Summary
Background
Over the last 20 years, percutaneous transluminal balloon coronary angioplasty (PTCA), bare metal stents (BMS) and drug eluting stents (DES) succeeded each other as catheter-based treatments for coronary artery disease (CAD). We present an overview of randomised trials comparing these interventions with each other and with medical therapy in patients with nonacute CAD.
Methods
We searched Medline for trials contrasting at least two of the aforementioned interventions. Outcomes of interest were death, myocardial infarction (MI), coronary artery bypass grafting (CABG), target lesion or vessel revascularisation (TLR/TVR), and any revascularisation. Random effects meta-analyses summarised head-to-head (direct) comparisons, and network meta-analyses integrated direct and indirect evidence.
Findings
61 eligible trials (25 388 patients) investigated 4 of 6 possible comparisons between the 4 interventions. No trials directly compared DES with medical therapy or PTCA. In all direct or indirect comparisons, succeeding advancements in PCI did not yield detectable improvements in deaths and MI. The risk ratio for indirect comparisons between DES and medical therapy was 0·96 (95% confidence interval: 0·60, 1·52) for death and 1·15 (0·73, 1·82) for MI. In contrast, there were sequential significant reductions in TLR/TVR with BMS compared to PTCA and with DES compared to BMS. The risk ratio for the indirect comparison between DES and PTCA for TLR/TVR was 0·30 (0·17, 0·51).
Interpretation
Sequential innovations in the catheter-based treatment of nonacute CAD showed no evidence of an impact on death or MI when compared with medical therapy.
Introduction
Since its introduction by Andreas Gruntzig in 1977, percutaneous coronary intervention (PCI) has revolutionised the care of patients with coronary artery disease (CAD) by permitting a catheter-based approach to revascularise hemodynamically-compromised coronary arteries. In the United States, more than one million patients are treated with PCI annually, often for nonacute CAD.1
Recent meta-analyses suggest that PCI improves outcomes in high-risk patients with acute coronary syndromes and ST-elevation myocardial infarction.2,3 However, their role in the management of patients with nonacute CAD has been more controversial. An early meta-analysis suggested percutaneous transluminal balloon coronary angioplasty (PTCA) was superior to medical therapy for symptom reduction, albeit with an increase in the rate of subsequent procedures.4 Later trials tested bare metal stents (BMS) against PTCA,5 and subsequently drug-eluting stents (DES) against BMS.6,7 Based on the results of these trials, which showed sequential reductions in the need for target vessel or lesion revascularization (TVR/TLR) with these technological advances, PCI is now performed almost exclusively with stents, most of which are DES in the United States.8
Despite the sequential testing of PTCA against medical therapy, BMS against PTCA, and DES against BMS, the cumulative benefits of technological innovations after 20 years of clinical trials in this field have not been systematically evaluated.
Herein we present a systematic overview of all RCTs comparing medical therapy, PTCA, BMS and DES in the treatment of patients with nonacute CAD. We explore the succession of these interventions over time using traditional meta-analysis and network meta-analysis.9,10 Network meta-analysis not only increases statistical power by incorporating evidence from both direct (head-to-head) and indirect comparisons across all 4 interventions, but can provide insights on the relative effectiveness of interventions that have never been directly compared, such as medical therapy and DES.
Methods
Literature search
We performed three incremental electronic Medline searches using the MeSH terms Stents, Angioplasty, and Coronary Disease (last search April 28, 2008) to identify English language publications from RCTs evaluating medical therapy, PTCA, BMS, or sirolimus or paclitaxel-eluting stents (DES) in patients with CAD. We used a methodological filter to select controlled trials.11 We complemented searches by perusing the reference lists of previous meta-analyses4–6,12,13 and set no geographical restrictions.
Eligibility criteria
Eligible were RCTs of patients with symptomatic or asymptomatic nonacute CAD. This included trials with patients with stable and unstable angina but excluded those with patients with a recent acute myocardial infarction (MI) within the prior 72 hours at the time of the first enrolment. We included trials with at least 10 patients in each arm, comparing at least two of the four treatments of interest (medical therapy, PTCA, BMS, and paclitaxel or sirolimus DES). We focused on sirolimus and paclitaxel eluting stents because these stents have been most widely studied among the various drug eluting stents. Eligible outcomes were death, fatal and non-fatal MI, TVR or TLR, any subsequent PCI (revascularisation) and coronary artery bypass grafting (CABG). We took care to avoid duplication of information from multiple reports on the same trial, by only considering data from the report with the longest follow-up.
We excluded RCTs that enrolled only patients with diabetes mellitus and those that enrolled any patients with acute MI within the prior 72 hours. We also excluded RCTs focusing only on venous bypass grafts, in-stent restenosis, or left main disease. We excluded trials comparing two different types of non-stenting techniques (e.g., cutting balloon angioplasty or directional coronary atherectomy vs. PTCA), two different BMS or two different DES classes. Finally, we excluded trials that used different PCI types in the same arm (e.g., either PTCA or BMS), unless more than 85% of patients in the corresponding arm were treated with one type of PCI (e.g., with BMS).
Data extraction
Data were extracted directly into electronic summary tables by four investigators. All extractions were verified against the primary paper by a different investigator, and any discrepancies were resolved by discussion among the data extractors.
From each trial we recorded information on the publication (first author, journal and year of publication); patient demographics (mean age, proportion of males, diabetics, and patients with multivessel disease or unstable angina); the type of PCI strategies that were compared and number of patients randomised to each arm; years of patient enrolment; whether the trial was blinded; follow-up duration; and the number of events of interest in each arm. Specifically, we extracted the number of deaths, non-fatal or fatal MI, CABG, and the total number of subsequent revascularizations, where available. When applicable (i.e., for patients undergoing PCI) we also extracted the number of TVRs and TLRs, according to each trial's definition. Typically, TVR was defined as any subsequent revascularisation of the same vessel that was initially treated, and TLRs as any subsequent revascularisation within 5 mm from the boundaries of the original lesion.
Statistical analyses
We explored graphically the temporal accumulation of randomised evidence per comparison, by plotting the cumulative number of patients enrolled to the different comparisons over time. For studies that did not report the start of patient enrolment, we calculated an approximate starting year of enrolment based on a linear regression that used follow-up duration and publication year as predictors.
First, we performed separate random effects meta-analyses14 for all available direct comparisons (head-to-head comparisons of 2 treatments in the same RCTs). We quantified the extent of between-study heterogeneity with I2. I2 ranges between 0 and 100% and expresses the proportion of between-study variability that is attributable to heterogeneity rather than chance.15
We then examined the network formed by the four alternative strategies (medical therapy, PTCA, BMS, and DES). In this network, one can also calculate indirect comparisons between two strategies, by examining RCTs that contrast each strategy against a third “reference” intervention (e.g., one can indirectly compare DES versus medical therapy by using trials that compare DES versus BMS, and a separate set of trials comparing BMS versus medical therapy).16,17
We combined all direct and indirect evidence in a single joint analysis (network meta-analysis), using a two level linear mixed effects model with heteroskedastic errors.10 Network meta-analysis incorporates indirect evidence by assuming that the effects of different interventions are transitive. Simply put, if DES is more effective than BMS with respect to a given outcome, and BMS is more effective than PTCA, then DES should be more effective than PTCA. The extent of disagreement between direct and indirect evidence is quantified by the incoherence of the network.10 For network comparisons addressed only by direct or only by indirect evidence incoherence could not be estimated10 and was fixed at 0.16
Main analyses used all trials with available quantitative information for each outcome. For all calculations we performed subgroup analyses according to the type of DES (sirolimus, paclitaxel), the proportion with unstable angina (below or above the median), and by excluding trials of stents vs. medical therapy in stable patients with a recent acute MI (between 72 hours and 28 days from enrolment). For all direct comparisons we performed random effects meta-regressions to examine the association of the proportion of unstable angina with the effect size. In sensitivity analyses we included trials with 0 events in both arms in the RR calculations (by adding 0.5 to all cells of the pertinent 2 by 2 tables), and examined earlier follow-up data as well (for trials reporting multiple follow-up times). Finally, we examined the robustness of the main analyses by including in the PTCA vs Medical comparison 5 trials with mixed interventions in their PCI arm (less than 85% of PCI belonged to our prespecified intervention; see Appendix).
Analyses were conducted in Intercooled Stata 8.2 (Stata Corp, College Station, TX) using the metan routine and in R 2.6.0 (R Foundation for Statistical Computing, Vienna, Austria) using the nlme package. We did not perform any adjustments for the fact that the evaluated outcomes are correlated. Unless otherwise stated, all p-values are two tailed and are considered significant for p<0.05.
Results
Search results
The electronic searches yielded 2559 citations, which were screened at the abstract level. Of these, 91 publications were reviewed in full text, and 61 RCTs (described in 72 publications) were finally eligible for our comparative effectiveness overview (Appendix Figure 1; Appendix Tables 1 to 4). Two trials (one comparing PTCA vs. medical treatment in strata of patients with one- and two-vessel disease,18 and one comparing slow and moderate release paclitaxel eluting stents vs. BMS19) were entered as 4 entries in the meta-analyses. Therefore, we herein refer to 63 unique trial entries, with a total of 25 388 patients.
Eligible trials
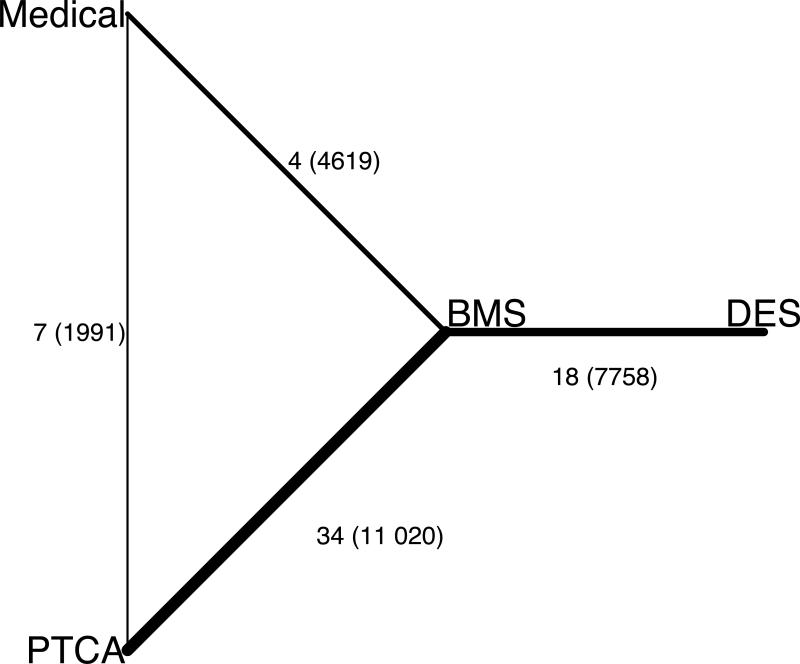
Enrolment in the first RCT started in 1987, and the included RCTs were published between 1994 and 2007. The median number of patients randomised was 238 (interquartile range, IQR: 110, 446), with 8 trials having more than 1000 patients. The median follow-up duration was 12 months (IQR: 6, 24). As shown in Figure 1, RCTs generally focused on comparisons between successive strategies: 7 compared PTCA vs. medical treatment, 34 BMS vs. PTCA, and 18 DES vs. BMS; the remaining 4 trials compared BMS with medical treatment. Among the 18 DES trial entries, 7 pertained to sirolimus- and 11 to paclitaxel-eluting stents (7 for polymeric and 4 for non-polymeric paclitaxel).
Figure 1.
Graphical representation of the network of eligible trials.
Lines connect the interventions that have been studied in head-to-head (direct) comparisons in the eligible randomised trials. The width of the lines represents the relative amount of information on each comparison in terms of the cumulative number of randomised patients. The numbers correspond to the number of trial entries (see text), and in the parentheses the cumulative number of randomised patients per comparison.
RCTs differed significantly in their follow-up duration, proportion of enrollees with unstable angina, mean participant ages and calendar years of enrolment and publication across the 4 comparisons (Table 1). Notably, trials comparing PTCA versus medical therapy had the longest follow up and routinely excluded patients with unstable angina. In contrast, trials comparing different modes of PCI (PTCA, BMS, and DES) included between 0 and 80% patients with unstable angina (median proportion 34%, IQR: 19, 48%; among 35 trials reporting this information). Mean participant ages were lowest for PTCA vs. medical therapy trials and highest for DES vs. BMS trials. The difference is consistent with the inclusion of older patients in later trials (Spearman's ρ=0·59 between mean age and year of trial initiation; p<0·01). There were no significant differences in the distribution of the proportions of diabetics, males or patients with multivessel disease among the 4 comparison sets (Table 1).
Table 1.
Characteristics of trials across different comparisons
| Characteristic | PTCA vs. Medical | BMS vs. PTCA | BMS vs. Medical | DES vs. BMS |
|---|---|---|---|---|
| Number of trials | 7 | 34 | 4 | 18 |
| Number of patients (median, IQR) | 201 (101, 227) | 249 (116, 407) | 1134 [66, 2286]* | 268 (175, 500) |
| Follow-up (median, IQR), months | 60 (36, 84) | 9 (6, 12) | 30 [12, 60]* | 9 (9, 12) |
| Calendar year | ||||
| Publication | 1998 (1997, 2003) | 2000 (1999, 2002) | 2005 [2002, 2007]* | 2004 (2003, 2005) |
| Enrolment | 1990 (1988, 1992) | 1996 (1994, 1997) | 1997 [1997, 2000]* | 2001 (2001, 2002) |
| Demographics | ||||
| % unstable angina (median, IQR) | 0 (0, 0) | 34 (18, 48) | 0 [0, 0]* | 34 (31, 50) |
| mean age (median, IQR) | 56 (56, 58) | 60 (58, 61) | 60 [59, 62]* | 62 (61, 65) |
| % males (median, IQR) | 85 (71, 94) | 78 (72, 82) | 83 [78, 100]* | 76 (70, 79) |
| % diabetics (median, IQR) | 11 (9, 18) | 16 (11, 21) | 22 [14, 34]* | 20 (17, 25) |
| % multivessel disease (median, IQR) | 0 (0, 40) | 38 (33, 57) | 60 [49, 70]* | 42 (40, 44) |
| Including only lesions in small arteries | 0 | 9 | 0 | 0 |
| Including only complex lesions | 0 | 0 | 0 | 3 |
Range.
BMS: bare metal stents; DES: drug eluting stents; IQR: interquartile range; ND: no data; PTCA: percutaneous transluminal coronary angioplasty.
Small arteries are arteries with a diameter of 3.0 mm or less. Complex lesions are lesions near bifurcations or in tortuous vessels.
Evolution of comparisons over time
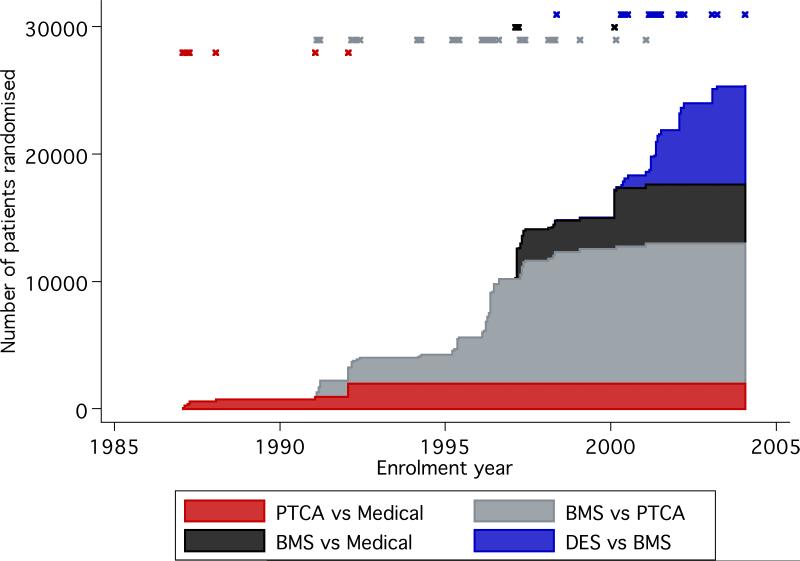
The cumulative number of randomised patients increased steeply in the last decade (Figure 2). Most were enrolled for comparisons between different strategies for PCI. Overall, each time a newer technology appeared, the focus of subsequent RCTs shifted to evaluate the latest technology versus its immediate predecessor. The only exceptions to this trend were 4 RCTs that compared BMS and medical therapy, which started enrolling patients as early as 1997, after at least 20 trials (on 7124 patients) had already begun comparing BMS with PTCA.
Figure 2.
Accumulation of randomised evidence per comparison type over time.
Shown is the cumulative number of patients randomised in each comparison (different colors) against the year of patient enrollment in each trial. The year of first patient enrollment in each trial is marked with an `x' in the upper part of the graph, with colors corresponding to comparison type.
Direct comparisons
The only comparisons for which there was no direct comparative evidence were DES vs. PTCA and DES vs. medical therapy (Appendix Figure 2). Depending on the outcome/comparison combination, the amount of the accumulated information on direct comparisons ranged from 2 to 32 trials, or 1991 to 10 709 randomised patients (Table 2).
Table 2.
Summary effects from direct comparisons and combined direct and indirect comparisons
| Trials (patients) | RR (95% CI) | ||||
|---|---|---|---|---|---|
| Direct only | Additional indirect | Direct only | I2 (%) | Combined direct and indirect* | |
| PTCA vs. Medical | |||||
| Death | 7 (1991) | 44 (21 566) | 0·82 (0·59, 1·15) | 18 | 0·91 (0·70, 1·18) |
| MI | 7 (1991) | 48 (21 631) | 1·09 (0·59, 1·99) | 70 | 1·23 (0·89, 1·70) |
| CABG | 5 (1646) | 38 (16 555) | 1·10 (0·81,1·49) | 1 | 1·06 (0·87,1·29) |
| Revascularisation | 7 (1991) | 6 (6818) | 1·08 (0·74, 1·56) | 81 | 0·92 (0·74, 1·14) |
| BMS vs. Medical | |||||
| Death | 3 (4518) | 49 (19 039) | 0·96 (0·79, 1·18) | 0 | 0·90 (0·70, 1·16) |
| MI | 4 (4619) | 51 (19 003) | 1·18 (0·97, 1·43) | 0 | 1·24 (0·88, 1·75) |
| CABG | 2 (2267) | 50 (15 934) | 0·97 (0·63, 1·50) | 0 | 1·04 (0·83, 1·29) |
| Revascularisation | 3 (4518) | 10 (4291) | 0·78 (0·58, 1·05) | 75 | 0·71 (0·58, 0·87) |
| DES vs. Medical | |||||
| Death | 0 (0) | 51 (23 557) | – | – | 0·96 (0·60, 1·52) |
| MI | 0 (0) | 55 (23 622) | – | – | 1·15 (0·73, 1·82) |
| CABG | 0 (0) | 43 (18 201) | – | – | 0·58 (0·38, 0·88) |
| BMS vs. PTCA | |||||
| Death | 26 (9720) | 25 (13 837) | 0·91 (0·64, 1·27) | 1 | 0·99 (0·76, 1·30) |
| MI | 30 (10 709) | 25 (12 913) | 0·97 (0·81, 1·16) | 2 | 1·01 (0·83, 1·23) |
| CABG | 28 (10 151) | 15 (8050) | 0·99 (0·80, 1·23) | 0 | 0·97 (0·83, 1·14) |
| TVR/TLR | 32 (10 568) | 0 (0) | 0·68 (0·60, 0·77) | 56 | 0·68 (0·60, 0·77) |
| Revascularisation | 3 (2300) | 10 (6509) | 0·79 (0·59, 1·06) | 62 | 0·77 (0·61, 0·99) |
| DES vs. PTCA | |||||
| Death | 0 (0) | 51 (23 557) | – | – | 1·05 (0·66, 1·69) |
| MI | 0 (0) | 55 (23 622) | – | – | 0·94 (0·65, 1·35) |
| CABG | 0 (0) | 43 (18 201) | – | – | 0·55 (0·37, 0·81) |
| TVR/TLR | 0 (0) | 50 (18 326) | – | – | 0·30 (0·17, 0·51) |
| DES vs. BMS | |||||
| Death | 15 (7328) | 36 (16 229) | 1·09 (0·73, 1·63) | 0 | 1·06 (0·71, 1·58) |
| MI | 14 (6303) | 41 (17 319) | 1·03 (0·79, 1·35) | 0 | 0·93 (0·68, 1·26) |
| CABG | 12 (4995) | 31 (13 206) | 0·56 (0·36, 0·88) | 0 | 0·56 (0·39, 0·80) |
| TVR/TLR | 18 (7758) | 0 (0) | 0·44 (0·35, 0·56) | 52 | 0·44 (0·35, 0·56) |
For the outcomes of MI, CABG and revascularisation the network meta-analysis had no incoherence (<0·0001). For death the incoherence parameter was small (0.039, see text); for TVR/TLR calculations of incoherence were not applicable.
BMS: bare metal stents; CABG: coronary artery bypass grafting; CI: confidence interval; DES: drug eluting stents; MI: acute myocardial infarction; PTCA: percutaneous transluminal coronary angioplasty; RR: risk ratio; TVR/TLR: target vessel/lesion revascularisation; UA: unstable angina.
Trials with no events in both arms have been excluded from these calculations. Including them using continuity corrections does not change these results appreciably.
There were no statistically significant differences for death in any of the direct comparisons. The summary RRs ranged between 0·82 and 1·09. The corresponding confidence intervals could not exclude RRs as extreme as 0·59 or 1·63 in all comparisons. There was little evidence for heterogeneity (I2<18% in all comparisons).
Similarly, for MI we found no statistically significant differences in any comparison. Summary RRs for MI ranged between 0·97 and 1·18; their confidence intervals could not exclude RRs as extreme as 0·59 and 1·99 in any comparison. There was evidence for heterogeneity in the MI outcome for PTCA vs. medical therapy (I2=70%).
The only statistically significant effect for subsequent CABG was found in the DES vs. BMS comparison (12 trials; 4995 patients). The summary RR was 0·56 (95% confidence interval, CI: 0·36 to 0·88; favoring DES), with no evidence for between-trial heterogeneity (I2=0).
The TLR/TVR outcome was not assessed in trials where medical therapy was a comparator. There were significant improvements in this outcome favoring BMS vs. PTCA (RR= 0·68, 95% CI: 0·60, 0·77), and DES vs. BMS (RR=0·44, 95% CI: 0·35, 0·56), with evidence for heterogeneity in both instances (I2=56% and 52%, respectively; Table 2).
The outcome of any revascularisation was assessed in comparisons between BMS, PTCA and medical therapy, and no significant differences were found in any comparison (Table 2). However, this outcome was reported only in 3 out of 34 RCTs in the BMS vs. PTCA comparison.
Network meta-analyses
We also performed joint analyses of all direct and indirect evidence in our network (Figure 3). In the network meta-analysis, all point estimates were similar to the direct comparisons. All statistically significant findings from the aforementioned direct comparisons persisted in these analyses as well (Table 2).
For all outcomes except for death, indirect and direct evidence were very consistent (network incoherence <0·0001). For death, the network incoherence parameter was small yet non-zero (network incoherence=0·039), implying that direct and indirect evidence were not entirely consistent for the comparisons between BMS, PTCA and medical therapy. Indeed, the direct RR for death in the PTCA vs. medical therapy comparison was 0·82 (95% CI: 0·59, 1·15), whereas the corresponding indirect was 1·05 (95% CI: 0·71, 1·57). The combined direct and indirect evidence was between these estimates (Table 2).
For almost all outcomes and comparisons, the network meta-analysis resulted in summary estimates with increased precision (narrower confidence intervals) compared to the corresponding direct comparisons. For example, for the death outcome, the confidence intervals from the network meta-analysis could exclude RRs more extreme than 0·70 or 1·30 for the comparisons between BMS, PTCA and medical therapy (Table 2).
We were also able to contrast DES with PTCA and medical therapy based on indirect evidence. For both comparisons there were no significant differences for death and MI. However, the corresponding 95% confidence intervals could not exclude RRs as extreme as 0·60 and 1·69 or 0·65 and 1·82, respectively (Figure 3; Table 2) for death or for MI. The summary indirect effects for the frequency of CABG favored DES over both PTCA and medical therapy beyond what is expected by chance (RR=0·55 and 0·58, respectively). Similarly, the risk for TVR/TLR was significantly lower for DES compared to PTCA (RR=0·30, 95% CI: 0·17, 0·51).
Subgroup and sensitivity analyses
Subgroup analyses agreed with the main analyses (not shown). Of note, when trials using sirolimus stents were excluded, we found no evidence that paclitaxel stents decreased death or MI compared to any of the other therapies. In meta-regressions, the proportion of patients with unstable angina was not associated with the RR for death (p=0·34 and p=0·21 for BMS vs. PTCA, and for DES vs. BMS, respectively; not estimable for other comparisons), for MI (corresponding p-values were p=0·12 and p=0·44) or for CABG (p=0·50 and p=0·60, respectively). For the TVR/TLR outcome, meta-regressions suggested potentially smaller differences between the BMS vs. PTCA strategies with increasing proportion of unstable angina patients in the trials (p=0·09).
Analyses were very similar when we included trials with 0 events in both arms in the calculations, when we added trials with mixed interventions in their PCI arm to the PTCA vs Medical comparisons, and when we examined alternative follow-up times for trials reporting multiple follow-up durations (not shown).
Discussion
Over the last two decades, over 25 000 patients were enrolled in 61 RCTs (63 meta-analysis entries) testing PCI for the treatment of nonacute CAD. While BMS and DES yielded sequential improvements in diminishing the need for revascularization, we found no evidence that serial innovations in PCI technologies yielded detectable improvements in the hard outcomes of death or MI, compared to medical therapy. Overall, these results support current recommendations to optimize medical therapy as an initial management strategy in patients with CAD in the non-acute setting. The broad range of included patients across the spectrum of people with nonacute CAD suggests that these findings are likely applicable to many real-life clinical scenarios where either PCI or medical therapy might be considered.
The geometry of our network of trials suggests that each technological innovation became the de facto standard for trials of future innovations, although no clear advantage in terms of hard clinical outcomes had been demonstrated against medical therapy. Only 4 trials involved head-to-head comparisons between stenting and medical therapy and, as of this writing, there are no trials comparing DES to medical therapy. Perhaps not surprisingly, of the six possible comparisons among the 4 interventions, the majority of RCTs (52/63 = 83% including 18 778/25 388 = 74% of total patients) were focused on comparing 2 types of PCI strategies —testing BMS to PTCA and testing DES against BMS.
For patients whose symptoms cannot be adequately relieved by medical therapy, PCI with stenting (drug eluting or bare metal) is a reasonable option for reducing symptoms and improving quality of life.20 In these cases, our indirect comparison suggests that DES substantially reduces the relative risk for TVR/TLR by approximately 70% compared to balloon angioplasty. However, while some medically-treated patients may eventually require PCI for symptom relief,5 many appear able to avoid the risk and expense associated with procedural management of their disease altogether, without incurring any higher risk of mortality or MI. The one caveat to this is that initial PCI with DES may reduce the need for CABG compared to initial medical therapy; this finding however should be confirmed in a head-to-head comparison.
We found that DES was associated with a significantly lower rate of CABG when compared to BMS. In accordance with previous meta-analyses,21 we found no evidence for differences in CABG rates between BMS and PTCA. Furthermore, indirect comparisons suggest that CABG rates favor DES over PTCA and medical therapy. To our knowledge, the present analysis is the first to suggest favorable effects of DES on CABG rates compared to other PCI interventions or medical treatment. For example, assuming control rates similar to those in COURAGE,22 34 patients (95% CI: 23,117) would have to be treated with DES rather than medical therapy to prevent one CABG, over 48 months of follow-up (Appendix Table 5). A head-to-head trial between DES and medical therapy may clarify differences in outcome between these therapies that were not apparent in this indirect comparison.
Furthermore, we observed that stents were associated with lower rates of subsequent PCIs, with DES favored over BMS for this outcome. This finding is in agreement with previous meta-analyses, but should be interpreted with several caveats in mind. A prior meta-analysis has shown that in the (necessarily unblinded) comparisons between BMS and PTCA, similar degrees of angiographic restenosis were more likely to be revascularized in the PTCA rather than the BMS arm.5 Secondly, there was a tendency for TVR/TLR outcome rates to be higher in the BMS control arms of BMS vs. DES trials, compared to rates in the experimental arm of PTCA vs. BMS trials.23,24 Lastly, in the BENESTENT II trial, which included randomisation between clinically-based and protocol-driven follow-up angiography, protocol-driven angiography itself doubled TVR/TLR rates.25 Taken together these findings suggest that the true reduction in clinically driven TVR/TLR rates from PTCA to DES may be less than the sum suggested by the sequential improvements.
Nevertheless, it remains curious why these substantial sequential reductions in TVR/TLR with sequential innovations in PCI have not translated into detectable improvements in hard outcomes like death or MI. A likely reason for the apparent failure of PCI to reduce death or MI in nonacute CAD is that PCI remains a local therapy, typically targeted at a few hemodynamically-compromising lesions. CAD though is a diffuse disease process that frequently involves the entire coronary vasculature, making it more likely to respond to systemic treatments like medical therapy.26 Additionally, radiographically detected restenotic lesions that may lead to revascularization in nonacute CAD may often be stable and less prone to cause MI or death.27
As with all null meta-analyses, the absence of a detected difference between therapies does not rule out the possibility of an undetected benefit (or harm), as described by our confidence intervals. Of note, a recent meta-analysis suggested a mortality reduction with PCI-based strategies compared to medical strategies.28 This meta-analysis, however, included data extracted from an erroneous report29 and no longer reached formal statistical significance when the corrected outcome data30 were used. There were important methodological differences between our network meta-analysis and the aforementioned study. The latter considered all catheter-based strategies as a single treatment, included trials with mixed interventions in their PCI arm, as well as trials in which substantial numbers of patients received CABG instead of PCI, and did not use any information from indirect comparisons.
Although many patients with unstable angina were included in this network meta-analysis, these results should be cautiously interpreted with respect to this specific population. Several relevant trials that included patients with unstable angina were excluded from the present analysis because they also enrolled patients with acute MI. In earlier studies, patients with unstable angina may actually have had evidence of myonecrosis by more contemporary markers (e.g., troponin). However, the inadvertent inclusion of patients with acute disease and indications for emergency PCI would presumably have biased this study to finding a treatment effect for intervention compared to medical therapy. In any case, our results are consistent with earlier observations that the benefits of PCI in patients with acute coronary syndromes (including those with unstable angina) may be limited to high-risk patients, with uncertain benefit observed in lower-risk patients with negative cardiac biomarkers (i.e. unstable angina).3,31 It also is important to emphasize that our analysis also excluded patients with ST-elevation MI treated with PCI, a clinical setting in which it has more consistently been demonstrated to be better than medical therapy.32
Several limitations of the present analysis are worth noting. First, indirect evidence is susceptible to confounding,33 and thus should be interpreted with due caution, as it does not always agree with the corresponding direct estimates.34,35 Although in our case direct and indirect evidence agreed for all outcomes except for death, this can be an artifact of the limited interconnections in our network (not all possible comparisons have been performed). For death direct and indirect evidence disagreed in their direction, but were both statistically nonsignificant in all comparisons and their confidence intervals overlapped greatly. Second, medical therapy has substantially evolved over the past two decades, and comparisons to medical treatments in some of the earlier trials may not be directly relevant to current practice. Third, crossover from medical therapy to PCI and from PTCA to stenting with BMS may have biased findings towards a neutral effect on some outcomes, and against PCI in general. Because here we are interested in effectiveness (i.e. in testing initial management strategies) rather than efficacy,36,37 we believe that this concern is only tangentially relevant. As with any meta-analysis, we can only analyse outcomes reported in the original RCTs and do not consider every possible clinically relevant outcome. We could not, for example, evaluate for changes in functional status or late in-stent thrombosis due to a lack of consistently reported data. Furthermore, we cannot fully explore treatment effects in different sub-populations without access to individual patient data. Finally, publication bias is an inherent limitation of meta-analyses, but this is more likely to affect the interpretation of the positive findings on revascularization, rather than the neutral finding on death and MI.
Conclusion
Over the past two decades, sequential innovations in PCI for the management of stable CAD showed no evidence of an impact on death or MI when compared to medical therapy. Technology appeared to develop in advance of data on hard clinical outcomes such as deaths and MI. Comparative effectiveness trials of DES versus medical therapy, examining clinically important outcomes are needed.
Supplementary Material
Results from network meta-analyses incorporating direct and indirect comparisons between the eligible interventions.
Matrix of summary risk ratio plots from the performed comparisons between the four interventions, with respect to 5 outcomes. Note that the DES vs. PTCA and DES vs. medical cells of the matrix are based only on indirect data. Each summary risk ratio is depicted by a filled circle, and the corresponding 95% confidence intervals are shown as horizontal lines. Red vertical lines correspond to the line of no effect. The Appendix shows the corresponding figure for direct effects only.
* TVR could not be assessed in medical therapy trials.
† The total number of revascularisations (not only those of the target vessel/lesion) was not extractable from DES vs. BMS trials, and was only extractable from 3 out of 34 BMS vs. PTCA trials.
BMS: bare metal stents; DES: drug-eluting stents; MI: Myocardial infarction, PTCA: percutaneous transluminal balloon angioplasty; Revasc: total number of revascularisations; TVR: target vessel (or lesion) revascularisation.
Acknowledgement
Dr. Alsheikh-Ali is currently a recipient of a faculty development award from Pfizer/Tufts Medical Center.
Footnotes
Conflict of interest statement We declare that none of the authors has a conflict of interest in this submission.
Role of the funding source No specific funding was obtained for this work. None other than the authors had any role in study design, collection, analysis, or interpretation of data, in the writing of the report, or the decision to submit the paper for publication.
References
- (1).Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- (2).Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- (3).Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA. 2005;293:2908–17. doi: 10.1001/jama.293.23.2908. [DOI] [PubMed] [Google Scholar]
- (4).Bucher HC, Hengstler P, Schindler C, Guyatt GH. Percutaneous transluminal coronary angioplasty versus medical treatment for non-acute coronary heart disease: meta-analysis of randomised controlled trials. BMJ. 2000;321:73–7. doi: 10.1136/bmj.321.7253.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Brophy JM, Belisle P, Joseph L. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann Intern Med. 2003;138:777–86. doi: 10.7326/0003-4819-138-10-200305200-00005. [DOI] [PubMed] [Google Scholar]
- (6).Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004;364:583–91. doi: 10.1016/S0140-6736(04)16850-5. [DOI] [PubMed] [Google Scholar]
- (7).Katritsis DG, Karvouni E, Ioannidis JP. Meta-analysis comparing drug-eluting stents with bare metal stents. Am J Cardiol. 2005;95:640–3. doi: 10.1016/j.amjcard.2004.10.041. [DOI] [PubMed] [Google Scholar]
- (8).Grines CL. Off-label use of drug-eluting stents putting it in perspective. J Am Coll Cardiol. 2008;51:615–7. doi: 10.1016/j.jacc.2007.10.028. [DOI] [PubMed] [Google Scholar]
- (9).Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–24. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- (10).Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313–24. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- (11).Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol. 2002;31:150–3. doi: 10.1093/ije/31.1.150. [DOI] [PubMed] [Google Scholar]
- (12).Ioannidis JP, Katritsis DG. Percutaneous coronary intervention for late reperfusion after myocardial infarction in stable patients. Am Heart J. 2007;154:1065–71. doi: 10.1016/j.ahj.2007.07.049. [DOI] [PubMed] [Google Scholar]
- (13).Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–48. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- (14).DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- (15).Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- (16).Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- (17).Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D'Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–iv. doi: 10.3310/hta9260. [DOI] [PubMed] [Google Scholar]
- (18).Folland ED, Hartigan PM, Parisi AF. Percutaneous transluminal coronary angioplasty versus medical therapy for stable angina pectoris: outcomes for patients with double-vessel versus single-vessel coronary artery disease in a Veterans Affairs Cooperative randomized trial. Veterans Affairs ACME InvestigatorS. J Am Coll Cardiol. 1997;29:1505–11. doi: 10.1016/s0735-1097(97)00097-1. [DOI] [PubMed] [Google Scholar]
- (19).Colombo A, Drzewiecki J, Banning A, Grube E, Hauptmann K, Silber S, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–94. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- (20).Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–68. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- (21).Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation. 2005;111:2906–12. doi: 10.1161/CIRCULATIONAHA.104.521864. [DOI] [PubMed] [Google Scholar]
- (22).Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- (23).Kent DM, Trikalinos TA. Are “treatment” bare metal stents superior to “control” bare metal stents? A meta-analytic approach. Am Heart J. 2008;155:624–9. 629. doi: 10.1016/j.ahj.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tung R, Kaul S, Diamond GA, Shah PK. Narrative review: drug-eluting stents for the management of restenosis: a critical appraisal of the evidence. Ann Intern Med. 2006;144:913–9. doi: 10.7326/0003-4819-144-12-200606200-00009. [DOI] [PubMed] [Google Scholar]
- (25).Serruys PW, van HB, Bonnier H, Legrand V, Garcia E, Macaya C, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II) Lancet. 1998;352:673–81. doi: 10.1016/s0140-6736(97)11128-x. [DOI] [PubMed] [Google Scholar]
- (26).Holmes DR, Gersh BJ, Whitlow P, King SB, Dove JT. Percutaneous coronary intervention for chronic stable angina: A reassessment. J Am Coll Cardiol Intv. 2008;1:34–43. doi: 10.1016/j.jcin.2007.10.001. [DOI] [PubMed] [Google Scholar]
- (27).Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–72. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- (28).Schomig A, Mehilli J, de WA, Seyfarth M, Pache J, Kastrati A. A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:894–904. doi: 10.1016/j.jacc.2008.05.051. [DOI] [PubMed] [Google Scholar]
- (29).Soares PR, Hueb WA, Lemos PA, Lopes N, Martinez EE, Cesar LA, et al. Coronary revascularization (surgical or percutaneous) decreases mortality after the first year in diabetic subjects but not in nondiabetic subjects with multivessel disease: an analysis from the Medicine, Angioplasty, or Surgery Study (MASS II) Circulation. 2006;114:I420–I424. doi: 10.1161/CIRCULATIONAHA.105.000679. [DOI] [PubMed] [Google Scholar]
- (30).Hueb W, Lopes NH, Gersh BJ, Soares P, Machado LA, Jatene FB, et al. Five-year follow-up of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2007;115:1082–9. doi: 10.1161/CIRCULATIONAHA.106.625475. [DOI] [PubMed] [Google Scholar]
- (31).Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- (32).Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- (33).Baker SG, Kramer BS. The transitive fallacy for randomized trials: if A bests B and B bests C in separate trials, is A better than C? BMC Med Res Methodol. 2002;2:13. doi: 10.1186/1471-2288-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Song F, Harvey I, Lilford R. Adjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. J Clin Epidemiol. 2008;61:455–63. doi: 10.1016/j.jclinepi.2007.06.006. [DOI] [PubMed] [Google Scholar]
- (36).Ernst E, Pittler MH. Efficacy or effectiveness? J Intern Med. 2006;260:488–90. doi: 10.1111/j.1365-2796.2006.01707.x. [DOI] [PubMed] [Google Scholar]
- (37).Prochaska JO, Evers KE, Prochaska JM, Van MD, Johnson JL. Efficacy and effectiveness trials: examples from smoking cessation and bullying prevention. J Health Psychol. 2007;12:170–8. doi: 10.1177/1359105307071751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results from network meta-analyses incorporating direct and indirect comparisons between the eligible interventions.
Matrix of summary risk ratio plots from the performed comparisons between the four interventions, with respect to 5 outcomes. Note that the DES vs. PTCA and DES vs. medical cells of the matrix are based only on indirect data. Each summary risk ratio is depicted by a filled circle, and the corresponding 95% confidence intervals are shown as horizontal lines. Red vertical lines correspond to the line of no effect. The Appendix shows the corresponding figure for direct effects only.
* TVR could not be assessed in medical therapy trials.
† The total number of revascularisations (not only those of the target vessel/lesion) was not extractable from DES vs. BMS trials, and was only extractable from 3 out of 34 BMS vs. PTCA trials.
BMS: bare metal stents; DES: drug-eluting stents; MI: Myocardial infarction, PTCA: percutaneous transluminal balloon angioplasty; Revasc: total number of revascularisations; TVR: target vessel (or lesion) revascularisation.