were of Asian origin. This distribution is comparable to the demographics of the region and was also comparable to the distribution for the experimental group. This is the same control group used in our previous study (MacWhinney et al., 2000). A full data set for this task was available for 141 children.
Parental consent was obtained for all participants. The Institutional Review Boards at Carnegie Mellon University and Children's Hospital of Pittsburgh approved the protocol.
2.2. Procedures
Task
The children listened to digitized recordings of sentences comprised of 2N (article plus noun) and one V (verb plus past tense marker). All of the children with LH and RH injury and 102 of the children in the C group processed 36 sentences; 48 children in the C group processed 18 sentences based on an early version of the protocol. A comparison of the results by number of sentences revealed no statistical differences and therefore the data were collapsed for the purposes of this paper. The sentences were in the form of NVN, VNN, and NNV, consistent with previous research using the Competition Model (Bates et al., 1984; Von Berger et al., 1996). The nouns were either animate (A) or inanimate (I) with three possible configurations (AA, AI, IA). Example sentences of the nine stimuli types are given in Table 2. The experiment was built using the PsyScope experiment generator system (Cohen, MacWhinney, Flatt, & Provost, 1993). Each N and V was digitized as a separate audio file and combined randomly by PsyScope during presentation. This procedure allowed us to eliminate any confounding of intonation patterns with sentence type.
Table 2.
Sample stimuli sentences in each category of word order (NVN, NNV, VNN) and animacy (AA, AI, IA)
| Category | Stimulus sentence |
|---|---|
| NVN–AA | The cat kissed the bear |
| NVN–AI | The lion watched the pencil |
| NVN–IA | The block hugged the camel |
| NNV–AA | The cat the bear kissed |
| NNV–AI | The lion the pencil watched |
| NNV–IA | The block the camel hugged |
| VNN–AA | Kissed the cat the bear |
| VNN–AI | Watched the lion the pencil |
| VNN–IA | Hugged the block the camel |
Equipment
The experiment was administered on a Macintosh 660AV with a 15-in. monitor. Auditory stimuli were presented across stereo headphones with a separate headset for the child and the experimenter. The children indicated their responses by pressing the PsyScope button box.
Testing
All S and C children were tested individually. The task described here was administered with additional computerized tasks of information processing (MacWhinney et al., 2000); most children required two testing sessions, each lasting about 30 min. Each of the experiments was presented in a game format. Before each experiment, the child was presented with both oral and written directions for the task and was given the opportunity to complete a few practice trials. Children were instructed to listen carefully and to respond as fast as they could. After the tester was confident that the child understood the instructions and had successfully completed the practice trials, the actual experiment was presented. Children were given as much time as necessary to make a response on each trial. Therefore, no data were eliminated because of long reaction times. After each session, the child was rewarded with his or her choice of a small prize.
2.3. Data analysis
We collapsed age into two categories: younger (5–8 years of age) and older (9 and over) because of the small number of children in the S group and the uneven distribution of their ages. The overall design permitted a 3 × 3 × 2 × 2 mixed model analysis of variance. Animacy (AA, AI, IA) and word order (NVN, NNV, VNN) were within-subject variables. Group (S, C) and age (younger, older) were between-subject variables. The first dependent variable was proportion of trials in which the first noun was chosen. Scores ranged from 0.00 to 1.00. The second dependent variable was reaction time, which represented the time from the initial presentation of the sentence to the button press. Each of these dependent measures was analyzed separately.
Maximum likelihood estimation (MLE) techniques were used to describe patterns of group differences and to determine if children in the S group were functioning like younger children in the C group. The MLE techniques use an iterative procedure in conjunction with a mathematical model to find values for the parameters in a model to best fit the data. This procedure provides estimations of the relative strength of cues for individuals and groups. The specific model used for this study (McDonald & MacWhinney, 1989) predicts the probability of choosing the first N as agent based on the strengths of word order and animacy cues. Separate parameters were estimated for each level of the cue, with three levels for animacy (AA, AI, IA) and three levels of word order (NVN, NNV, VNN). The cues were assumed to interact additively. Parameters were estimated using the Solver function in Microsoft Excel that computed a fit that minimized the squared deviations between the observed data points and those predicted by the model on the basis of the performance of children in the C group. This MLE analysis also provides goodness-of-fit statistics, including the root mean squared deviation (rmsd) and the correlation between the model's predictions and the data. These statistics are a measure of how well the model fits the data. A low rmsd indicates that the model closely matched the actual data from the estimated cue strengths.
To compare patterns of the cue strengths of children with RH damage to those with LH damage, the estimated parameters were converted into categories as follows: Random (.5 ± .25), Mature adult pattern (> .75 or < .25), Consistent adult pattern (> .90 or < .10), and Immature non-adult pattern (> .75 or < .25).
3. Results
3.1. Choice
Choice data revealed a significant main effect for word order, F(2, 298) = 134:380, p < .0001, and for animacy F(2, 298) = 4.534, p = .0115. The interaction of word order and animacy was also significant, F(4, 596) = 2.639, p = .0331. Group (S, C) approached significance, F(1, 149) = 3.639, p = .0584, and the interaction of word order and group was significant, F(2, 298) = 3.927, p = .0207. Age was not significant, F(1, 149) = .129, p = .7202.
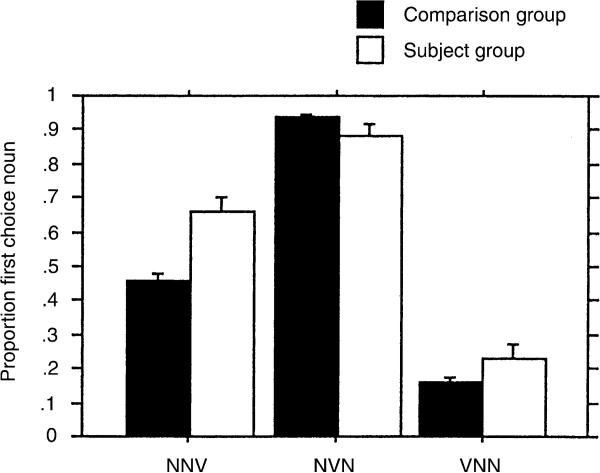
These findings can be understood by reference to the figures. Fig. 1 illustrates proportion of first noun choice for all word orders for the S and C groups. In the C group, first noun choice approached 1.0 for NVN and 0.0 in VNN. This is the usual pattern of results for adults on this task (Von Berger et al., 1996). However, in the NNV phrases, first noun choice was 0.45 in the C group, whereas it typically reaches 0.1–0.2in adults (Von Berger et al., 1996). The performance of the S group is similar to that of the C group in NVN and VNN, though the proportion of first noun choice is not as close to the adult pattern as is the C group's performance. In the NNV, the S group has a tendency toward first noun choice, with a score of almost 0.7.
Fig. 1.
Proportion of choice of first noun as agent in subjects with LH damage (S) and comparison children (C) as a function of sentence type.
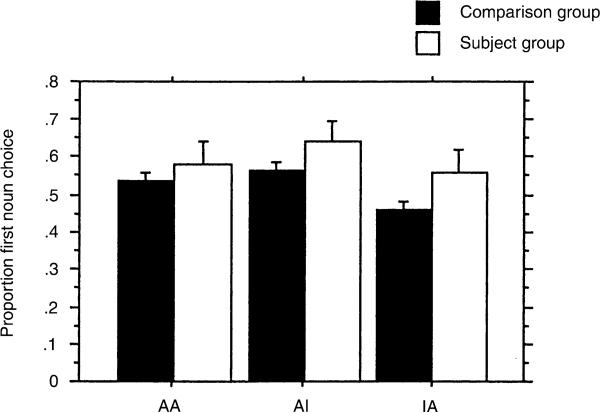
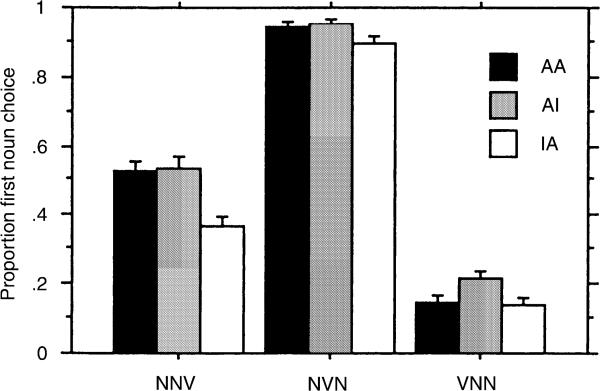
Fig. 2 demonstrates the effect of animacy on choice. The highest levels of first noun choice occur with the AI pattern and the lowest for the atypical IA pattern. The S and C groups have similar scores, though the C group's performance is closer to the adult pattern (Von Berger et al., 1996), which shows less influence of animacy and greater influence of word order on choice. Fig. 3 demonstrates the interaction of word order and animacy. In the NVN word order, animacy cues do not influence first noun choice. For both the NNV and VNN word orders, the AI configuration increases the proportion of first noun choice over the IA configuration, demonstrating that the animacy cue influences choice in the non-canonical word orders.
Fig. 2.
Proportion of choice of first noun as agent in subjects with LH damage (S) and comparison children (C) as a function of animacy cues (A animate, I inanimate).
Fig. 3.
Proportion of choice of first noun as agent as a function of sentence type and animacy cues (A animate, I inanimate).
Within the group with LH damage, we found no association of lesion type with the pattern of results.
3.2. Parameter estimations
Table 3 presents cue strengths for the S and C groups at younger and older ages. These data show that the strength of the animacy cue is comparable in the S and C groups at both ages. The cue strength for NVN word order is high in the younger C group and almost reaches 1.0 in the older C group. The NVN cue is less salient to the younger S group than to the younger C group, but similar in the older S group to the C group. These data suggest that the younger S group may be developmentally delayed in comparison to the C group in assigning strength to this cue. With the VNN word order, children in the C group are likely to choose the second N, so that their choice score is low. This tendency to choose the second N becomes stronger at the older ages in the C group than it is at the younger ages. The cue salience for the older S group is similar to that of the younger C group, again suggesting a developmental lag. The tendency for children in the C group to choose the second N in the NNV word order is extremely weak. Cue salience does not change substantially over this age range for the NNV word order cue in the C group. The S group favors a first noun strategy for NNV word order and the cue strength actually increases rather than decreases across the age range. Thus, for this cue the difference between the S and C groups provides no evidence that the S group is developmentally delayed. Rather, these data suggest that the NNV cue is interpreted differently in the S and C groups.
Table 3.
Parameter estimates for animacy (AA, AI, IA) and word order (NVN, NNV, VNN) for the Subject and Control groups, split into Younger (5–8 years) and Older (9 and older) subgroups
| Subjects | Comparison group |
Subject group |
||
|---|---|---|---|---|
| Younger n = 82 | Older n = 59 | Younger n = 7 | Older n = 5 | |
| Animacy | .5891 | .5782 | .5598 | .5374 |
| NVN | .9242 | .9720 | .8131 | 1.000 |
| VNN | .1955 | .0967 | .2460 | .1891 |
| NNV | .4746 | .4428 | .6428 | .6684 |
| rmsd | .0257 | .0183 | .0389 | .0657 |
Performance of the children with RH damage spanned from immature to mature performance and overlaps the performance of children with LH damage. Table 4 demonstrates the developmental patterns of all the children with brain injury. One child, EMF, aged 7 years, relied on animacy cues for sentence comprehension and showed random choice with the NVN word order, as did 2of the 4 children aged 5 and 6 years in the LH group. JOD, at the age of 8 years, had a first noun choice for NVN and a second noun choice for VNN, like the children with LH damage who were aged 7 and 10. One of the children with RH damage consistently chose the second noun in the NNV word order, the only child with brain injury to show the typical adult response on this word order.
Table 4.
Developmental patterns of parameter estimates for three word orders in the children with early brain injury
| Lesion side | Age | Word order |
|||
|---|---|---|---|---|---|
| NVN | VNN | NNV | |||
| LH | 5 | STEW | R | R | R |
| 6 | JOR | R | R | R | |
| 6 | BRAS | M | R | R | |
| 6 | ELS | MM | M | R | |
| 7 | RYB | M | MM | I | |
| 7 | MAM | MM | M | I | |
| 7 | DUP | MM | MM | R | |
| 10 | TID | MM | M | I | |
| 10 | JUS | MM | M | I | |
| 10 | DES | MM | MM | R | |
| 10 | KAM | MM | MM | I | |
| 12 | MAG | M | R | R | |
| RH | 7 | EMF | R | R | R |
| 7 | KAD | MM | MM | MM | |
| 8 | JOD | M | MM | R | |
| | |||||
| N = noun phrase, V = verb phrase | |||||
| Parameter Estimate Range | Description | Letter code | |||
| .5 ± .25 | Random | R | |||
| >.75 or <.25 | Adult pattern (Mature) | M | |||
| >.90 or <.10 | Consistent adult pattern (Mature) | MM | |||
| >.75 or <.25 | Non-adult (Immature) | I | |||
3.3. Reaction time
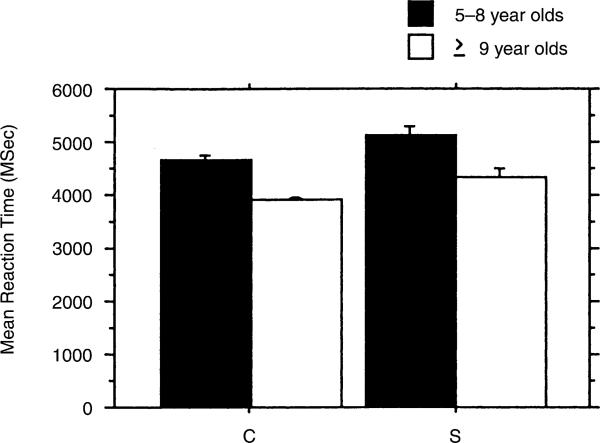
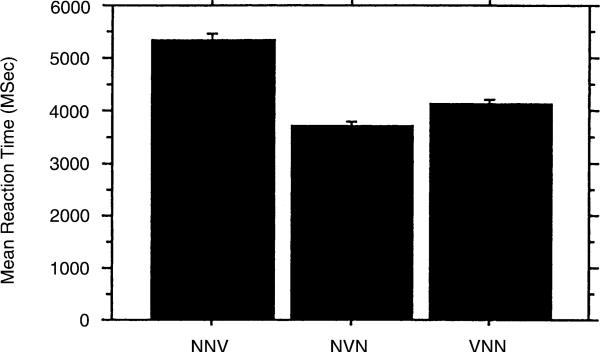
Reaction time data revealed a significant main effect for age group F(1, 149) = 4.424, p = .0371 and word order F(2, 298) = 29.803, p < .0001. Fig. 4 shows the mean reaction times for the younger and older S and C groups and demonstrates that mean reaction time is faster for the C group at both ages. Fig. 5 shows the reaction time for the three word orders. The reaction time is faster for the NVN word order than for VNN, which has a faster mean reaction time than the NNV word order. This pattern was similar within both groups. Thus, the NNV word order, most difficult from the standpoint of age of adult understanding, is also the slowest in terms of reaction time.
Fig. 4.
Mean reaction time as a function of age in subjects with LH damage (S′) and comparison children (C).
Fig. 5.
Mean reaction time as a function of sentence type.
Children with brain injury were slower on this task than controls. The difference reached statistical significance when the subjects with LH and RH damage were combined and compared with controls F(2, 153) = 6.071, p = .0029 but not when children with LH damage alone were compared to controls. Interestingly, the child with RH damage who showed mature performance on the NNV word order had the longest reaction times compared with the other two children with RH damage who were comparable to children with LH damage.
4. Discussion
The purpose of the present study was to compare the sentence comprehension strategies of children with prelinguistic LH and RH damage to children developing typically using an on-line sentence-comprehension task. We analyzed the children's choice about who was the agent of the action in sentences that varied systematically by word order and by animacy and their reaction times to make that decision. The data showed that word order and animacy were important cues in the choice of which N is the agent for children with brain damage and for children developing typically. The interactions of word order with both age and group were significant. Moreover, the strength of the word order cues varied across groups. Taken together, these results suggest that children with LH damage had developmental delays in sentence comprehension. We did not find differences in results as a function of the specific type of LH lesion. Of the three children with RH damage, one showed a response pattern typical of adults, but the other two showed similar delays as the children with LH injury. Reaction time on these tasks was a function of word order and age. Children made decisions about the NVN word order faster than they did about the other word orders and older children were faster than younger children. Children with LH and RH damage were generally slower than were children in the C group, though differences only achieved statistical significance when the children with LH and RH damage were combined. This may have been due to the variability of reaction times in the children.
We found similar developmental patterns for the children developing typically as have been previously reported. Von Berger et al. (1996) found that children aged 7 and 8 choose the first noun as agent approximately 85% of the time in the NVN condition, 25% of the time in the VNN condition, and 55% of the time in the NNV condition. By the ages of 9 and 10, the percentages have changed to 95%, 8%, and 25%, respectively (Von Berger et al., 1996). The main difference between our C group and the previous report was the results for the NNV word order. The older C group in this study, aged 9–10 years, remained approximately at chance levels in assigning agent in the NNV condition. The reason for this difference is not clear but may be related to the actual stimuli in the experiments, the particular nouns and verbs used. Children in the C group were more likely to choose the second noun if the animacy cues were complementary. Thus, we suspect that if we were to test a somewhat older cohort, we would see a gradual shift from chance performance to second noun choice, regardless of animacy cues. We cannot compare our reaction time results directly to this previous study because of differences in the time frame measured as reaction time. Our method was actual reaction time whereas Von Berger and colleagues created an adjusted reaction time by subtracting out a mean button press time for each child calculated from baseline measures (Von Berger et al., 1996). Nonetheless, the rank order of reaction times is the same in both studies and both studies show a definite developmental trend in this age range.
The data suggested that the children with LH damage have developmental delays in the use of syntactic cues for sentence processing. The results of the parameter estimation are particularly useful to make this point. In children developing typically, animacy is mastered earlier than is word order as a cue in the choice of the agent of an action. In this study, the animacy cue had similar cue strength in both the S and C groups and in both younger and older age groups, suggesting mature use of this cue, even at the youngest ages. Within the word orders, the NVN cue strength was higher in the C group than in the S group at the younger ages. The cue strength of NVN for five of the six 5- and 6-year-olds in the subject group was less than .8000 whereas the average cue strength for 5- and 6-year-olds in the C group was .9029 and .8792, respectively. The youngest children with both LH and RH damage did not reliably use the NVN word order in sentence comprehension. The older S group did show consistent use of the NVN cue. These results suggest that the younger children with brain injury were experiencing a developmental delay and not a permanent deficit in the use of this cue. As further evidence of developmental delay in sentence comprehension, the cue strength for first noun choice in the VNN word order for the older S group was comparable to that of the younger C group.
Performance on the NNV differentiated the S from the C group. This construction is relatively rare in English. The word order might arise if a speaker wanted to emphasize the object of the action rather than the subject such as in the sentence “it was the zebra (not the gazelle) that the lion attacked.” In this study, both the S and C groups failed to demonstrate the usual adult pattern of responding, second noun choice. In the C group, children remained at chance on choice of the agent in the AA and AI conditions. However, they tended to show the adult pattern (second noun choice) when the animacy cue was IA, that is, when the word order and animacy cues were complementary and converged on the choice of the second noun. The S group showed a definite tendency for first noun choice in the NNV phrases. They did not show any change in the NNV cue as a function of age. They did not show any change in first noun choice, even in those phrases that used the IA order of animacy cues. They assimilated the NNV order to the more common NVN order.
In terms of the choice data, only one child with brain injury showed the mature adult response and she was a child with RH injury. However, the other two children with RH injury had similar profiles as children with LH injury, with evidence of developmental delays. Though we did not have many subjects with RH damage, these preliminary results suggest that these children are likely to show patterns similar to children with LH damage.
Reaction time results were similar to the choice data. Children responded more quickly to the canonical NVN sentences than to the other word orders. Similarly, they were faster with the VNN sentences than the NNV sentences. As expected, older children had shorter mean reaction times than younger children. The LH subject group performed the task from 400 to 1200 milliseconds more slowly than C groups, though this difference did not reach statistically significance. In our previous work, children with early brain injuries were slower in reaction times to information processing (MacWhinney et al., 2000) and we suspect that in a larger group the differences in reaction time would be statistically significant. Interestingly, the two children in the RH group with immature sentence comprehension choice data had reaction times similar to the children with LH damage. However, the child with mature performance had the longest reaction times, demonstrating a time-accuracy trade-off.
How do these results contribute to the understanding about the effects of pre-linguistic unilateral brain injury on language development? The developmental delays in sentence comprehension of children with focal lesions to either hemisphere are somewhat unexpected, given that the same children experience success in acquiring a solid, functional use of language. We suspect that they do better when the linguistic task places few explicit demands on the speaker for precise word choice or syntactic construction. Sentence comprehension during conversation is aided by the redundancy of information within an exchange and may be compromised to some degree without obvious communication breakdowns. Moreover, the time constraints for production and comprehension within routine conversation are also minimal. Generally, in conversation, individuals can take the time required to comprehend what they hear and formulate their response. However, the on-line sentence comprehension task requires an immediate interpretation of a familiar or unfamiliar sentence construction. For children with prelinguistic brain injury, it appears that decrements in performance are more likely to occur when tasks demand precision, rapid responding, or integration and organization of considerable information (Bates & Roe, 2001; Bates et al., 1999; Bates, Wulfeck, Opie, Fenson, & Kriz, 1999; MacWhinney et al., 2000). The added burden of LH damage seems to be greater in children with hemispherectomy or acquired strokes (Aram & Ekelman, 1987; Aram et al., 1986; Dennis, 1976; Dennis & Kohn, 1975; Dennis & Whitaker, 1976; Kiessling et al., 1983; Rankin et al., 1981; Stark, Bleile, Brandt, Freeman, & Vining, 1995; Stark & McGregor, 1997; Vargha-Khadem & Polkey, 1992) than in children with prelinguistic injuries.
The findings here do not support the concept that the LH has a privileged congenital specialization for language in general or syntactic processing in particular (Aram & Ekelman, 1987; Aram et al., 1986; Kiessling et al., 1983; Rankin et al., 1981; Vargha-Khadem & Polkey, 1992). Though the children with LH damage showed developmental delays, two of the three children with RH damage showed similar developmental delays. An alternative explanation is that the children with early unilateral damage to either hemisphere are forced to rely on a smaller than usual neural network for language learning and processing. Connectionist models of development show that small neural networks require more exposures than larger networks to learn particular skills and that the effects of such resource restriction are most apparent in the development of skills that are not easily predictable from the input (Marchman, 1993; Seidenberg & McClelland, 1989). In this study, the children with brain injury showed developmental delay in using the most common word order cue, NVN, and also a less common but not infrequent VNN word order cue. However, they showed immature responses to the most infrequent word order cue, NNV, and no evidence of developmental changes in the age range studied here.
The results here are also compatible with the concept of bilateral hemispheric participation in sentence comprehension, at least for challenging sentences, as has been documented in imaging studies (Booth et al., 2000; Just et al., 1996). If in fact bilateral involvement assists in sentence comprehension, then children with prelinguistic injury to either hemisphere would be at high risk for some disruptions in these tasks.
This interpretation of the results leads to a set of testable hypotheses. We hypothesize that greater exposure than available to 9- and 10-year-olds may be required to develop maturity in the NNV word order. We could test this hypothesis by replicating this study in a group of adolescents or young adults with prelinguistic injuries to determine if they eventually learn to use the NNV cue as do adults and older children developing typically. Alternatively, we could provide training in the low frequency structures or feedback regarding their interpretation. We would predict that experimental exposure would also speed up the learning process. Finally, we predict that testing a larger group of children with RH lesions would validate the fact that developmental delays in sentence comprehension occur after injury to either hemisphere. A larger sample might be able to document differences in the results as a function of other variables, such as intrahemispheric location or size of lesion.
Footnotes
This research was supported in part by Grant R01 DC01903 from the National Institutes of Health. We thank the children who participated in this study, their families, the teachers, and comparison subjects in the Mt. Lebanon Montesorri School, the Cathedral School, the Carnegie Mellon Child Care Center, St. Edmund's Academy, and the Seton Center School. We also thank Allison Rowland and Emma Belton for helpful comments on a previous version.
References
- Aram D, Meyers SC, Ekelman B. Fluency of conversational speech in children with unilateral lesions. Brain and Language. 1990;38:105–121. doi: 10.1016/0093-934x(90)90104-o. [DOI] [PubMed] [Google Scholar]
- Aram DM, Ekelman BL. Unilateral brain lesions in childhood: performance on the Revised Token Test. Brain and Language. 1987;32(1):137–158. doi: 10.1016/0093-934x(87)90121-0. [DOI] [PubMed] [Google Scholar]
- Aram DM, Ekelman BL, Rose DF, Whitaker HA. Verbal and cognitive sequelae following unilateral lesions acquired in early childhood. Journal of Clinical and Experimental Neuropsychology. 1985;7(1):55–78. doi: 10.1080/01688638508401242. [DOI] [PubMed] [Google Scholar]
- Aram DM, Ekelman BL, Whitaker HA. Spoken syntax in children with acquired unilateral hemisphere lesions. Brain and Language. 1986;27(1):75–100. doi: 10.1016/0093-934x(86)90006-4. [DOI] [PubMed] [Google Scholar]
- Banker B, Larrouche J. Periventricular leukomalacia of infancy. Archives of Neurology. 1962:32–57. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- Bates E, Elman J. Parker ST, Langer J, editors. The ontogeny and phylogeny of language: A neural network perspective. Biology and behavior: The evolution of human development. 2000. pp. 89–130.
- Bates E, MacWhinney B, Caselli C, Devescovi A, Natale F, Venza V. A cross-linguistic study of the development of sentence interpretation strategies. Child Development. 1984;55(2):341–354. [PubMed] [Google Scholar]
- Bates E, McDonald J, MacWhinney B, Appelbaum M. A maximum likelihood procedure for the analysis of group and individual data in aphasia research [see comments]. Brain and Language. 1991;40(2):231–265. doi: 10.1016/0093-934x(91)90126-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Roe K. Language development in children with unilateral brain injury. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. MIT Press; Cambridge, MA: 2001. pp. 281–307. [Google Scholar]
- Bates E, Thal D, Trauner D, Fenson J, Aram D, Eisele J, Nass R. From first words to grammar in children with focal brain injury. Developmental Neuropsychology. 1997;13:447–476. [Google Scholar]
- Bates E, Vicari S, Trauner D. Neural mediation of language development: perspectives from lesion studies of infants and children. In: Tager-Flusberg H, editor. Neurodevelopmental disorders. MIT Press; Cambridge, MA: 1999. pp. 533–581. [Google Scholar]
- Bates E, Wulfeck B, Opie M, Fenson J, Kriz S. Comparing free speech in children and adults with left-versus right-hemisphere injury (Abstract). Brain and Language. 1999;69:377–379. [Google Scholar]
- Bishop D. Linguistic impairment after left hemidecortication for infantile hemiplegia? A reappraisal. Quarterly Journal of Experimental Psychology. 1983;35A:199–207. doi: 10.1080/14640748308402125. [DOI] [PubMed] [Google Scholar]
- Booth JR, Macwhinney B, Thulborn KR, Sacco K, Voyvodic J, Feldman HM. Functional organization of activation patterns in children: whole brain fMRI imaging during three different cognitive tasks. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1999;23(4):669–682. doi: 10.1016/s0278-5846(99)00025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic J, Feldman HM. Patterns of brain activation in children with strokes engaged in three cognitive tasks. Developmental Neuropsychology. 2000;18(2):139–169. doi: 10.1207/S15326942DN1802_1. [DOI] [PubMed] [Google Scholar]
- Chomsky N. Aspects of the theory of syntax. The MIT Press; Cambridge, MA: 1965. [Google Scholar]
- Chomsky N. Syntactic structures. Mouton; The Hague: 1972. [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. PsyScope: an interactive graphical system for designing and controlling experiments in the Psychology laboratory using Macintosh computers. Behavior Research Methods, Instrumentation, and Computers. 1993;25:257–271. [Google Scholar]
- Dall'Oglio AM, Bates E, Volterra V, Di Capua M, Pezzini G. Early cognition, communication and language in children with focal brain injury. Developmental Medicine and Child Neurology. 1994;36(12):1076–1098. doi: 10.1111/j.1469-8749.1994.tb11810.x. [DOI] [PubMed] [Google Scholar]
- Dennis M. Impaired sensory and motor differentiation with corpus callosum agenesis: a lack of callosal inhibition during ontogeny? Neuropsychologia. 1976;14(4):455–469. doi: 10.1016/0028-3932(76)90074-9. [DOI] [PubMed] [Google Scholar]
- Dennis M. Capacity and strategy for syntactic comprehension after left or right hemidecortication. Brain and Language. 1980;10(2):287–317. doi: 10.1016/0093-934x(80)90058-9. [DOI] [PubMed] [Google Scholar]
- Dennis M, Kohn B. Comprehension of syntax in infantile hemiplegics after cerebral hemidecortication: left-hemisphere superiority. Brain and Language. 1975;2(4):472–482. doi: 10.1016/s0093-934x(75)80084-8. [DOI] [PubMed] [Google Scholar]
- Dennis M, Lovett M, Wiegel-Crump CA. Written language acquisition after left or right hemidecortication in infancy. Brain and Language. 1981;12(1):54–91. doi: 10.1016/0093-934x(81)90005-5. [DOI] [PubMed] [Google Scholar]
- Dennis M, Whitaker HA. Language acquisition following hemidecortication: linguistic superiority of the left over the right hemisphere. Brain and Language. 1976;3(3):404–433. doi: 10.1016/0093-934x(76)90036-5. [DOI] [PubMed] [Google Scholar]
- Dick F, Wulfeck B, Bates E, Saltzman N, Naucler N, Dronkers N. Interpretation of complex syntax in aphasic adults and children with focal lesions or specific learning impairment (Abstract). Brain and Language. 1999;69:335–336. [Google Scholar]
- Dunn L, Dunn L. Peabody picture vocabulary test. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Eisele J, Aram D. Comprehension and imitation of syntax following early hemisphere damage. Brain and Language. 1994;46:212–231. doi: 10.1006/brln.1994.1013. [DOI] [PubMed] [Google Scholar]
- Evans JL, MacWhinney B. Sentence processing strategies in children with expressive and expressive-receptive specific language impairment. International Journal of Language and Communication Disorders. 1999;34(2):117–134. doi: 10.1080/136828299247469. [DOI] [PubMed] [Google Scholar]
- Feldman HM, editor. Language development after early unilateral brain injury: A replication study. Erlbaum; Hillsdale, NJ: 1994. [Google Scholar]
- Feldman HM, Holland AL, Kemp SS, Janosky JE. Language development after unilateral brain injury. Brain and Language. 1992;42(1):89–102. doi: 10.1016/0093-934x(92)90058-m. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Glass P, Bulas DL, Wagner AE, Rajasingham SR, Civitello LA, Coffman CE. Pattern of neuropsychological deficit at age five years following neonatal unilateral brain injury. Brain and Language. 1998;63(3):346–356. doi: 10.1006/brln.1998.1956. [DOI] [PubMed] [Google Scholar]
- Goodglass D. Understanding aphasia. Academic Press; San Diego: 1993. [Google Scholar]
- Just M, Carpenter P, Keller T, Eddy W, Thulborn K. Brain activation modulated by sentence comprehension. Science. 1996;274(5284):114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kiessling L, Denckla M, Carlton M. Evidence for differential hemisphere function in children with hemiplegic cerebral palsy. Developmental Medicine and Child Neurology. 1983;25:727–734. doi: 10.1111/j.1469-8749.1983.tb13840.x. [DOI] [PubMed] [Google Scholar]
- Leiter RG. Leiter international performance scales. Stoelting; Chicago: 1979. [Google Scholar]
- Lenneberg EH. Biological foundations of language. Wiley; New York: 1967. [Google Scholar]
- Levy Y, Amir N, Shalev R. Morphology in a child with a congenital, left-hemisphere brain lesion: implications for normal acquisition, constraints on language acquisition: studies of atypical children. Erlbaum; Hillsdale, NJ: 1994. [Google Scholar]
- MacWhinney B, Bates E, editors. The crosslinguistic study of sentence processing. Cambridge University Press; Cambridge: 1989. [Google Scholar]
- MacWhinney B, Feldman H, Sacco K, Valdes-Perez R. Online measures of basic language skills in children with early focal brain lesions. Brain and Language. 2000;71(3):400–431. doi: 10.1006/brln.1999.2273. [DOI] [PubMed] [Google Scholar]
- MacWhinney B, Osman-Sagi J, Slobin DI. Sentence comprehension in aphasia in two clear case-marking languages. Brain and Language. 1991;41(2):234–249. doi: 10.1016/0093-934x(91)90154-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchman VA. Constraints on plasticity in a connectionist model of the English past tense. Journal of Cognitive Neuroscience. 1993;5(2):215–234. doi: 10.1162/jocn.1993.5.2.215. [DOI] [PubMed] [Google Scholar]
- Marchman VA, Miller R, Bates E. Babble and first words in children with focal brain injury. Applied Psycholinguistics. 1991;12:1–22. [Google Scholar]
- McDonald J, MacWhinney B. The acquisition of cue-category mappings. In: MacWhinney B, Bates E, editors. The crosslinguistic study of sentence processing. Cambridge University Press; Cambridge: 1989. pp. 397–421. [Google Scholar]
- Muller RA, Rothermel RD, Behen ME, Muzik O, Chakraborty PK, Chugani HT. Language organization in patients with early and late left-hemisphere lesion: a PET study. Neuropsychologia. 1999;37(5):545–557. doi: 10.1016/s0028-3932(98)00109-2. [DOI] [PubMed] [Google Scholar]
- Muller RA, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chakraborty PK, Chugani HT. Brain organization of language after early unilateral lesion: a PET study. Brain and Language. 1998;62(3):422–451. doi: 10.1006/brln.1997.1931. [DOI] [PubMed] [Google Scholar]
- Pinker S. The language instinct. Alien Lane, the Penguin Press; London: 1994. [Google Scholar]
- Rankin JM, Aram DM, Horwitz SJ. Language ability in right and left hemiplegic children. Brain and Language. 1981;14(2):292–306. doi: 10.1016/0093-934x(81)90081-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left brain injury in determining implications for models of language development. Annals of the New York Academy of Sciences. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Reilly JS, Bates EA, Marchman VA. Narrative discourse in children with early focal brain injury. Brain and Language. 1998;61(3):335–375. doi: 10.1006/brln.1997.1882. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, McClelland J. A distributed, developmental model of word recognition and naming. Psychological Review. 1989;96(4):523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Stark RE, Bleile K, Brandt J, Freeman J, Vining EPG. Speech-language outcomes of hemispherectomy in children and young adults. Brain and Language. 1995;51(3):406–421. doi: 10.1006/brln.1995.1068. [DOI] [PubMed] [Google Scholar]
- Stark RE, McGregor KK. Follow-up study of a right-and a left-hemispherectomized child: implications for localization and impairment of language in children. Brain and Language. 1997;60(2):222–242. doi: 10.1006/brln.1997.1800. [DOI] [PubMed] [Google Scholar]
- Thal D, Marchman V, Stiles J, Aram D, Trauner D, Nass R, Bates E. Early lexical development in children with focal brain injury. Brain and Language. 1991;40(4):491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Crivello F, Mellet E, Nkanga-Ngila B, Mazoyer B. Functional anatomy of dominance for speech comprehension in left handers vs right handers. Neuroimage. 1998;8(1):1–16. doi: 10.1006/nimg.1998.0343. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Carr LJ, Isaacs E, Brett E, Adams C, Mishkin M. Onset of speech after left hemispherectomy in a nine-year-old boy. Brain. 1997;120(Pt 1):159–182. doi: 10.1093/brain/120.1.159. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, Muter V. A review of cognitive outcome after unilateral lesions sustained during childhood. Journal of Child Neurology. 1994;9(Suppl 2):67–73. [PubMed] [Google Scholar]
- Vargha-Khadem F, Polkey CE. A review of cognitive outcome after hemidecortication in humans [Review] [51 refs]. Advances in Experimental Medicine and Biology. 1992;325:137–151. doi: 10.1007/978-1-4615-3420-4_8. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Walters GV, O'Gorman AM. Development of speech and language following bilateral frontal lesions. Brain and Language. 1985;25(1):167–183. doi: 10.1016/0093-934x(85)90128-2. [DOI] [PubMed] [Google Scholar]
- Von Berger E, Wulfeck B, Bates E, Fink N. Developmental changes in real-time sentence processing. First Language. 1996;16:193–222. [Google Scholar]
- Woods B, Carey S. Language deficits after apparent clinical recovery from aphasia. Annals of Neurology. 1978;6:405–409. doi: 10.1002/ana.410060505. [DOI] [PubMed] [Google Scholar]
- Woods B, Teuber H. Changing patterns of childhood aphasia. Annals of Neurology. 1977;3:273–280. doi: 10.1002/ana.410030315. [DOI] [PubMed] [Google Scholar]