Abstract
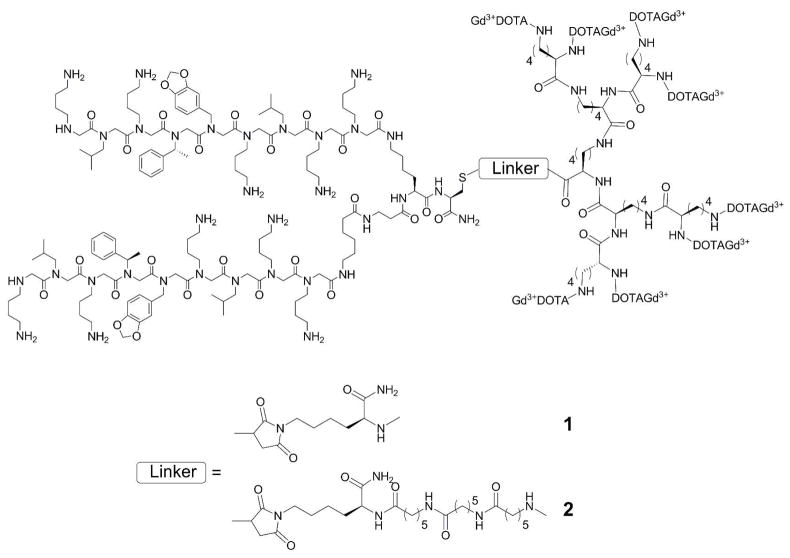
The synthesis of a polylysine dendron containing eight GdDOTA units conjugated to peptoid dimer known to have a high affinity for the vascular endothelial growth factor receptor 2 (VEGFR2) is described. This simple low molecular weight system with a molecular r1 relaxivity of ~48 mM−1s−1 is shown to enhance MR images of tumors grown in mice in vivo.
Magnetic resonance imaging (MRI) is widely used for anatomical imaging of soft body tissues and for measuring dynamic processes such as perfusion, diffusion and chemical exchange. Paramagnetic complexes (largely Gd3+, Fe2+, Mn2+) are commonly used to enhance contrast differences by altering the inherent relaxation properties (T1, T2, T2*) of tissue water. MR contrast agents are typically given in high doses (0.1 mmol/kg) and consequently are generally considered too insensitive for molecular imaging applications. Consequently, MR contrast agents designed to target specific biostructures are often based on nanoparticle or dendrimer platforms that allows significant amplification by additive effect of multiple paramagnetic centers over a single center.1 Although this approach does improve the sensitivity of MR agents, changing from a simple low molecular weight complex to a large particle can have a substantial effect on tissue biodistribution and clearance of the agent. Other factors that can be optimized to decrease the amount of agent needed for detection include increasing its affinity for a target (lowest KD).2 We recently demonstrated that a single Gd3+-peptide conjugate targeted to a specific protein attached to agarose beads could be detected my MRI at a local concentration of ~4 μM and, based on those results, predicted that an Gd3+-based agent with a molecular r1 ~ 100 mM−1 s−1 should be able to detect biological targets present in at ~690 nM.3 However, creating a single low MW agent with an r1 ~ 100 mM−1 s−1 has proven difficult even with highly motionally restricted systems.4 A simpler approach would be to attach a few Gd3+ chelates each having a more typical r1 to a targeting moiety such that the molecular r1 sums to ~100 mM−1 s−1. This has been achieved by attaching several Gd3+ complexes to a variety of functionalized scaffolds (eg. polymers, hyperbranched polymers, dendrimers)5–7 or to nanoparticles8 but such large structures can add new complexity by slowing renal filtration rates9 (glomerular filtration threshold MW ≤ 45 kDa)5 and even altering biodistribution of the agent. For example, a PAMAM G4 dendrimer with ~21 GdDTPA plus four biotins on its surface for targeting (~29 kD) is retained in the vasculature of a tumor at 24 hr simply due to the inherent enhanced permeability and retention (EPR) property characteristic of macromolecules.10 This feature could make it difficult if not impossible to differentiate a targeted versus a non-targeted macromolecule in tumors. Polylysine-based dendrimers also show promise in that they can be synthesized in specific sizes, are biocompatible and their blood clearance rates can be tuned by molecular weight to allow sufficient time to reach their target.11 Antibodies and peptides have been most widely used for targeting specific biomarkers but such systems often suffer from poor in vivo stability or costly production. More recently, β-peptides and peptoids have gained interest as targeting moieties because they are easy to prepare, cost effective and stable toward peptidase and protease activity. Furthermore, screening methods to identify highly specific peptoid targeting agents for specific biomarkers on living cells have been reported.12 A strategy we have adopted for creating targeted agents for molecular imaging by MRI consists of: 1) selection of target specific moiety from a peptoid library, 2) affinity optimization, 3) attachment of a small poly(GdDOTA)lysine dendrimer scaffold to the targeting peptoid, 4) affinity optimization of the final conjugate, and 5) in vivo testing. As an initial demonstration, we utilized a dimeric form of 9-residue peptoid sequence (GU40C4) known to bind with high affinity to the vascular endothelial growth factor receptor 2 (VEGFR-2),12 an important target for tumor metastasis. The fluorescein-tagged GU40C4 peptoid displayed a KD = 91 ± 20 nM for VEGFR-2 (See supporting information). A third generation poly(DOTA-lysine) dendron with a lysine linker (Fig. 1) was prepared by standard solid phase and solution synthesis followed by addition of a maleimide group to the free α-amino of the linker (Scheme S1, supporting information). DOTA was selected as the Gd3+ chelate since it forms complexes with high thermodynamic and kinetic stability thus eliminating problems associated with metal release, especially for applications in vivo.13 Dendron conjugation to the GU40C4 peptoid was accomplished by reaction of the thiol group of Cys residue of the peptoid and the maleimide moiety of the dendron. The r1 relaxivity of the peptoid-Gd8-dendron conjugate (1 in Fig. 1) was 13.8 ± 0.2 mM−1s−1 (37°C, pH 7, 23 MHz) per Gd3+ ion, slightly higher than the r1 of the unconjugated Gd8-dendron (12.3 ± 0.5 mM−1s−1). These r1 relaxivities are similar to that reported for Gadomer 17 (16.5 mM−1s−1 at 25°C, 20 MHz), a dendritic contrast agent composed of three third generation lysine dendrons attached to a trimesoyl triamide central core.14 The affinity between conjugate 1 and VEGFR-2, as measured by displacement of the fluorescein-peptoid, was about 7-fold weaker (KD = 703 ± 172 nM) than unmodified GU40C4, presumably reflecting the steric bulk introduced by the Gd8-dendron. To test this, a longer linker was introduced between the peptoid and the Gd8-dendron (2 in Fig. 1). This modification yielded a conjugate with an increased binding affinity (KD = 215 ± 67 nM) and somewhat higher r1 (15.1 ± 0.2 mM−1s−1, 37°C, pH 7, 23 MHz). At 9.4T, the measured r1 relaxivity of 2 was 6.1 mM−1s−1 per Gd at 37°C so this translates to a molecular relaxivity of ~48 mM−1s−1 under the conditions used for in vivo imaging. The r1 value of 2 bound to VEGFR2 on agarose beads did not change significantly so one can reasonably assume that this will be its relaxivity in vivo as well.
Figure 1.
Gd8-dendron GU40C4 conjugates with different linker chains.
Porcine aortic endothelial (PAE) and PAE cells expressing human VEGFR2 (PAE/KDR) (2.5×105 receptors per cell) were chosen for initial MR imaging. The receptor concentration per cell volume (~5 μm cell radius) was ~790 nM for PAE/KDR while the control PAE cells had human VEGFR2. Thus, the local concentration of VEGFR2 in PAE/KDR cells was predicted to be within the detection limit for a Gd3+ agent having a molecular r1 of 100 mM−1s−1.3 T1 weighted images of both cell lines exposed to 2 or the Gd8-dendron lacking the peptoid (Fig. S4) showed that the image intensity of the PAE cell sample did not change when exposed to either 1.5 μM 2 or the non-conjugated Gd8-dendron while the PAE/KDR cells showed a significant increase in image contrast only when exposed to 2, reflecting specific binding of 2 to VEGFR2 on these cells. ICP-MS analysis of the cells post imaging showed there was negligible Gd3+ associated with either cell line exposed to the non-conjugated Gd8-dendron while the PAE/KDR cells exposed to 2 had ~900 nM Gd3+. These results show that cell receptors expressed at this level can be detected by MRI using these simple targeted agents.
Next, we moved to an animal model to evaluate whether VEGFR2 expression could be detected in vivo using this low MW agent (~ 8.6 kD). Mice with MDA-MB-231 xenografts known to express VEGFR215 were imaged before and after administration of 2 (0.008 mmol/kg). Those results were compared with images of other mice after injection of a scrambled 9-residue peptoid-Gd8-dendron conjugate (~ 8.4 kD) (0.008 mmol/kg) that does not bind VEGFR2 (Fig. S3). As shown in Fig. 2 (left panel), tumors in mice treated with 2 were maximally enhanced at ~4 hr post injection (Fig 2A) while the image intensity in tumors after injection of the control peptoid Gd8-dendron conjugate (Fig 2B) returned to baseline levels by 4 hr (Fig 2 right panel). The kidneys of these animals were also monitored during the study. After injection of the control peptoid Gd8-dendron, the kidneys quickly darkened as a result of a reduction T2 as expected during clearance of a Gd3+ agent at high imaging fields (Fig 2B).16 The kidney intensity in animals post injection of an equivalent dose of 2 did not darken significantly until much later in the study (Fig. 2A). This indicates that 2 is largely sequestered elsewhere during these early time points. It has been reported that VEGFR2 is also expressed in other tissues (e.g. kidney, liver) in mice17 which might explain some of the time dependent contrast differences. The kidney signal intensity in mice treated with 2 and the control peptoid returned to pre-treatment levels at 48–72 hours post injection.
Figure 2.
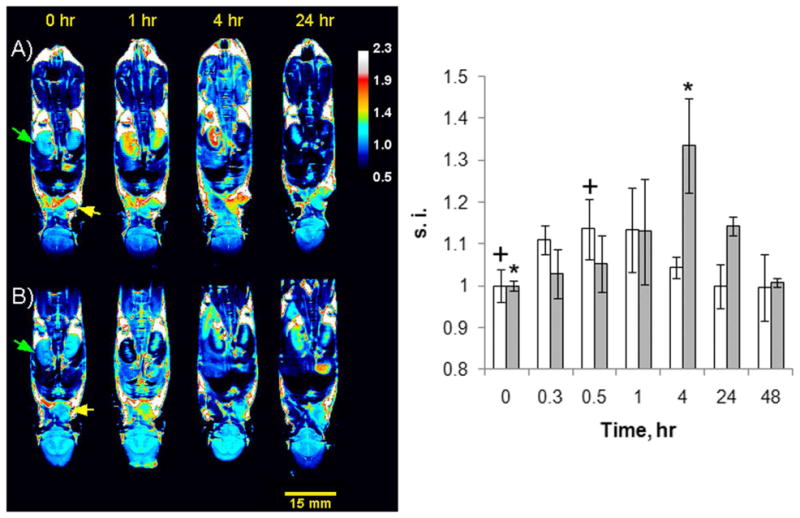
(left). 9.4 T MR T1-weighted coronal images of nude mice with subcutaneous cell tumor MDA-MB-231 xenografts at various timepoints following the intravenous (i.v.) tail injection of Gd8-dendron peptoid conjugates. A) images of a tumor-bearing mouse before and after i.v. tail injection of 0.008 mmol/kg of conjugate 2. B) images of a different tumor-bearing mouse before and after tail i.v. injection with 0.008 mmol/kg of an indentical Gd8-dendron conjugated to a control scrambled peptoid. The green and yellow arrows point to a kidney and tumor, respectively. (right.) Tumor signal intensities (s.i.) in mice injected with 2 (gray bars) or the control peptoid (white bars) (s.i. is the average of 3 mice) normalized to the tumor intensity prior injection. A t-test (two-tailed, unequal variance) was used to compare s.i. before injection and at 0.5 hr or 4 hr post injection of the animals injected with the control peptoid (+) or 2 (*) respectively. A p<0.05 was considered statistically significant.
In conclusion, we have presented a low molecular weight general targeting platform by combining a peptoid sequence derived from a chemical library with a Gd8-dendron to generate a high T1 relaxivity probe for molecular imaging of VEGFR2 in vivo by MRI. We anticipate that this platform technology will be generally useful for other targets that exist in tissues at these levels as well.
Supplementary Material
Acknowledgments
Financial support from the National Institutes of Health (RR02584, CA115531 and CA126608) is gratefully acknowledged.
Footnotes
Supporting Information Available. Calculations, detailed experimental procedures and characterization data for the compounds discussed in this work. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Villaraza LAJ, Bumb A, Brechbiel MW. Chem Rev. 2010;110:2921. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S, Xie J, Chen X. Biochemistry. 2010;49:1364. doi: 10.1021/bi901135x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanaoka K, Lubag AJM, Castillo-Muzquiz A, Kodadek T, Sherry AD. Magn Reson Imaging. 2008;26:608. doi: 10.1016/j.mri.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kielar F, Tei L, Terreno E, Botta M. J Am Chem Soc. 2010 doi: 10.1021/ja101518v. [DOI] [PubMed] [Google Scholar]
- 5.Zarabi B, Borgman MP, Zhuo J, Gullapalli R, Ghandehari H. Pharm Res. 2009;26:1121. doi: 10.1007/s11095-009-9830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanson SD, Kukowska-Latallo JF, Patri AK, Chen C, Ge S, Cao Z, Kotlyar A, East AT, Baker JR., Jr Int J Nanomedicine. 2008;3:201. [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Z, Thorek DLJ, Tsourkas A. Angew Chem Int Ed Engl. 2010;49:346. doi: 10.1002/anie.200905133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warsi MF, Adams RW, Duckett SB, Chechik V. Chem Commun. 2010;46:451. doi: 10.1039/b915223g. [DOI] [PubMed] [Google Scholar]
- 9.Ye F, Jeong EK, Jia Z, Yang T, Parker D, Lu ZR. Bioconjug Chem. 2008;19:2300. doi: 10.1021/bc800211r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W, Okollie B, Bhujwalla ZM, Artemov D. Magn Reson Med. 2008;59:679. doi: 10.1002/mrm.21508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langereis S, Dirksen A, Hackeng TM, Van Genderen MHP, Meijer EW. New J Chem. 2007;31:1152. [Google Scholar]
- 12.Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T. J Am Chem Soc. 2008;130:5744. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- 13.Cabella C, Crich SG, Corpillo D, Barge A, Ghirelli C, Bruno E, Lorusso V, Uggeri F, Aime S. Contrast Media Mol Imaging. 2006;1:23. doi: 10.1002/cmmi.88. [DOI] [PubMed] [Google Scholar]
- 14.Nicolle GM, Tóth E, Schmitt-Willich H, Radüchel B, Merbach AE. Chem--Eur J. 2002;8:1040. doi: 10.1002/1521-3765(20020301)8:5<1040::aid-chem1040>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Roland CL, Dineen SP, Lynn KD, Sullivan LA, Dellinger MT, Sadegh L, Sullivan JP, Shames DS, Brekken RA. Mol Cancer Ther. 2009;8:1761. doi: 10.1158/1535-7163.MCT-09-0280. [DOI] [PubMed] [Google Scholar]
- 16.Caravan P, Farrar CT, Frullano L, Uppal R. Contrast Media Mol Imaging. 2009;4:89. doi: 10.1002/cmmi.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maharaj ASR, Saint-Geniez M, Maldonado AE, D’Amore PA. Am J Pathol. 2006;168:639. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.