Abstract
The goal of transplantation is the induction of immunologic tolerance. At present, nonspecific immunosuppression is used to prevent graft rejection and, commonly, graft-versus-host disease (GVHD). Nevertheless, nonspecific immunosuppressive therapy is frequently complicated by infection, malignant tumors, and drug toxicity. In order to examine whether hematopoietic chimerism can be used to induce specific allograft tolerance, we have reconstituted lethally irradiated Lewis rats with ACI bone marrow that has been depleted of T cells with use of immunomagnetic beads. This technique consists of binding OX-19, a mouse anti-rat pan-T lymphocyte monoclonal antibody, to magnetic polymer beads. Mixing of bone marrow or splenocytes with the bead/OX-19 complexes, followed by magnetic separation, results in significant depletion of T cells with minimal nonspecific cell loss. Immunomagnetic T-cell depletion of bone marrow, followed by reconstitution of a lethally irradiated host, allows for the development of stable, mixed hematopoietic chimerae without evidence of GVHD. These hosts are immunocompetent by clinical criteria. Recipients of untreated donor bone marrow that did or did not receive nonspecific immunosuppression demonstrated varying degrees of GVHD and reduced survival. The ability to rapidly and simply deplete T lymphocytes from bone marrow and produce stable, immunocompetent hematopoietic chimerae without GVHD may be an important method for tolerance induction to vascularized allografts.
The successful induction of specific tolerance continues to be an elusive goal in the postoperative treatment of patients after organ transplantation. Despite the use of a variety of techniques, there has been no conclusive evidence supporting the achievement of a permanent state of tolerance to vascularized organs in human beings. At present, the control of allograft rejection depends on the use of nonspecific immunosuppressive agents, which induce a generalized paralysis of the immune system and predispose the recipient to the development of secondary infections.1, 2
It has recently been demonstrated that the induction of hematopoietic chimerism, after bone marrow transplantation in the mouse, is associated with the development of a specific tolerance to donor skin allografts and xenografts.3 In order to establish whether hematopoietic chimerism is capable of inducing tolerance to vascularized organ allografts, we have begun a series of experiments that examine the influence of chimerism on immunologic tolerance in rats. The rat represents a more suitable model than the mouse in which to study the rejection process of vascularized organ allografts. In preliminary studies we have demonstrated the prevention of graft-versus-host disease (GVHD) with use of immunomagnetic T-lymphocyte depletion (ITLD) in a rat model.4 This report demonstrates the usefulness of ITLD to induce stable chimerism after bone marrow transplantation between fully allogeneic, strongly histoincompatible donor-recipient strain combinations.
MATERIAL AND METHODS
Animals
The animals used were 200 to 300 gm inbred male ACI (RT1a) and Lewis (RT11) strain rats purchased from Harlan Sprague Dawley (Indianapolis, Ind.). They were given acidified water containing tetracycline hydrochloride (100 mg/L) and neomycin sulfate (10 mg/L) and fed rat food (Wayne Lab Blox F-6, Chicago, Ill.) ad libitum. The rats were allowed to acclimatize to the conventional facility for 2 weeks before experimentation.
Immunomagnetic bead preparation
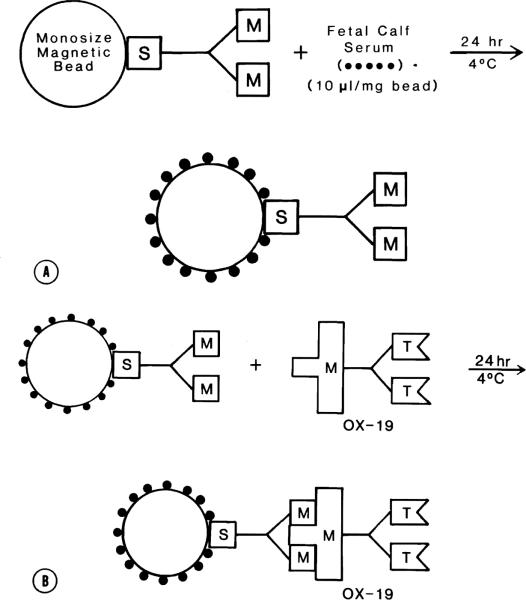
The initial step of bead preparation was performed as previously described.5 Magnetic, monosized polymer beads (20% magnetite by weight) are commercially available with covalently bound, affinity-purified sheep anti-mouse IgG directed against all mouse IgG subclasses (M450-11001, Dynal-Dynabeads, Great Neck, N.Y.). The beads were incubated with 10 μl of fetal calf serum (FCS) per mg of beads for 24 hours at 4° C, and washed three times with Hanks' balanced salt solution (HBSS) (Fig. 1, A). Then, OX-19, a mouse anti-rat pan-T lymphocyte monoclonal antibody, was mixed with the immunomagnetic beads at 1 μl/mg bead. The incubation conditions were the same as described above (Fig. 1, B).
Fig. 1.
Immunomagnetic bead preparation. A, Step 1: Monosized magnetic beads bound with sheep anti-mouse IgG are incubated with fetal calf serum to prevent nonspecific depletion of bone marrow cells. B, Step 2: The beads are then mixed with OX-19, a mouse anti-rat monoclonal antibody specific for rat T lymphocytes and thymocytes.
T-Lymphocyte depletion of bone marrow
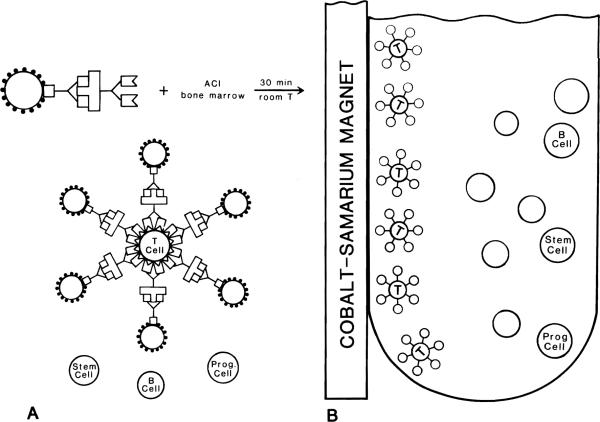
Donor bone marrow was depleted of T lymphocytes by means of immunomagnetic bead/OX-19 complexes. Bone marrow was harvested from the tibias and femurs of ACI rats by a previously described technique.6 Cell counts and viability were determined by trypan blue exclusion, and the viability of the cell suspensions always exceeded 90%. The bone marrow was mixed with the OX-19–coated beads in 5 to 10 ml of HBSS containing 0.05 mg/ml of gentamicin in a round-bottomed glass test tube (Fig. 2, A) and incubated at room temperature for 30 minutes. Gentle mixing was performed six times during the incubation. Magnetic separation was accomplished with a cobalt-samarium magnet. The bead/OX-19/T-lymphocyte complexes were polarized to the magnet and immobilized against the test tube wall (Fig. 2, B). The supernatant—containing stem cells, progenitor cells, and B cells—was extracted and used for flow cytometric analysis or bone marrow transplantation.
Fig. 2.
T-Lymphocyte depletion of rat bone marrow. A, Step 1: The bead/OX-19 complexes are mixed with ACI bone marrow, and binding of the antibody results in rosette formation. B cells, stem cells, and other progenitor cells remain in solution. B, Step 2: The bead/OX-19/T-cell complexes are polarized toward an applied magnetic force. The supernatant (containing B cells, stem cells, and other progenitor cells) is easily removed.
Spleen preparation
The spleens of ACI strain rats were removed after rats were killed by CO2 asphyxiation. The spleen cells were prepared by mincing the organ in RPMI solution. Splenocytes were isolated, counted, and viability established by trypan blue exclusion technique.
Flow cytometry
The analysis of cell surface–associated markers by flow cytometry was conducted by placing 1 × 106 bone marrow or spleen cells (±ITLD) in 12 × 75 mm glass tubes in 0.1 ml of staining buffer (phosphate buffered saline solution, pH 7.4, 0.1% Naazide, 2% FCS). The marker antisera (OX-19) or normal sera were added (1:10 to 1:100 final dilution) for 30 minutes at 4° C. The cells were washed twice and resuspended in fluoroscein isothiocyanate–labeled anti-IgG directed against the primary antibody (rat anti-mouse IgG, Hand L chain specific; Boehinger-Mannheim, Indianapolis, Ind.). After a 30-minute incubation period at 4° C, the cells were washed twice and analyzed for fluorescent staining in a flow cytometer (FAC-STAR, Becton-Dickinson, Mountain View, Calif.).
Bone marrow reconstitution
Total body irradiation (1000 rad) was administered to recipient rats 4 to 6 hours before bone marrow reconstitution. Induction of anesthesia was performed by intraperitoneal injection of 3.6% chloral hydrate. An upper midline celiotomy was performed. The intestines were eviscerated and retracted toward the left upper quadrant. A total of 50 × 106 bone marrow cells from ACI rats (±ITLD), suspended in 1.5 ml of HBSS containing 0.05% gentamicin, was injected into the exposed infrahepatic vena cava above the renal veins. Hemostasis was ensured, and the viscera were restored to their normal intra-abdominal position. The fascia and skin were closed in two layers with 4-0 polyglycolic acid (Dexon) suture. This method of bone marrow inoculation through the exposed inferior vena cava was used to provide the appropriate controls for experiments involving the placement of intra-abdominal vascularized allografts.
Drug immunosuppression
Two drugs, FK-506 and cyclosporine (CyA), were used to produce nonspecific immunosuppression.
FK-506, generously supplied in powder form by Fujisawa Pharmaceutical Company, Ltd., Osaka, Japan, was reconstituted in normal saline solution. The drug was administered orally at 0.5, 1.6, and 3.2 mg/kg on days 0 to 28 using an No. 14 gauge feeding gavage catheter. When used in a two-drug regimen, the FK-506 was used at 0.5 mg/kg/day and CyA at 5.0 mg/kg/day.
CyA was generously supplied by the Sandoz Pharmaceutical Corporation, East Hanover, N.J. The liquid form (100 mg/ml) was mixed with olive oil and administered at 5.0 mg/kg/day through a No. 14 feeding gavage catheter 3 hours after the administration of FK-506 (0.5 mg/kg/day only). When used alone, the powder form of CyA was mixed in a Miglyol base to a concentration of 25 mg/ml and administered in a deep intramuscular depot at a dose of 25 mg/kg/day from days −1 to 18.
In vivo comparison of ITLD versus nonspecific immunosuppression (Table I)
Table I.
Treatment groups
| Group | n | Donor | Recipient | Pre-BMTx host Rx (rad) All | BM treatment | Post BMTx* host treatment |
|---|---|---|---|---|---|---|
| I | 10 | — | Lewis | 1000 | — | — |
| II | 20 | ACI | Lewis | 1000 | — | — |
| III | 10 | ACI | Lewis | 1000 | ITLD | — |
| IV | 10 | ACI | Lewis | 1000 | — | FK-506 |
| V | 10 | ACI | Lewis | 1000 | — | CyA |
| VI | 10 | ACI | Lewis | 1000 | — | FK-506 + CyA |
Legend: BMTx, Bone marrow transplantation.
For doses, see text.
All hosts were Lewis strain rats that had received 1000 rad of total body irradiation. Group I received no bone marrow reconstitution. Groups II and IV through VI received untreated ACI bone marrow. Group III received ITLD-treated ACI bone marrow. Group IV received oral FK-506 at 1.6 mg/kg/day (n = 5) or 3.2 mg/kg/day (n = 5) from days 0 to 28. Group V received CyA 25 mg/kg/day intramuscularly from days −1 to 18. Group VI received oral FK-506 (0.5 mg/kg/day) and oral CyA (5.0 mg/kg/day) from day 0 through 28.
Assessment of chimerism
The presence of hematopoietic chimerism was established with a hemagglutination assay for the expression of donor MHC class I histocompatibility antigens. Lewis anti-ACI antisera to RT1. A histocompatibility antigens was used in a standard hemagglutination protocol7. Red cell chimerism was assessed by the demonstration of donor erythrocytes in the peripheral blood of animals surviving longer than 2 months. The levels of chimerism were estimated by comparison to control experiments in which erythrocytes from the donor and recipient strains were mixed in 10% ratios from 0% to 100% each and then tested for hemagglutination.
Assessment of GVHD
The recipients of donor bone marrow were assessed for GVHD on a daily basis. Rats were weighed every 2 to 3 days. The diagnosis of GVHD was based on previously described clinical and histopathologic findings.6, 8, 9 An animal was considered to exhibit acute GVHD if at least four of the following signs were observed: diffuse erythema, hyperkeratosis of the foot pads, dermatitis, weight loss, generalized unkempt appearance, or diarrhea. Necropsy specimens from the non-ITLD groups were taken from the ear, tongue, liver, spleen, small intestine, and mesenteric lymph nodes. Five ear biopsy specimens from the ITLD group taken on day 34 were also examined. The specimens were processed routinely for light microscopy, with use of hematoxylin-eosin staining, and examined for the histopathologic features associated with acute GVHD.8,9
Statistics
Survival data were compared with Kaplan-Meier plots using a generalized Savage (Cox-Mantel) test. The incidence of engraftment and GVHD within respective groups was compared by χ2 analysis. A value of p < 0.05 was considered statistically significant.
RESULTS
T-Lymphocyte depletion of bone marrow
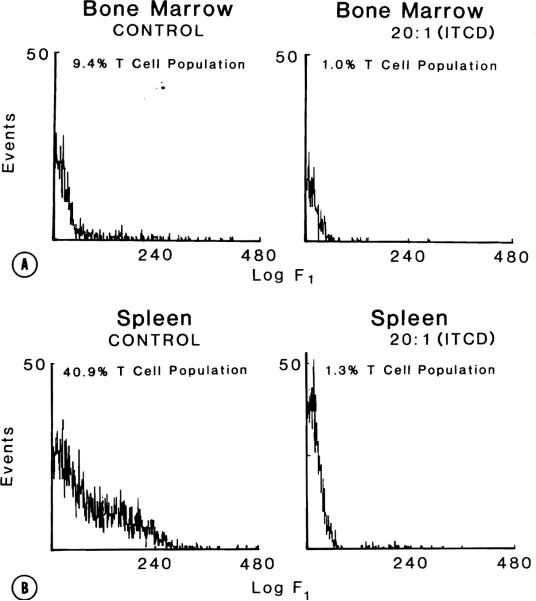
Flow cytometry of both spleen and bone marrow was used to test the effectiveness of the lTLD technique. Normally, rat bone marrow contains approximately 5% to 6% of its lymphocyte population as T cells, and rat spleen, approximately 50%. Various bead/T lymphocyte ratios were examined (1:1, 2:1, 5:1, 20:1, 60:1) to determine satisfactory depletion with minimal nonspecific cell loss. ITLD (20 beads:1 T cell) of ACI bone marrow reduced the population at T cells from 9.4% to 1.0% most efficiently (Fig. 3, A). With this same ratio, the ACI spleen T-lymphocyte population was decreased from 40.3% to 1.3% (Fig. 3, B). Nonspecific cell loss after ITLD was in the range of 2% to 9%. Less than a 20:1 bead/T lymphocyte ratio resulted in inefficient removal of T cells. A 60:1 bead/T lymphocyte ratio was associated with a 31% nonspecific cell depletion.
Fig. 3.
Single-color flow cytometric assessment of T-lymphocyte depletion. A, Bone marrow: With 20 beads per T cell, ACI bone marrow is depleted to a 1% T-cell population from the control levels of 9.4%. B, Spleen: Lymphocytes in ACI spleen account for 40.9% of its lymphocyte population. Following the same conditions as in A, ITLD results in a depletion of T lymphocytes to 1.3%.
In vivo assessment of T-lymphocyte depletion
All 10 lethally irradiated control Lewis rats that did not receive bone marrow reconstitution (Table II; group I) died, as expected, by day 13. Eleven rats irradiated and reconstituted with untreated ACI bone marrow, all had clinical signs of acute GVHD (group II). Of these animals, 9 of 11 were dead by day 25, and the remaining two rats died by 48 days with clinical and pathologic lesions consistent with acute GVHD.
Table II.
Chimerism induction and prevention of graft-versus-host disease in lethally irradiated Lewis rats
| Group | n | Donor | BM Rx | Host Rx | BM engraft* | Clin. GVHD* | Hist. GVHD | Surv at 90d† | Chimerism |
|---|---|---|---|---|---|---|---|---|---|
| I | 10 | — | — | — | — | — | — | 0/0 | No |
| II | 20 | ACI | — | — | 11/20 | 11/11 | ++ | 0/20 | N/A |
| III | 10 | ACI | ITLD | — | 10/10‡ | 0/10§ | 0 | 10/10§ | Yes; stable |
| IV | 10 | ACI | — | FK-506 | 9/10 | 0/9§ | + | 1/10 | Yes; 2 mo |
| V | 10 | ACI | — | CyA | 9/10 | 0/9§ | + | 0/10 | N/A |
| VI | 10 | ACI | — | FK-506 + CyA | 9/10 | 0/9§ | + | 0/10 | Yes; 2 mo only |
χ2 test.
Survival at 90 days; Cox-Mantel test.
p < 0.05.
p < 0.001.
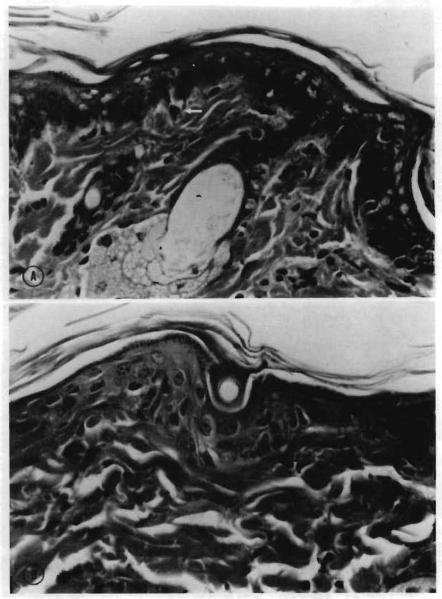
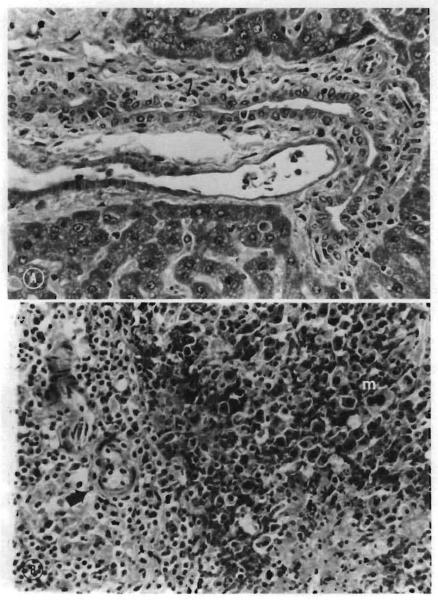
The most common manifestation of clinical acute GVHD was erythema of the ears. The most specific clinical determinant appeared to be the combination of erythema, hyperkeratosis of the foot pads, and severe weight loss. The most consistent and sensitive histopathologic lesion of acute GVHD in these animals was the appearance of single basal epithelial cell necrosis with adjacent lymphocyte infiltration (satellitosis) in the tongue and ear (Fig. 4, A). The ear was unaffected in rats receiving nonspecific immunosuppression. The livers of affected animals frequently exhibited lymphocytic infiltration of the portal triads, with injury to the bile duct epithelium ranging from disorganization with focal cellular injury to complete destruction and satellitosis (Fig. 5, A). Other histopathologic features included lymphocytic depletion of lymph node paracortical areas and splenic arteriolar sheaths, with lympho-blast or histocyte infiltration of the mantle zone and extramedullary hematopoiesis (Fig. 5, B). Cryptitis was present in sections of the small intestine.
Fig. 4.
Photomicrographs of an ear of a lethally irradiated Lewis rat. A, Recipient of untreated ACI bone marrow (25 days after reconstitution). Single cell necrosis in basal layers of epithelium (arrow) and mononuclear cell infiltrate. (Hematoxylin and eosin stain. Original magnification ×160.) B, Recipient of ITLD-treated ACI bone marrow (34 days after reconstitution). Normal ear skin. (Hematoxylin and eosin stain. Original magnification ×160.)
Fig. 5.
A, Microscopic section of liver: Recipient of untreated ACI bone marrow. Note the presence or lymphocytes and single cell necrosis in the bile duct epithelium (arrow). (Hematoxylin and eosin stain. Original magnification ×100.) B, Microscopic section of spleen: Recipient of untreated ACI bone marrow. Note depletion of lymphocytes in periartcrior sheath (arrow). Infiltration of mantle zone (right half of photo) by primitive cells and megakaryocytes (m), indicating repopulation by marrow elements and possible GVHD.
Ten lethally irradiated Lewis rats received ACI bone marrow treated with ITLD (group III). There was successful engraftment in all animals and 100% survival for more than 165 days. No clinical or histopathologic evidence for acute or chronic GVHD was present during any period of their posttransplant course. The recipients of ITLD-treated ACI bone marrow had normal ear biopsy specimens at 34 days after transplantation (Fig. 4, B). The recipient rats that received nonspecific immunosuppression in the postoperative period (groups IV through VI) did not develop clinical signs of acute GVHD. In some animals in all three groups, however, mild histologic evidence of GVHD developed, and the survival in the three groups was poor, with all but one animal dead by 90 days. There was no apparent difference between FK 1.6 mg/kg/ day and 3.2 mg/kg/day.
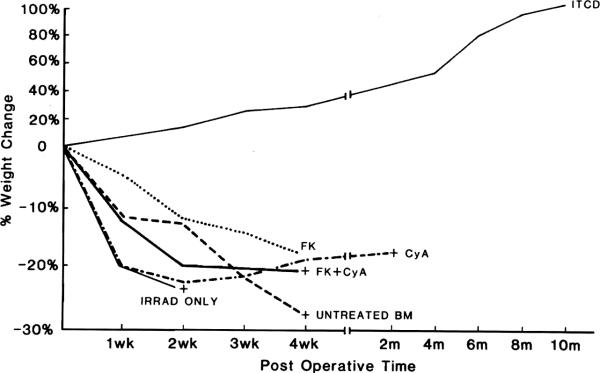
All of the recipients of ITLD-treated marrow gained weight by day 7. This progressed steadily, and 10 months after bone marrow transplantation, the chimerae not used in other experimental protocols had gained 100% of their body weight. All of the recipients in the control and other treatment groups lost weight and died, except for a single rat in group IV (FK-506 alone) (Fig. 6).
Fig. 6.
Postoperative weight change after bone marrow transplantation. Only those animals receiving ITLD-treated ACI bone marrow gained weight and survived as long-term stable chimerae. All animals in control and other treatment groups lost weight and died within 90 days, except for a single recipient receiving FK-506.
Assessment of chimerism
All of the recipients of ACI bone marrow treated with ITLD were chimeric and exhibited 20% to 80% donor-type erythrocytes (Table III). The animals were tested at 2, 4, 7, and 11 months. The initial level of chimerism in an individual animal was maintained throughout its posttransplant course (± 10% ). Control Lewis strain rats and Lewis recipients of non-T-depleted syngeneic bone marrow were not chimeric as assessed by this technique (data not shown).
Table III.
Erythrocyte chimerism in lethally irradiated Lewis rats receiving ITLD-treated ACI bone marrow: Hemagglutination assay
| No. of chimeric rats |
|||
|---|---|---|---|
| % Chimerism | 4 mo | 7 mo | 11 mo |
| 100 | — | — | — |
| 90 | — | — | — |
| 80 | — | 1 | 1 |
| 70 | 2 | 2 | 1 |
| 60 | 1 | — | 2 |
| 50 | — | — | — |
| 40 | — | — | — |
| 30 | 1 | 2 | 1 |
| 20 | 1 | — | — |
| 10 | — | — | — |
| 0 | — | — | — |
DISCUSSION
All forms of nonspecific immunosuppression are associated with serious toxic side effects. Although immunosuppression with cyclosporine has revolutionized the field of transplantation, microbial infections, viral-associated lymphomas, nephrotoxicity, and hepatotoxicity are common complications.2, 10, 11 Using mixed syngeneic/allogeneic T lymphocyte-depleted bone marrow to reconstitute lethally irradiated mice, Ildstad and Sachs produced immunocompetent chimerae with specific hyporeactivity to donor-type skin grafts and concordant rat xenografts.3, 12–14
In order to establish whether a similar model of mixed chimerism in rats would allow for transplanting donor-type vascularized organ grafts, we have used reconstitution of lethally irradiated Lewis rats with ACI bone marrow to induce hematopoietic chimerism. To reproducibly develop stable chimerae and prevent the graft-versus-host response, the mouse anti-rat pan-T lymphocyte monoclonal antibody OX-1915 was used to deplete T cells from ACI (donor) bone marrow.
The monosized beads used as a carrier for the T-lymphocyte depletion were initially developed to improve resolution in liquid chromatography. The addition of magnetite (20% by weight) to the beads allowed them to be used for magnetic separation. The surface of the bead is partly hydrophobic, allowing antibodies to bind by physical adsorption, and partly hydrophilic because of the presence of hydroxyl groups, which can be chemically activated to covalently bind antibodies.16 These beads have been used in a variety of situations, including lymphoma cell removal,16 mononuclear cell selection,17 and purging of neuroblastoma cells18 from bone marrow.
Using a bead/T-Iymphocyte ratio of 20:1 (assuming a bone marrow population of 5% T lymphocytes for the bone marrow and 50% for the spleen) a rapid and effective depletion of T cells was accomplished with minimal nonspecific cell loss. Lower bead/T-cell ratios were less effective in depleting T lymphocytes, based on the percentage of cells removed as measured by flow cytometry, and the use of larger numbers of beads was accompanied by significant nonspecific cell loss.
Depletion of the donor T lymphocytes was associated with the appearance of a stable chimerism without evidence of GVHD. As we have described earlier,6 rat bone marrow contains sufficient numbers of mature T lymphocytes to induce GVHD across a variety of histocompatibility differences, and transplantation of marrow containing T lymphocytes results in fatal acute GVHD. Treatment with the immunosuppressive drugs CyA and/or FK-506 was not sufficient to prevent the high mortality rates, presumably because of immunoincompetence (drug plus lethal irradiation) or subclinical GVHD, when the recipients received bone marrow containing T lymphocytes. The animals that received T lymphocytedepleted marrow began normal weight gain 1 week later and steadily progressed to 100% of their body weight 10 months after reconstitution. Weight gain, long-term survival, a healthy appearance, and absence of GVHD—all are consistent with a functionally intact immune system.
We have not observed any evidence that immunomagnetic T-lymphocyte depletion with OX-19 is associated with a higher-than-expected failure of the depleted bone marrow to engraft. This complication of bone marrow depletion has been observed in mice, dogs, and human beings,19 suggesting that differences in the types of T-lymphocyte subsets, the number of residual T lymphocytes, or NK cells may have an important influence on the efficiency of bone marrow reconstitution.
In summary, immunomagnetic T-lymphocyte depletion is a novel technique, rapidly performed in vitro, that is nontoxic to the recipient. It is relatively inexpensive and is effective with minimal manipulation of the donor marrow. This technique allows the production of hematopoietic chimerism without GVHD and may have important clinical applications for bone marrow and solid organ transplantation.
Acknowledgments
Supported by research grants from the Veterans Administration and project grant No. DK-29961 from the National Institutes of Health.
We appreciate the technical assistance of Judy Wargo, MS, and Jo Harnaha, BS. We also thank Donna Ross for assistance with the manuscript.
Biographies
Dr. Collin J. Weber (New York, N.Y.). Have you tried this with xenografts, that is, xenogeneic marrow into the rat? How soon after injecting purified allogeneic marrow can you do a graft of, let us say, skin and get it to take? Is it immediate, or is there a lag phase?
Dr. Hoffman. We have not done xenografts in our model. It has been done by Ildstad and Sachs using mixed syngeneic/xenogeneic bone marrow reconstitution to develop mixed xenogeneic chimerae in a mouse/rat model. Though having but a small percentage of donor type cells within the host animal, these chimerae accepted skin grafts in 2 to 3 months after bone marrow reconstitution.
Dr. Weber. I would urge you to consider doing that because you seem poised to answer an important question: How stable is a xenochimera? If I understand Dr. Sachs' data correctly, most of his murine chimerae did not hold long term. Perhaps there is something peculiar to the mouse. If you can show it in the rat, it would push us ahead to do it in, let us say, primates.
Dr. Michael A. Grosso (Denver, Colo.). There has been a resurgence of the passenger lymphocyte theory in rejection of solid organ transplantation. Lymphocytes from the donor organ participate not just in graft-versus-host disease but in presenting antigens that initiate allograft rejection. Can you see any way to apply this technique to T-cell deplete solid organs so as to possibly influence allograft rejection?
Dr. Hoffman (closing). As far as the issue of graft-versus-host disease, it may not be a matter of just T-cell depletion. There may be some interaction with NK cells, and, in addition to which, in the mixed chimeric model there appears to be a null cell population present in the syngeneic component, which may inhibit the graft-versus-host reaction. The immunomagnetic beads have not been used in solid organ research or in vivo protocols.
Footnotes
Presented at the Fiftieth Annual Meeting of the Society of University Surgeons, Feb. 9–11, Baltimore, Md.
REFERENCES
- 1.Thomson AW, Whiting PH, Simpson JG. Cyclosporine: immunology, toxicity and pharmacology in experimental animals. Agents Actions. 1984;15:306–27. doi: 10.1007/BF01972366. [DOI] [PubMed] [Google Scholar]
- 2.Tolkoff-Rubin NE, Rubin RH. The impact of cyclosporine therapy on the occurrence of infection in the renal transplant recipient. Transplant Proc. 1986;2(suppl 1):168–76. [PubMed] [Google Scholar]
- 3.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts. Nature. 1984;307:168–70. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman AH, Makowka L, Cramer DV, et al. Immunomagnetic T lymphocyte depletion of rat bone marrow using OX-19 monoclonal antibody. J Invest Surg. doi: 10.3109/08941938909057430. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lea T, Vartdal F, Davies C, Ugelstad J. Magnetic monosized polymer particles for fast and specific fractionation of human mononuclear cells. Scand J Immunol. 1985;22:207–16. doi: 10.1111/j.1365-3083.1985.tb01873.x. [DOI] [PubMed] [Google Scholar]
- 6.Oaks MK, Cramer DV. The genetics on bone marrow transplantation in the rat. Transplantation. 1985;39:69–76. doi: 10.1097/00007890-198501000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Cramer DV, Davis BK, Shonnard JW, et al. Phenotypes of the major histocompatibility complex in wild rats of different geographical origins. J Immunol. 1978;120:179–87. [PubMed] [Google Scholar]
- 8.Beschorner WE, Tutschka PJ, Santos GW. Sequential morphology of graft-versus-host disease in the rat radiation chimera. Clin Immunol Immunopathol. 1982;22:203–23. doi: 10.1016/0090-1229(82)90038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavin RE, Woodruff JM. The pathology of bone marrow transplantation. In: Sommers SC, editor. Pathology annual. Appleton-Century Crofts; New York: 1974. pp. 291–344. [PubMed] [Google Scholar]
- 10.Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583–7. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Buren CT. Cyclosporine: progress, problems and perspectives. Surg Clin North Am. 1986;66:435–49. doi: 10.1016/s0039-6109(16)43931-9. [DOI] [PubMed] [Google Scholar]
- 12.Ildstad ST, Wren SM, Bluestone JA, et al. Characterization of mixed allogeneic chimeras. Immunocompetence, in vitro reactivity and genetic specificity of tolerance. J Exp Med. 1985;162:231–44. doi: 10.1084/jem.162.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ildstad ST, Wren SM, Bluestone JA, et al. Effect of selective T cell depletion of host and/or donor bone marrow on lymphopoietic repopulation, tolerance, and graft versus host disease in mixed allogeneic chimeras (B10 + B10.D2 → B10) J Immunol. 1986;136:28–33. [PubMed] [Google Scholar]
- 14.Ildstad ST, Wren SM, Sharrow SO, et al. In vivo and in vitro characterization of species hyporeactivity to skin xenografts in mixed xenogeneically reconstituted mice (B10 + F344 rat → B10) J Exp Med. 1984;160:1820–35. doi: 10.1084/jem.160.6.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallman MJ, Mason DW, Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982;12:511–8. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- 16.Kvalheim G, Fodstad O, Pihl A, et al. Elimination of B-lymphoma cells from human bone marrow: model experiments using monodisperse magnetic particles coated with primary monoclonal antibodies. Cancer Res. 1987;47:846–51. [PubMed] [Google Scholar]
- 17.Egeland T, Lea T. A rapid rosette technique for quantitation and separation of mononuclear cell subsets using monoclonal antibodies. J Immunol Methods. 1982;55:213–9. doi: 10.1016/0022-1759(82)90033-3. [DOI] [PubMed] [Google Scholar]
- 18.Treleaven JG, Ugelstad J, Phillips T, et al. Removal of neuroblastoma cells from bone marrow with monoclonal antibodies conjugated to magnetic microspheres. Lancet. 1984;1:70–3. doi: 10.1016/s0140-6736(84)90004-7. [DOI] [PubMed] [Google Scholar]
- 19.Martin PJ, Hansen JA, Buckner CD, et al. Effects of in vitro depletion of T cells in HLA-identical allogeneic marrow grafts. Blood. 1985;66:664–72. [PubMed] [Google Scholar]