Abstract
Physiological events in the initial inflammatory stage of cutaneous wound healing influence subsequent stages. Proinflammatory cytokines coordinate molecular and cellular processes during the inflammatory stage. Polyunsaturated fatty acids (PUFA) alter proinflammatory cytokine production, but how this phenomenon specifically influences wound healing is not clearly understood. In the present study, effects of marine-derived ω-3 eicosapentaenoic and docosahexaenoic PUFA on proinflammatory cytokines in wound serum and time to complete healing in healthy, human skin were evaluated. We compared plasma fatty acid levels in two groups (N=30) at baseline and after 4 weeks of eicosapentaenoic/ docosahexaenoic PUFA supplements (active) or placebo (control). Eight small blisters on participants’ forearms were created. Proinflammatory cytokines interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α were quantified in blister fluid at 5 and 24 hours after creation. Wound area was calculated daily. Eicosapentaenoic and docosahexaenoic plasma fatty acid levels were significantly higher in the active group. Additionally, we found significantly higher IL-1β levels in blister fluid in the active group and time to complete wound closure was somewhat longer. These results suggest that eicosapentaenoic and docosahexaenoic PUFA may increase proinflammatory cytokine production at wound sites and thus, depending on the clinical context, have noninvasive, therapeutic potential to affect cutaneous wound healing
Wound healing is a complex, sequential, biological process that occurs in at least three overlapping stages, the first being inflammation.1 Molecular and cellular processes during the inflammatory stage of skin healing are initiated and amplified to a large degree by a group of protein mediators known as proinflammatory cytokines.1,2 Proinflammatory cytokine synthesis and activity are affected by the concentration of ω-3 polyunsaturated fatty acids (PUFA) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), obtained primarily from fish oil, in plasma, tissue and cellular membranes as well as the ω-3 to ω-6 ratio.3
The ω-6 arachidonic acid (AA) and the ω-3 EPA are released from the phospholipid bilayer of cellular membranes in response to stimuli such as wounding and are competitively metabolized with EPA being the preferential substrate.4,5 A general assertion can be made that as dietary quantities of marine-derived EPA and DHA increase and are metabolized more lipid mediators known as eicosanoids result that are less biologically potent for inciting cellular responses than those from AA metabolism, which subsequently influences proinflammatory cytokine production.5,6
In addition, EPA and DHA are believed to affect the actual gene expression of proinflammatory cytokines at the level of transcription by altering cellular membrane fluidity, cell to cell signaling, mobility of cells, interaction of receptors with their agonist, membrane function such as capping, and formation of secondary signals.4,7 The effects of ω-3 PUFA on the locally produced proinflammatory cytokines during the inflammatory stage of cutaneous wound healing has been only minimally studied in human in vivo wounds and thus, is not yet clearly understood. Depending on the clinical context, manipulating proinflammatory cytokine production locally, either positively or negatively, during the inflammatory stage could noninvasively assist the wound healing process. The purpose of this study was to examine the effects of EPA and DHA dietary supplementation on proinflammatory cytokine production at blister wound sites during the inflammatory stage of healing and the subsequent time to complete cutaneous wound healing in a healthy human population.
The primary proinflammatory cytokines are interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α (TNF-α). They are secreted by neutrophils, macrophages, mast cells, fibroblasts and endothelial cells and have autocrine, paracrine and endocrine effects.2,8–10 These cytokines play essential roles in the signaling of biological processes during the inflammatory stage of wound healing by binding to receptors on target cells.1,2,11 Collectively, this network of proinflammatory cytokines assists in controlling infection and preparing tissue for further repair by enhancing phagocytic activity, stimulating keratinocyte migration at wound edges, fibroblast chemotaxis and proliferation, breakdown of extracellular matrix proteins and by regulating the release of additional cytokines and growth factors.1,2 An increased expression of proinflammatory cytokines within a few hours after tissue injury has been shown to correspond to the inflammatory stage of wound healing and normal repair.12 Conversely, a diminished production of proinflammatory cytokines in the initial stage of wound healing was found in glucocorticoid-treated mice and was associated with impaired wound healing.11
Studies have shown that increasing dosages of the ω-3 PUFA EPA and DHA in the form of encapsulated fish oil to a concentration of 1% of the total phospholipid fatty acids will decrease systemic lymphocyte proliferation and IL-1β, TNF-α, and IL-6 by as much as 90%, 70%, and 60%, respectively.13 A total daily intake of 1.6 g of EPA and 1.1 g of DHA resulted in a significant reduction in proinflammatory cytokine production systemically after 4 weeks of consumption.14 Based on dietary estimates for the US population, the mean adult intake for EPA and DHA is only 0.03–0.06 g/day and 0.06–0.09 g/day, respectively,15 but a therapeutic threshold is quickly reached with dietary supplementation.16 Dietary supplements of EPA and DHA could affect local cellular and molecular activity, such as the gene expression of proinflammatory cytokines, during the inflammatory stage of healing and thus, subsequent stages.
Numerous studies exist regarding the correlation between increased levels of ω-3 PUFA and diminished proinflammatory cytokine production in peripheral blood monocytes and plasma, but a few studies have shown enhancement in the production of certain proinflammatory cytokines in cells such as fibroblasts and peritoneal macrophages treated with ω-3 PUFA.17–19 Furthermore, a positive correlation was found between collagen production in EPA-treated porcine medial collateral ligament fibroblasts and proinflammatory IL-6 production in in vitro wounds.20 The mixed study findings concerning the effects of ω-3 PUFA on proinflammatory cytokine levels may be related to variations in study designs and cell types, but collectively the results point to their potential ability to affect epidermal wound healing because of their influence on proinflammatory cytokine levels and thus, cell types that are important to the healing process.
We attempted to clarify the effects of ω-3 EPA and DHA PUFA on cutaneous healing in vivo in healthy individuals to address a gap in the literature regarding potential relationships among increased EPA/DHA consumption, proinflammatory production, and cutaneous wound healing. Our hypotheses were that individuals consuming ω-3 EPA/DHA dietary supplements for 4 weeks before blistering would have significantly lower levels of proinflammatory cytokines at blister wound sites and a slower time to complete wound healing than those in the control group consuming a placebo for the same interval of time.
MATERIALS AND METHODS
Procedures
This randomized, double-blind, repeated measures, experimental study was conducted at the General Clinical Research Center (GCRC) at The Ohio State University. The majority of the sample was recruited from the surrounding academic area and medical center. Based on a power analysis with an estimated large effect size of .40, power at .80, and .05 significance, 14 participants per group were necessary to conduct a two-group repeated measures analysis of variance (ANOVA).21 Effect size was based on documented reductions in systemic proinflammatory cytokine levels due to ingestion of EPA and DHA.13,22,23 One additional subject (11%) per group was added to account for possible attrition based on previous studies using a blister initiation procedure24 for a total number of 30 subjects. Thirteen men and 17 women were assigned by computerized random sort to either the EPA/ DHA supplement or placebo group and blinded as to treatment.
Participants were healthy individuals between 18 and 45 years of age with the ability to read and write English. Individuals were excluded if they were taking nonsteroidal anti-inflammatory drugs, aspirin, lipid-lowering medications, nutritional supplements or corticosteroids and those experiencing chronic inflammatory skin diseases or were pregnant or lactating. Individuals who had immunologic related health problems such as cancer, autoimmune diseases, diabetes mellitus or peripheral vascular disease, difficulties with wound healing, surgery in the past year, self-reported current smokers or those reporting drinking 10 or more alcoholic beverages per week were also excluded.
After Institutional Review Board (IRB) approval, participants were recruited through advertisements placed in the university newspaper and on university bulletin boards. Following a complete explanation of the study including potential risks and benefits, informed written consent was obtained from eligible individuals. In addition to IRB approval, all research methods conducted were in compliance with the ethical rules for human experimentation stated in the 1975 Declaration of Helsinki.
Visit one took place at the GCRC at which time demographic data were collected that included age, gender, ethnicity, and education level. Body measurements consisted of height, weight, sagittal abdominal diameter (SAD), and body mass index (BMI). The primary investigator (PI), who was blinded to group assignment, provided verbal and written instructions to participants to take five softgels (Group 1- ω-3 softgels, Group 2 – placebo softgels) at bedtime until study completion. A specific date to begin the softgels was assigned to each participant. A total daily intake of 1.6 g of EPA and 1.1 g DHA has been determined to decrease proinflammatory cytokine production in peripheral blood after 4 weeks of consumption and, thus was the chosen dosage and time frame for this study.14 The placebo softgel contained 2.4mL of mineral oil. All softgels were the same in appearance and packaged in like containers by J.R. Carlson Laboratories, Inc. (Arlington Heights, IL). Verbal and written instructions were given to participants to maintain their usual diets, but to exclude fish, seafood, kelp and flaxseeds until study completion. Blood was collected for plasma fatty acid analysis after an 8-hour fast. Food frequency questionnaires (FFQ) were completed, which reflected micro and macro nutrients for the three months before study enrollment.
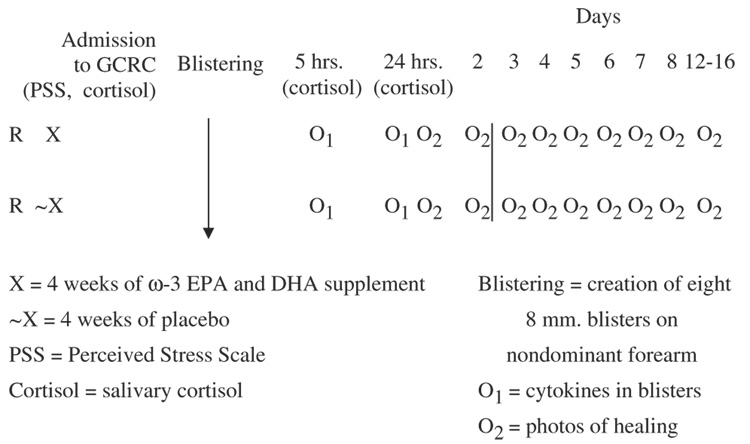
Four weeks after beginning the softgels, each participant was admitted before 9 a.m. for a 26-hour stay at the GCRC and was discharged the following morning. Parking (or bus) expenses and controlled meals were provided. Participants received $150 after completing the 5-week study. An illustration of the design and variable measurement points during the GCRC stay and postblistering period is provided (Figure 1).
Figure 1.
Stugy design.
Blister initiation
A suction blister protocol was modeled after one utilized in studies at the National Institute of Allergy and Infectious Diseases and at The Ohio State University.24,25 A similar suction blister device was also used (Electronic Diversities, Finksburg, MD). The blister wound model has been used extensively to examine, among other processes, epidermal regeneration.26 Two templates, possessing a total of eight circular orifices 8mm in diameter, were taped to the volar surface of the nondominant forearm. A vacuum of 350 mmHg was applied through a pump attached to a regulator until blisters formed 1 − 1½ hours (Electronic Diversities). The dermoepidermal junction was separated by the gentle suction and eight sterile 8-mm blisters were formed. Fluid was aspirated from each blister (0.05–0.1 mL) with a 27 gauge needle and syringe; the blister roof (epidermis) was removed with sterile scissors. Two plastic templates, which contained a total of eight wells, were placed directly over the blister wounds. Using a syringe with an attached angiocath the template wells were filled with 0.8–1.0 mL of 70% autologous serum, consisting of serum from the subjects’ own blood combined with Hanks’ balanced salt solution, and the eight well tops sealed with sterile tape. The autologous serum-buffer solution was aspirated via an angiocath attached to a syringe from four of the wells 5 hours after blister formation. In 24 hours the remaining solution was aspirated from the other four wells and the template removed. A standard wound care protocol was initiated before discharge.
After discharge
Daily assessments of the healing of the superficial blister sites occurred for 8 consecutive days after discharge and then every 4 days until complete healing. The PI, blinded to group assignment, utilized the single digital camera photogrammetry (SCP) method of wound assessment to quantify daily wound area yet to be healed for all eight blisters (Verge Videometer Measurement Documentation [VeV MD], Winnipeg, Manitoba, Canada).
Plasma fatty acid assay
The fatty acids evaluated in plasma included total ω-3 PUFA, ω-3 EPA and DHA, total ω-6 PUFA, ω-6 AA, and the ω-6/ω-3 ratio and were quantified by gas chromatography/ mass spectrometry (GC/MS) at Metametrix Clinical Laboratory (Norcross, GA). Fasting blood samples (1.0 mL) were collected in EDTA vacutainers and centrifuged at 720 g for 30 minutes at room temperature to isolate the plasma fraction. The plasma samples were stored a −80°C before analysis. Sample preparation consisted of a methyl esterification reaction followed by liquid/liquid extraction before analysis. The samples were analyzed using an Agilent 6890N GC with autosampler and an Agilent 5973N mass spectrometer (Agilent Technologies, Inc., Santa Clara, CA). The analytical separation was performed on a HP-23 (Cis/Trans FAME capillary column) 60 mm × 0.25 mm × 0.25 mm film thickness.
Stress assays
Increased stress has been associated with diminished proinflammatory production and delayed wound healing and thus, is essential to consider in an in vivo wound healing study.24,27 In this study all subjects completed a Perceived Stress Scale (PSS) on admission to the GCRC (after 4 weeks of treatment or placebo). The PSS is a 10-item questionnaire with a scale of 0 (never) to 4 (very often) that measures how unpredictable, uncontrollable and overloaded one perceives their daily life in the previous month. Possible scores range from 0 to 40 with a higher score suggesting greater perceived stress.
Salivary cortisol was measured on admission to the GCRC and at 5 and 24 hours postblistering to appraise the potential stressful effects of the blistering procedure. Saliva was obtained from a dental cotton roll and assayed by the GCRC core lab using the solid-phase radioimmunoassay (RIA) procedure (Diagnostic Products Corporation, Los Angeles, CA). Salivary cortisol is a valid and reliable measure of unbound hormone in the blood and reflects the physiological stress response.28
Proinflammatory cytokine assay
Locally produced proinflammatory cytokines (IL-1β, TNF-α, and IL-6) were measured in the blister fluid obtained at 5 and 24 hours postblister formation. Determinations were made by the GCRC core lab using electrochemiluminescence Multiplex System Sector 2400 imager (Meso Scale Discovery [MSD], Gaithersburg, MD). Samples were assayed in duplicate using the Human Proinflammatory II 4-Plex Ultra-Sensitive Kits.
Macroscopic analysis of wound healing
Wound healing was defined as the advance of the wound margins toward the wound center. Daily area yet to be healed was measured by a noncontact method using SCP and the wound measurement software (VeV MD, Vista Medical, Winnipeg, Manitoba, Canada) on 8 consecutive days after blister initiation and every 4 days thereafter until complete (100%) healing occurred. An orientation card of known dimensions was placed next to blister sites in view of the camera. The blister images were then downloaded to the VeV MD computer software program. Blister wounds were compared to the known size of the orientation card per the computer software. The wound perimeters were outlined on the computer with a cursor by the PI and the sum of the areas yet to be healed for all eight blisters was then calculated.
Total surface area yet to be healed for all eight blisters at each time point for 15 randomly selected subjects was recalculated by the same PI who was blinded to group assignment (active or placebo) and compared to initial calculations to evaluate intrarater reliability of the VeV measurement system. Significant Pearson’s correlation coefficients ranged from 0.59 to 0.99 with an average of 0.88 signifying that the VeV measurement system demonstrated high test–retest intrarater reliability in the current study.
Statistical analyses
Statistical analyses were conducted using the SPSS statistical package.
A two-group (with and without ω-3 fatty acid EPA/DHA supplement) across time (at GCRC admission, 5 and 24 hours after blistering) within and between group ANOVA was used to compare salivary cortisol levels. t-tests were used for between group comparisons of socio-demographic data, body measurements, plasma fatty acid levels (0 and 4 weeks), proinflammatory cytokines at blister sites (5 and 24 hours postblistering), total area yet to be healed (cm2/day) for all eight blisters, total lapsed time (days) to 0 cm2 wound closure, PSS scores and nutritional data generated by the FFQs. Within group comparisons of plasma fatty acid levels and proinflammatory cytokines in blister sites were evaluated with paired t-tests. Significance levels were set a priori at α=0.05.
RESULTS
Demographics, nutritional, and stress influences
Demographic characteristics describing participants in the active and placebo groups are displayed in Table 1 with similar data for both groups. Baseline nutritional data obtained from the FFQs showed no statistically significant differences between the active and placebo groups in regard to nutrients that could potentially influence wound healing (Table 2).
Table 1.
Demographic characteristics of participants (N=30)
| Activea (n=16) |
Placeboa (n=14) |
|
|---|---|---|
| Age, mean years (SD) | 23 (5.4) | 28 (8.4) |
| Male (%) | 44 | 43 |
| White (%) | 76 | 79 |
| African American (%) | 12.5 | 7 |
| Asian (%) | 12.5 | 14 |
| Educational level (%) | ||
| College graduates | 39 | 57 |
| Undergraduate students | 56 | 43 |
| High school graduates | 6 | 0 |
| Height, centimeters (SD) | 168.6 (8.8) | 172.1 (9.5) |
| Weight, kilograms (SD) | ||
| Baseline | 74.6 (24.8) | 76.7 (18.5) |
| 4 weeks | 75.6 (26.0) | 76.7 (19.1) |
| BMI, kg/m2(SD) | ||
| Baseline | 26.1 (7.5) | 25.7 (4.4) |
| 4 weeks | 26.4 (7.8) | 25.6 (4.5) |
| SAD, centimeters (SD) | ||
| Baseline | 19.4 (4.9) | 18.6 (2.7) |
| 4 weeks | 19.8 (5.1) | 18.7 (3.3) |
No significant differences between groups; BMI, body mass index; SAD, sagittal abdominal diameter.
Table 2.
Dietary characteristics of participants at baseline (N=30)
| Mean (SD) | ||
|---|---|---|
| Active (n=16) |
Placebo (n=14) |
|
| FFQ data (visit 1) estimates daily intake for preceding 3 mos. | ||
| Mean vitamin C mg/day (SD) | 125.3 (69.0) | 136.9 (84.5) |
| (RDAs 75–90 mg/day) | ||
| Mean protein g/day (SD) | 91.3 (32.1) | 91.4 (53.5) |
| (RDAs 46–56 g/day)* | ||
| Mean kilocalories/day (SD) | 2046.6 (562.6) | 2188.0 (1084.4) |
| (RDAs 1,848–3,141 kcal/day)** | ||
| Mean EPA g/day (SD) | .09 (.17) | .04 (.04) |
| Mean DHA g/day (SD) | .24 (.40) | .09 (.07) |
| (AI 0.50 g/day EPA+DHA)*** | ||
| Data below from demographic questionnaire (visit 1) | ||
| Mean cups of caffeine/day (SD) | 1.4 (1.8) | 1.6 (1.5) |
| Mean alcoholic drinks/week (SD) | 1.2 (2.2) | 1.2 (2.2) |
Based on 0.8 g/kg body weight for reference body weight for adults 18– > 70 years of age.
Estimated energy requirements (EER) for men and women 30 years of age; For each year below 30, add 7 kcal/day for women and 20 kcal/day for men. For each year above 30, subtract 7 kcal/day for women and 20 kcal/day for men. Requirements vary with BMI and physical activity level.
Source: International Society for Study of Fatty Acids and Lipids; Recommendations for Dietary Intake of Polyunsaturated Fatty Acids in Healthy Adults (2004).
Source: Food and Nutrition Board, Institute of Medicine, National Academies; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (2002).
RDA, Recommended Dietary Allowances; AI, Acceptable Intake.
Statistical analyses of PSS scores (at GCRC admission) and salivary cortisol levels (at GCRC admission and at 5 and 24 hours postblistering) demonstrated that participants in both groups were similar in their psychological and physiological reactions to stress. Salivary cortisol levels at times 1 and 3 were significantly higher than at time 2 for all participants (F=17.558, df=1, p < 0.001), however the interaction between treatment group and time was not significant (Table 3). The correlation between PSS scores and salivary cortisol levels was not significant perhaps because PSS scores reflected perceived psychological stress in the preceding month while salivary cortisol levels represented the physiological stress response at the time the salivary sample was collected.
Table 3.
Stress characteristics of participants (N=30)
| Mean (SD) | ||
|---|---|---|
| Active (n=16) |
Placebo (n=14) |
|
| Mean PSS Scores (SD) | 13 (3.4) | 12 (6.5) |
| Higher score (0–40)=greater stress over preceding month | ||
| Mean salivary cortisol levels (µg/dL; SD) | ||
| Time 1 (GCRC entry-a.m.) | 0.40 (0.2) | 0.39 (0.2) |
| Time 2 (5 hours postblistering-p.m.) | 0.16 (0.2)* | 0.14 (0.1)* |
| Time 3 (24 hours postblistering-a.m.) | 0.34 (0.2) | 0.39 (0.3) |
Significantly different from both Times 1 and 3 within groups (p < .001).
PSS, Perceived Stress Scale.
Plasma fatty acid measures
As expected, at 4 weeks postenrollment, those who received the EPA/DHA supplement for 4 weeks, had significantly higher plasma EPA (t=8.17, df=16, p < .000), DHA (t=8.25, df =21, p < .001), and total ω-3 PUFAs (t=8.50, df=19, p < .001), and significantly lower ω-6 PUFAs (t= −2.67, df=28, p < .01), AA to EPA ratio (t= −6.023, df=13, p < .001) and total ω-6 to ω-3 ratio (t= −10.50, df=18, p < .001) than the placebo group (Table 4).
Table 4.
Measures of plasma fatty acid for active and placebo group (Mean±SD)
| Baseline | Week 4 | Change | ||||
|---|---|---|---|---|---|---|
| Fatty acids (µM/L) | AC (n=16) | PL (n=14) | AC (n=16) | PL (n=14) | AC (n=16) | PL (n=14) |
| EPA | 20.9±14.9 | 22.0±29.7 | 125.2±53.1a,b | 15.1±8.6b | 99.9±57.8a,b | −6.9±30.0b |
| DHA | 110.1±38.4 | 102.2±41.2 | 179.4±42.2a,b | 83.8±17.9b | 67.3±43.9a,b | −18.4±35.5b |
| AA | 668.7±144.1 | 644.9±84.4 | 576.8±121.1a,b | 651.0±102.2b | −77.8±105.5a,b | 6.1±70.4b |
| Total | ||||||
| ω-6 | 2490.6±482.1 | 2599.6±357.0 | 2218.7±403.1a,b | 2589.8±349.9b | −259.1±290.5a,b | −9.8±255.2b |
| ω-3 | 182.2±57.3 | 187.7±85.0 | 371.8±94.7a,b | 158.3±31.2b | 181.3±107.7a,b | −29.3±83.9b |
| Ratios | ||||||
| AA/EPA | 43.8±24.3 | 49.0±21.8 | 5.5±2.7a,b | 57.2±32.0b | −38.1±25.7a,b | 8.3±22.9b |
| ω-6/ω-3 | 14.4±3.2 | 15.2±4.0 | 6.3±1.6a,b | 16.8±3.5b | −8.0±3.7a,b | 1.6±2.6b |
Significantly different from baseline–within group (p < .05).
Significantly different between groups @ week 4 (p < .05).
AC, active; PL, placebo; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid.
Fatty acid plasma levels were also examined for each individual to evaluate within group change from baseline to 4 weeks. Not surprisingly, the change in plasma fatty acid measures for participants who received the EPA/DHA supplement was significantly higher than the placebo group for EPA (t=6.30, df=21, p < .001), DHA (t=5.75, df=27, p < .001), and total ω-3 PUFAs (t= 5.85, df=27, p < .001), and significantly lower for AA (t= −2.50, df=27, p < .02), total ω-6 PUFAs (t= −2.45, df=27, p < .02), ratio of AA to EPA (t= −5.12, df=27, p < .001) and ratio of total ω-6 to total ω-3 fatty acids (t= −8.04, df=27, p < .001). From these data it can be surmized that the EPA/DHA supplements were taken appropriately.
Proinflammatory cytokine responses
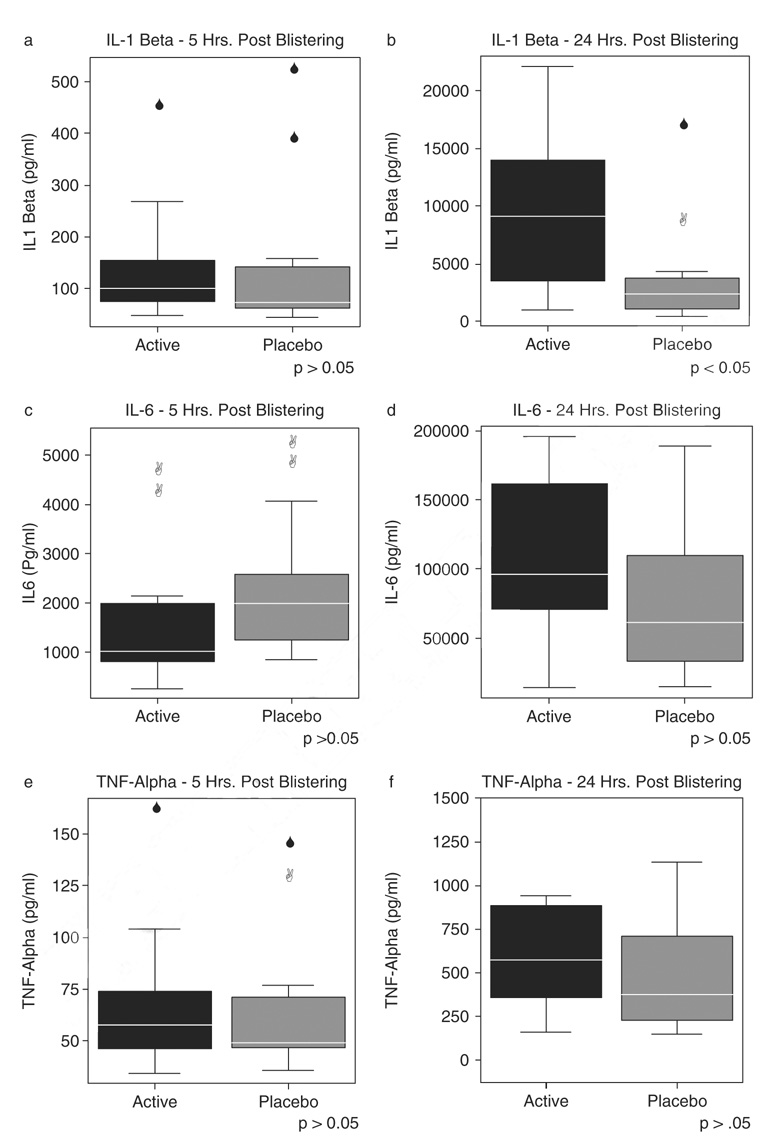
As anticipated, IL-1β, IL-6, and TNF-α proinflammatory cytokines levels in blister wound serum increased across the two time points, 5 and 24 hours postblistering, for both groups. Unexpectedly however, and contrary to our original hypothesis, participants who received EPA/DHA supplementation to their diet for 4 weeks, had significantly higher rather than lower levels of the IL-1β proinflammatory cytokines in the blister fluid at 24 hours postblistering than the placebo group (t=2.52, df=25, p < .05) (Figure 2B).
Figure 2.
Proinflammatory cytokine production-blister fluid in active (n=16) and placebo (n=14) groups. Upper and lower bars represent largest and smallest values that are not outliers.
To examine the relationships among predictor variables and IL-1β values at 24 hours postblistering, 15 possible variables were chosen for a forward stepwise multiple regression procedure. Interestingly, using that procedure the model chose gender and the ratio of AA/EPA at week 4 as the two primary predictors of IL-1 levels at 24 hours post-blistering Together those two variables explained 47.4% of the variance in the IL-1β values at that time point. Males and participants with lower AA:EPA ratios had higher IL-1β levels. Lower AA:EPA ratios were significantly correlated (p < .001) with the active treatment group.
There were higher quantities of IL-6 and TNF-α cytokines (Figure 2D and F) in blister fluid for both groups across time, but again, participants who consumed the EPA/DHA supplement for 4 weeks produced nonsignificantly higher levels of IL-6 and TNF-α cytokines in blister fluid at 24 hours postblistering.
Wound healing measures
The overall wound size in centimeter square for participants who received the ω-3 fatty acid EPA/DHA (fish oil) supplement for 4 weeks compared with those who consumed the placebo for the same interval, was not significantly different at any time point.
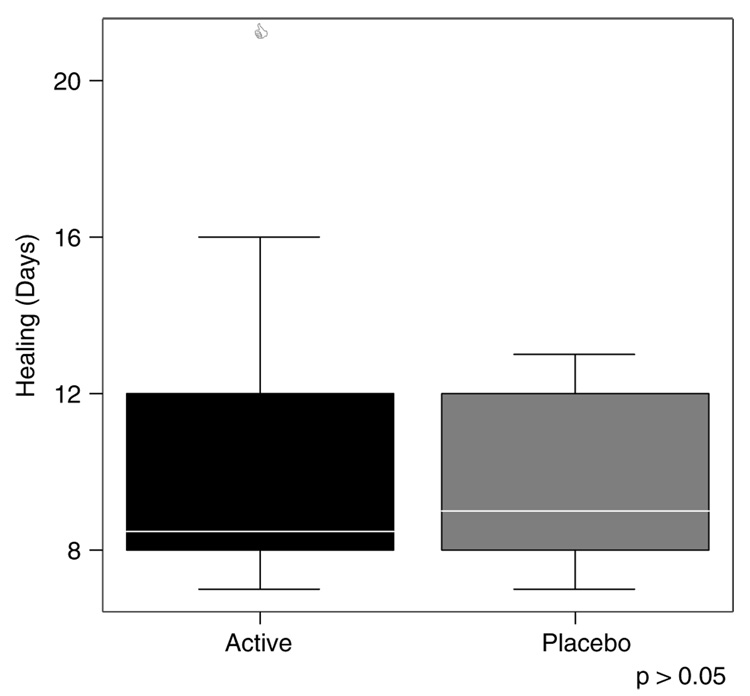
Time to complete wound healing was then evaluated (Figure 3). The number of days to complete wound healing (100% closure) was not significantly different between the two groups, although the mean number of days to complete healing for those who consumed the EPA/DHA supplement for 4 weeks before the blistering procedure, was slightly longer (10.7 days) than the mean (9.8 days) for those in the placebo group.
Figure 3.
Days to complete healing by active (n=16) and placebo (n=14) groups. Upper and lower bars represent largest and smallest values that are not outliers.
DISCUSSION
In the present work, we observed that with an EPA/DHA dose of 1.6 and 1.2 g/day respectively, there were significant increases in plasma fatty acids levels for both EPA and DHA from baseline to four weeks for individuals in the active group, which were basically consistent with several previous studies.16 In addition, there were significantly diminished quantities of AA and ratio of total ω-6–ω-3 fatty acids in individuals consuming the EPA/DHA supplement for 4 weeks, which were outcomes also aligned with some earlier research findings.14,29 Collectively, the plasma fatty acid results from the present study validated the use of the supplements by participants in the active group during the study interval and that the supplied dose was adequate to raise plasma fatty acid levels from baseline. There were no significant changes in plasma fatty acid levels for participants in the control group who consumed the placebo, mineral oil, for 4 weeks.
Interestingly, a recent study revealed that when EPA and DHA were provided as supplements to the diet in a total dose as low as 1 g/day in the form of ethyl esters, which are taken up much more slowly than the more rapidly absorbed triacyglycerols from fish, EPA, and DHA plasma levels rose from 0.6% to 1.4% and 2.9% to 4.3% respectively within only 10 days.30 Additionally, both EPA and DHA values returned to near baseline measures within 10 days of discontinuing the supplements. In the current study, the decision to use the chosen EPA/ DHA dose was based on work by Caughey et al.,14 which reported plasma proinflammatory cytokine inhibition after 4 weeks with the same dose. It may be beneficial in future wound studies to include smaller EPA/DHA dosages, in the form of both ethyl esters and triacyglycerols, for shorter time periods in a stratified design. Correlating data from this type of design may clarify the minimal EPA/DHA doses that affect the target mediators, such as proinflammatory cytokines, that control inflammatory responses during wound healing.
Not surprisingly, we found that IL-β, IL-6, and TNF-α proinflammatory cytokine levels in blister fluid were significantly increased across time from 5 to 24 hours after blister wound initiation in both groups in response to tissue trauma, which is consistent with earlier studies using suction blister models.11,24,25
Although the findings of significantly elevated IL-1 levels at 24 hours postblistering in the active group over the placebo group were not consistent with our original hypothesis, the results were in concert with a study that observed higher IL-1 expression in EPA treated nonirradiated and UVB-irradiated keratinocytes.31 Interleukin-1 assists in regulating fibroblast proliferation and collagen synthesis2 therefore, it can be posited that its initial upregulation at the wound site, as a result of EPA/DHA dietary supplementation, could be a pathway to regulate collagen formation. Improved production of healthy collagen is advantageous for efficient healing of skin with minimal scarring and for providing strength for connective tissues such as ligaments.17 In addition, initial IL-1 expression increases keratinocyte growth, important for reepithelialization,32 induces the production of IL-6 and has a stimulatory effect on angiogeneses.
Unexpectedly, the other primary predictor variable that contributed to the 47.4% of the variance in IL-1 values at 24 hours postblistering was gender (males), which could be related to the possible modulatory effects of local and systemically produced steroid hormones. Though the understanding of the role of estrogen in cytokine production is limited, it has been postulated that estrogen has an inhibitory effect on proinflammatory cytokine activity that could vary with tissue type.33 This is one explanation for the naturally occurring sexual dimorphisms of various inflammatory-related neurological disorders. It may be beneficial for future wound healing studies that explore the effects of EPA/DHA consumption on proinflammatory cytokine production to include measures of estrogen and testosterone, so that potential variations in data correlated to gender can be analyzed more thoroughly.
Although not statistically significant, higher quantities of IL-6 and TNF-α were also found in the wound fluid in the active group at 24 hours postblistering when compared with those in the placebo group. These identified trends may have translated to statistical significance if a larger sample size had been utilized. Again, these findings were not aligned with our original hypotheses, but were similar to a few previous studies that investigated the effects of EPA and DHA on tissue healing in animal models and cell cultures.20,31 For example, a study by Hankenson et al.20 found that EPA significantly elevated IL-6 release in medial collateral ligament cells in a bovine model. The researchers also found a significant linear correlation between IL-6 levels and collagen production. This suggests that EPA dietary supplementation may facilitate healing in tissues that particularly benefit from increased collagen production, such as ligaments. An interesting result in the present study was that increased levels of TNF-α in blister fluid at 24 hours postblistering were associated with slightly slower healing in the active group, which differs from an earlier study that demonstrated diminished local levels of TNF-α in healing-impaired glucocorticoid-treated mice.11
In the present study, we established that the time to complete wound healing (100% closure) for all eight blisters did not differ significantly between the two groups, however, those in the active group who consumed the EPA/DHA supplement for 4 weeks took approximately 1 day longer to heal than those in the placebo group. Considering the small size of the blister wounds, this finding could be of clinical significance. The higher levels of proinflammatory cytokines in blister fluid, found at 24 hours postblistering in the active group, suggest a more vigorous early inflammatory reaction. Therefore, it may have taken longer for the exudative stage of the inflammatory response to resolve, which may have resulted in a slightly longer time to complete wound closure. A previous study that measured wound healing in dog models also observed a longer time for reepithelialization in surgical wounds after ω-3 PUFA supplementation when compared with other dietary alterations.34 Furthermore, another research paper reported that topical administration of ω-3 PUFA to surgical wounds of mice was associated with slower wound closure in the first 10 days after surgery.35 Conversely, Ruthig et al.36 found improved reconstitution of epithelial integrity with both ω-3 and ω-6 PUFA-treated intestinal cells in rats following mucosal injury. Once again, these variable findings support the need for additional research studies to elucidate the effects PUFA have on wound healing and compare outcomes among similarly designed endeavors.
In summary, this study is the first to examine the effects of ω-3 (EPA/DHA) fatty acid dietary supplements on proinflammatory cytokine production in blister wound fluid and subsequently on cutaneous healing in a healthy human population. The results presented in this paper linked the EPA/DHA dietary supplements, taken by the participants in the active group, to a decreased plasma AA:EPA ratio from baseline and a significantly higher production of the proinflammatory cytokine IL-1β at blister wound sites at 24 hours postblistering, when compared with the placebo group, and nonsignificantly slower wound healing. Although not consistent with the original hypotheses, the study findings support the proposition that dietary ω-3 PUFA demonstrate ability to affect the local production of inflammatory mediators, such as proinflammatory cytokines, that regulate the wound healing process. Future research will hopefully clarify whether they are beneficial or detrimental to various wound types and populations of patients. This knowledge is desirable because the use of fish oil supplements, as well as other forms of complementary/ alternative medical (CAM) or integrative therapies, is growing briskly in the US
In 2002, 36% of adults in the US used some form of CAM therapy, such as dietary supplements, for health reasons.37 Approximately 12% of the adult population adds fish oil/ω-3 fatty acids to their diet and that number is on the rise.38 Essential fatty acid sales increased 50.3% in 2003 over 2002 and represented 11.5% of supplements’ total dollar sales.39 Thus, many individuals are consuming varying doses of EPA and DHA through supplements, which may alter cellular and molecular activities that influence wound healing. As our understanding of their bioactivity expands, it may be found increasingly important for clinicians to evaluate patients’ ω-3 fatty acid use when a wound is anticipated or already present, particularly if negative effects are identified.
ACKNOWLEDGMENTS
Supported by NIH/NINR Predoctoral NRSA Fellowship Awards 1 F31 NR009134-01, Sigma Theta Tau International, Epsilon Chapter and Alumni Grants for Graduate Research & Scholarship, The Ohio State University.
Glossary
- AA
arachidonic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- IL-1β
interleukin-1beta
- IL-6
interleukin-6
- PUFA
polyunsaturated fatty acids
- TNF-α
tumor necrosis factor-alpha
REFERENCES
- 1.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 2.Efron P, Moldawer L. Cytokines and wound healing: the role of cytokine and anticytokine therapy in the repair response. J Burn Care. 2004;25:149. doi: 10.1097/01.bcr.0000111766.97335.34. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm H, Mayer K, Mayser P, Eigenbrodt E. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br J Nutr. 2002;87 Suppl. 1:S59–S67. doi: 10.1079/bjn2001457. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JW. Immunonutrition: the role of omega-3 fatty acids. Nutrition. 1998;14:627–633. doi: 10.1016/s0899-9007(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 6.Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345–358. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DR, Urakaze M, Huang R, Taki H, Sugiyama E, Knoell CT, Xu L, Yeh ET, Auron PE. Dietary marine lipids suppress continuous expression of interleukin-1 beta gene transcription. Lipids. 1996;31 Suppl:S23–S31. doi: 10.1007/BF02637046. [DOI] [PubMed] [Google Scholar]
- 8.Miossec P, Ziff M. Immune interferon enhances the production of interleukin 1 by human endothelial cells stimulated with lipopolysaccharide. J Immunol (Baltimore, MD) 1986 137##. [PubMed] [Google Scholar]
- 9.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 10.Le JM, Weinstein D, Gubler U, Vilcek J. Induction of membrane-associated interleukin 1 by tumor necrosis factor in human fibroblasts. J Immunol. 1987;138:2137–2142. [PubMed] [Google Scholar]
- 11.Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoidtreated mice. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 12.Grellner W, Georg T, Wilske J. Quantitative analysis of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int. 2000;113:251–264. doi: 10.1016/s0379-0738(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 13.Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 14.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 15.Ervin RB, Wright JD, Wang C, Kennedy-Stephenson J. Dietary intake of fats and fatty acids for the United States population 1999–2000. Adv Data. 2004;348:1–6. [PubMed] [Google Scholar]
- 16.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83 Suppl:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 17.Jia Y, Turek JJ. Inducible nitric oxide synthase links NF-kappaB to PGE2 in polyunsaturated fatty acid altered fibroblast in-vitro wound healing. Lipids Health Dis. 2005;4:14. doi: 10.1186/1476-511X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardardottir I, Kinsella JE. Tumor necrosis factor production by murine resident peritoneal macrophages is enhanced by dietary n-3 polyunsaturated fatty acids. Biochim Biophys Acta. 1991;1095:187–195. doi: 10.1016/0167-4889(91)90098-i. [DOI] [PubMed] [Google Scholar]
- 19.Petursdottir DH, Olafsdottir I, Hardardottir I. Dietary fish oil increases tumor necrosis factor secretion but decreases interleukin- 10 secretion by murine peritoneal macrophages. J Nutr. 2002;132:3740–3743. doi: 10.1093/jn/132.12.3740. [DOI] [PubMed] [Google Scholar]
- 20.Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223:88–95. doi: 10.1046/j.1525-1373.2000.22312.x. [DOI] [PubMed] [Google Scholar]
- 21.Stevens JP. Applied multivariate statistics for the social sciences. 4th ed. Mahnah, N.J.: Lawrence Erlbaum Associates, Inc.; 2002. [Google Scholar]
- 22.Blok WL, Katan MB, van der Meer JW. Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. J Nutr. 1996;26:1515–1533. doi: 10.1093/jn/126.6.1515. [DOI] [PubMed] [Google Scholar]
- 23.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 24.Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry. 1999;56:450–456. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- 25.Kuhns DB, DeCarlo E, Hawk DM, Gallin JI. Dynamics of the cellular and humoral components of the inflammatory response elicited in skin blisters in humans. J Clin Invest. 1992;89:1734–1740. doi: 10.1172/JCI115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottrup F, Agren MS, Karlsmark T. Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen. 2000;8:83–96. doi: 10.1046/j.1524-475x.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 27.Head CC, Farrow MJ, Sheridan JF, Padgett DA. Androstenediol reduces the anti-inflammatory effects of restraint stress during wound healing. Brain Behav Immunol. 2006;20:590–596. doi: 10.1016/j.bbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinol. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 29.Mayer K, Fegbeutel C, Hattar K, Sibelius U, Krämer HJ, Heuer KU, Temmesfeld-Wollbrück B, Gokorsch S, Grimminger F, Seeger W. Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med. 2003;29:1472–1481. doi: 10.1007/s00134-003-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rupp H, Wagner D, Rupp T, Schulte L, Maisch B. Risk stratification by the “EPA + DHA level” and the “EPA/AA ratio” focus on anti-inflammatory and antiarrhythmogenic effects of long-chain omega-3 fatty acids. Herz. 2004;29:673–685. doi: 10.1007/s00059-004-2602-4. [DOI] [PubMed] [Google Scholar]
- 31.Pupe A, Moison R, De Haes P, van Henegouwen GB, Rhodes L, Degreef H, Garmyn M. Eicosapentaenoic acid, a n-3 polyunsaturated fatty acid differentially modulates TNF-alpha, IL-1alpha, IL-6 and PGE2 expression in UVB-irradiated human keratinocytes. J Invest Dermatol. 2002;118:692–698. doi: 10.1046/j.1523-1747.2002.01615.x. [DOI] [PubMed] [Google Scholar]
- 32.Sauder D. Interleukin-1 enhances epidermal wound healing. Lymphokine Res. 1990;9:465. [PubMed] [Google Scholar]
- 33.Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Estrogen and cytokines production – the possible cause of gender differences in neurological diseases. Curr Pharm Des. 2005;11:1017–1030. doi: 10.2174/1381612053381693. [DOI] [PubMed] [Google Scholar]
- 34.Mooney MA, Vaughn DM, Reinhart GA, Powers RD, Wright JC, Hoffman CE, Swaim SF, Baker HJ. Evaluation of the effects of omega-3 fatty acid-containing diets on the inflammatory stage of wound healing in dogs. Am J Vet Res. 1998;59:859–863. [PubMed] [Google Scholar]
- 35.Cardoso CR, Souza MA, Ferro EA, Favoreto S, Jr, Pena JD. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12:235–243. doi: 10.1111/j.1067-1927.2004.012216.x. [DOI] [PubMed] [Google Scholar]
- 36.Ruthig DJ, Meckling-Gill KA. Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6. J Nutr. 1999;129:1791–1798. doi: 10.1093/jn/129.10.1791. [DOI] [PubMed] [Google Scholar]
- 37.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;27:1–19. [PubMed] [Google Scholar]
- 38.National Center for Health Statistics. National health interview survey (NHIS) Public Use Data Release, NHIS survey description. 2002
- 39.Uhland V, Lewis K, Spehar C. American top 25: supplements climbing the charts. Natural Foods Merchandiser. 2004;25:44–52. [Google Scholar]
- 40.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]