Abstract
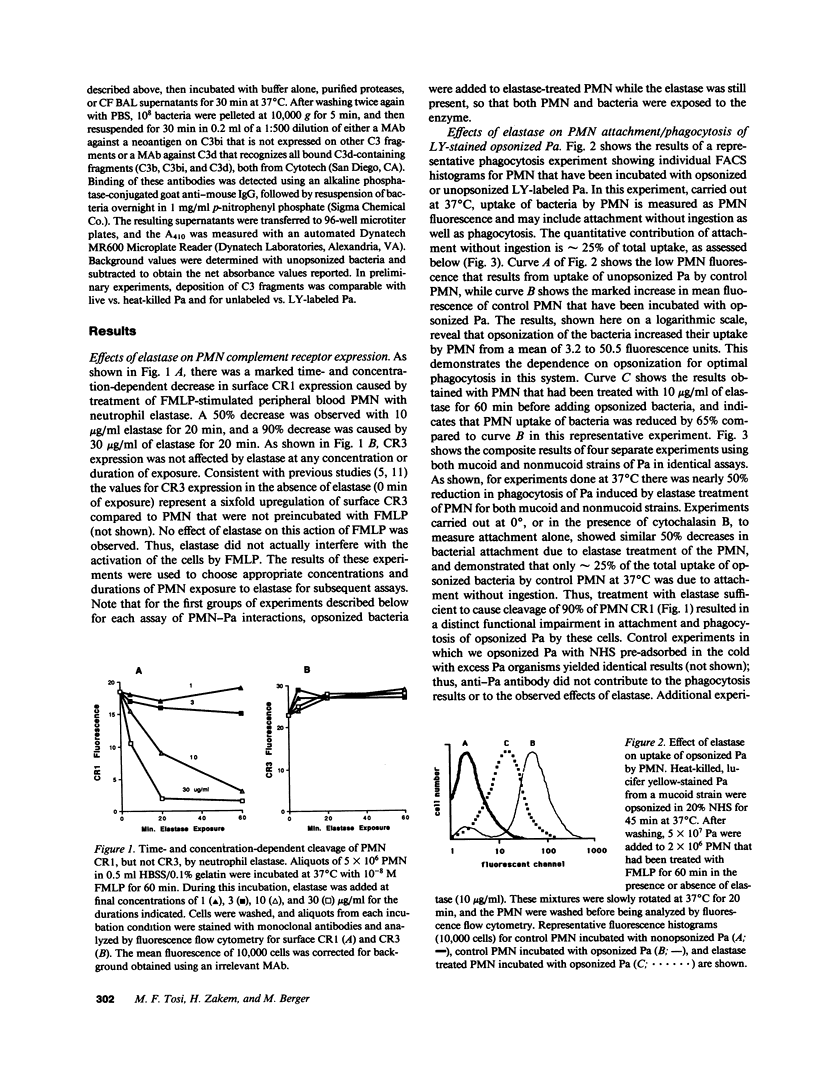
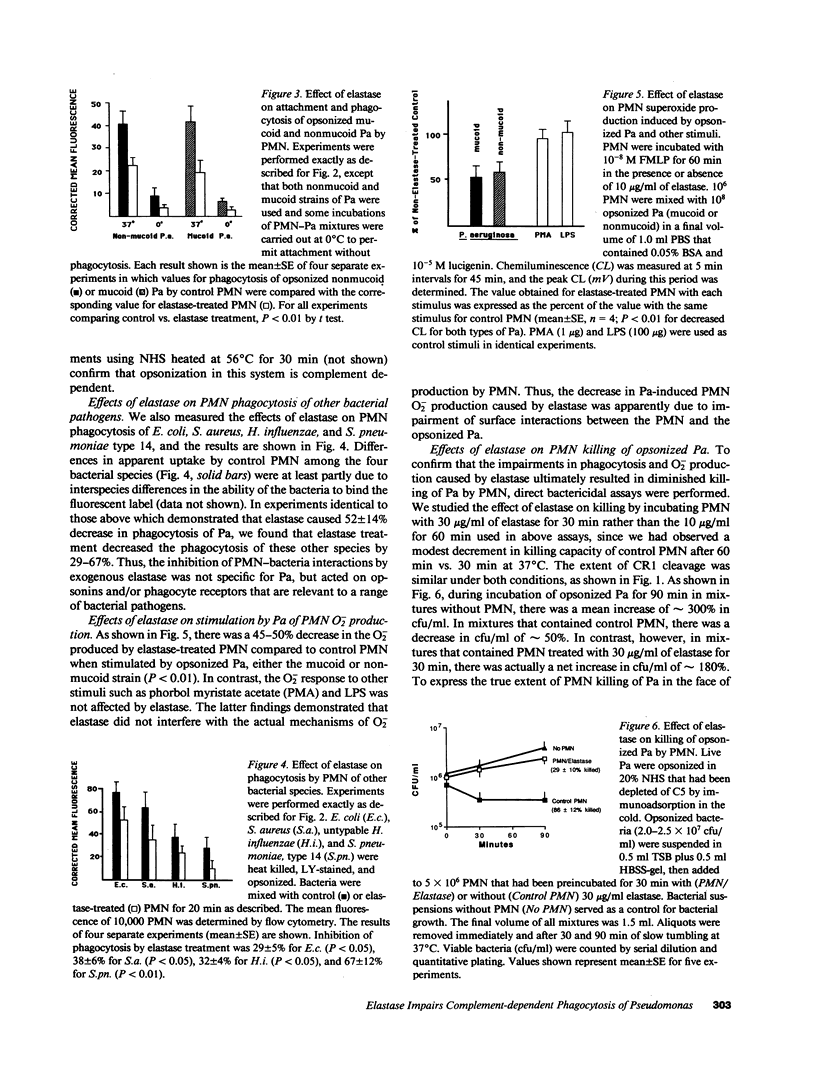
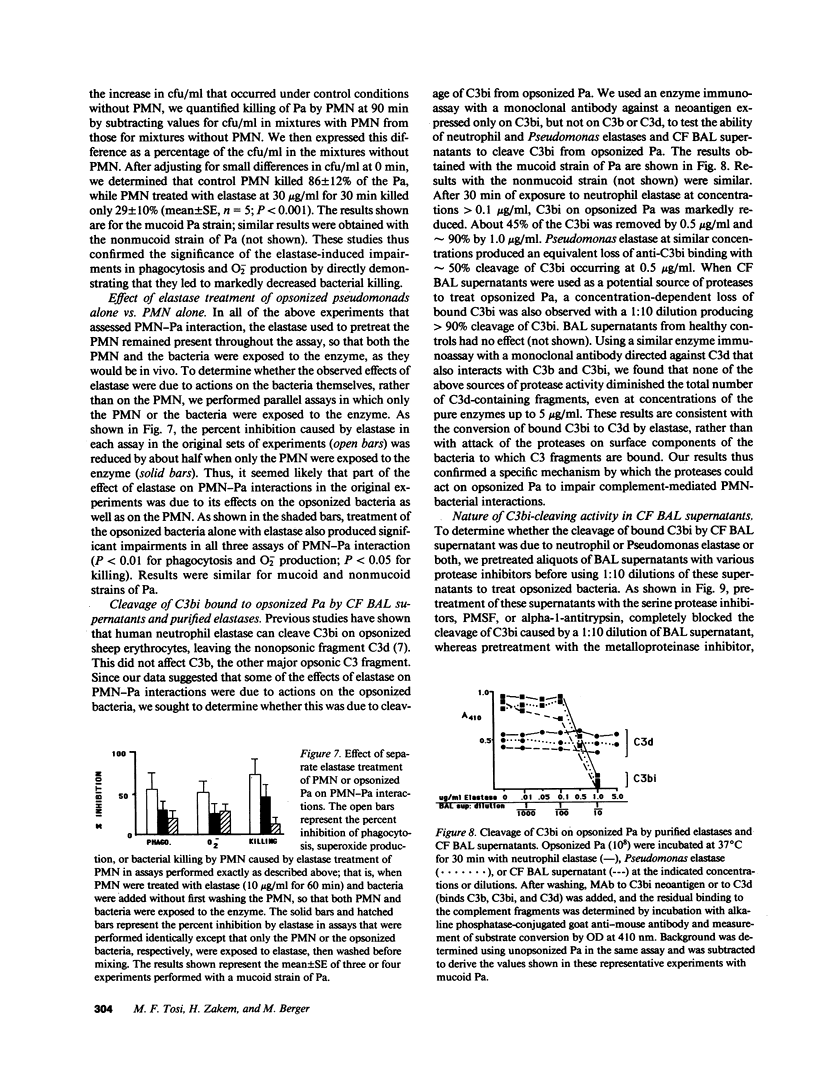
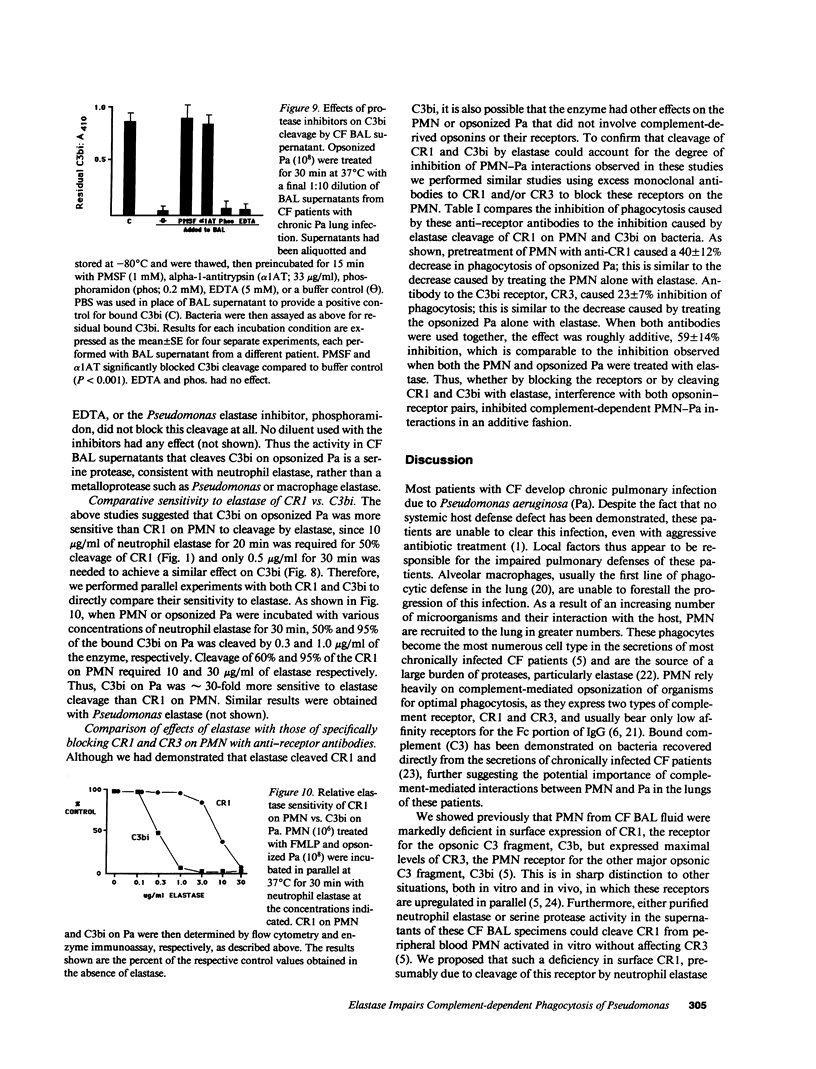
Neutrophil elastase has been implicated as a factor that impairs local host defenses in chronic Pseudomonas aeruginosa (Pa) lung infection in cystic fibrosis (CF). We recently showed that this enzyme cleaves the C3b receptor, CR1, from neutrophils (PMN) in the lungs of infected CF patients. The C3bi receptor on these cells, CR3, is resistant to elastase. We now show that purified neutrophil elastase markedly impairs complement-mediated PMN-Pa interactions including phagocytosis of opsonized Pa, stimulation by opsonized Pa of PMN superoxide production, and killing of opsonized Pa by PMN. When PMN and opsonized Pa were treated separately with elastase, additive levels of inhibition were observed in each of the above assays. The effects on the bacteria were due to cleavage of the bound C3bi from the surface of opsonized Pa by neutrophil elastase. C3bi was also cleaved by pseudomonas elastase, or bronchoalveolar lavage fluid from CF patients with chronic Pa lung infection. Inhibitors of neutrophil elastase eliminated C3bi cleavage by BAL fluid, while inhibitors of pseudomonas elastase had no effect. Blocking CR1 and CR3 on PMN with specific monoclonal antibodies reduced phagocytosis of opsonized Pa to an extent similar to that caused by elastase cleavage of CR1 on PMN and C3bi on Pa. We conclude that neutrophil elastase in the lungs of chronically infected CF patients cleaves C3bi from opsonized Pa as well as CR1 from PMN, creating an "opsonin-receptor mismatch" that severely impairs complement-mediated phagocytic host defenses against these bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Medof M. E. Increased expression of complement decay-accelerating factor during activation of human neutrophils. J Clin Invest. 1987 Jan;79(1):214–220. doi: 10.1172/JCI112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., O'Shea J., Cross A. S., Folks T. M., Chused T. M., Brown E. J., Frank M. M. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984 Nov;74(5):1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Sorensen R. U., Tosi M. F., Dearborn D. G., Döring G. Complement receptor expression on neutrophils at an inflammatory site, the Pseudomonas-infected lung in cystic fibrosis. J Clin Invest. 1989 Oct;84(4):1302–1313. doi: 10.1172/JCI114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. C., Poncz L., Klinger J. D., Stern R. C., Tomashefski J. F., Jr, Dearborn D. G. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985 Sep;132(3):529–535. doi: 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- Carlo J. R., Spitznagel J. K., Studer E. J., Conrad D. H., Ruddy S. Cleavage of membrane bound C3bi, an intermediate of the third component of complement, to C3c and C3d-like fragments by crude leucocyte lysosomal lysates and purified leucocyte elastase. Immunology. 1981 Oct;44(2):381–391. [PMC free article] [PubMed] [Google Scholar]
- Davies P., Fox R. I., Polyzonis M., Allison A. C., Haswell A. D. The inhibition of phagocytosis and facilitation of exocytosis in rabbit polymorphonuclear leukocytes by cytochalasin B. Lab Invest. 1973 Jan;28(1):16–22. [PubMed] [Google Scholar]
- Döring G., Buhl V., Høiby N., Schiøtz P. O., Botzenhart K. Detection of proteases of Pseudomonas aeruginosa in immune complexes isolated from sputum of cystic fibrosis patients. Acta Pathol Microbiol Immunol Scand C. 1984 Oct;92(5):307–312. doi: 10.1111/j.1699-0463.1984.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Döring G., Goldstein W., Botzenhart K., Kharazmi A., Schiøtz P. O., Høiby N., Dasgupta M. Elastase from polymorphonuclear leucocytes: a regulatory enzyme in immune complex disease. Clin Exp Immunol. 1986 Jun;64(3):597–605. [PMC free article] [PubMed] [Google Scholar]
- Döring G., Goldstein W., Röll A., Schiøtz P. O., Høiby N., Botzenhart K. Role of Pseudomonas aeruginosa exoenzymes in lung infections of patients with cystic fibrosis. Infect Immun. 1985 Sep;49(3):557–562. doi: 10.1128/iai.49.3.557-562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W., Endert J., Van Boven C. P. A quantitative method for assessing the third complement factor (C3) attached to the surface of opsonized Pseudomonas aeruginosa: interrelationship between C3 fixation, phagocytosis and complement consumption. J Immunol Methods. 1985 Jul 16;81(1):43–53. doi: 10.1016/0022-1759(85)90120-6. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Squier S. U., Wood R. E., Gee J. B., Reynolds H. Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984 Jul;74(1):236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S., Holsclaw D. S. Interactions of Pseudomonas aeruginosa with immunoglobulins and complement in sputum. Infect Immun. 1976 Jul;14(1):114–117. doi: 10.1128/iai.14.1.114-117.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson L., Venge P. Kinetic studies of neutrophil phagocytosis. V. studies on the co-operation between the Fc and C3b receptors. Immunology. 1982 Dec;47(4):687–694. [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Brown E. J., Frank M. M. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol. 1984;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- Looney R. J., Ryan D. H., Takahashi K., Fleit H. B., Cohen H. J., Abraham G. N., Anderson C. L. Identification of a second class of IgG Fc receptors on human neutrophils. A 40 kilodalton molecule also found on eosinophils. J Exp Med. 1986 Apr 1;163(4):826–836. doi: 10.1084/jem.163.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkenberg I., Ferber E. Lucigenin-dependent chemiluminescence as a new assay for NAD(P)H-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J Immunol Methods. 1984 Jun 8;71(1):61–67. doi: 10.1016/0022-1759(84)90206-0. [DOI] [PubMed] [Google Scholar]
- Moore F. D., Jr, Davis C., Rodrick M., Mannick J. A., Fearon D. T. Neutrophil activation in thermal injury as assessed by increased expression of complement receptors. N Engl J Med. 1986 Apr 10;314(15):948–953. doi: 10.1056/NEJM198604103141503. [DOI] [PubMed] [Google Scholar]
- Moss R. B., Hsu Y. P., Sullivan M. M., Lewiston N. J. Altered antibody isotype in cystic fibrosis: possible role in opsonic deficiency. Pediatr Res. 1986 May;20(5):453–459. doi: 10.1203/00006450-198605000-00015. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Suter S., Nydegger U. E., Roux L., Waldvogel F. A. Cleavage of C3 by neutral proteases from granulocytes in pleural empyema. J Infect Dis. 1981 Dec;144(6):499–508. doi: 10.1093/infdis/144.6.499. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Morgenthaler J. J., Chevallier I., Schnebli H. P. Fibronectin-cleaving activity in bronchial secretions of patients with cystic fibrosis. J Infect Dis. 1988 Jul;158(1):89–100. doi: 10.1093/infdis/158.1.89. [DOI] [PubMed] [Google Scholar]
- Sveum R. J., Chused T. M., Frank M. M., Brown E. J. A quantitative fluorescent method for measurement of bacterial adherence and phagocytosis. J Immunol Methods. 1986 Jun 24;90(2):257–264. doi: 10.1016/0022-1759(86)90083-9. [DOI] [PubMed] [Google Scholar]
- Tosi M. F., Anderson D. C., Barrish J., Mason E. O., Jr, Kaplan S. L. Effect of piliation on interactions of Haemophilus influenzae type b with human polymorphonuclear leukocytes. Infect Immun. 1985 Mar;47(3):780–785. doi: 10.1128/iai.47.3.780-785.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M. F., Berger M. Functional differences between the 40 kDa and 50 to 70 kDa IgG Fc receptors on human neutrophils revealed by elastase treatment and antireceptor antibodies. J Immunol. 1988 Sep 15;141(6):2097–2103. [PubMed] [Google Scholar]
- Welsh M. J., Fick R. B. Cystic fibrosis. J Clin Invest. 1987 Dec;80(6):1523–1526. doi: 10.1172/JCI113237. [DOI] [PMC free article] [PubMed] [Google Scholar]


