Abstract
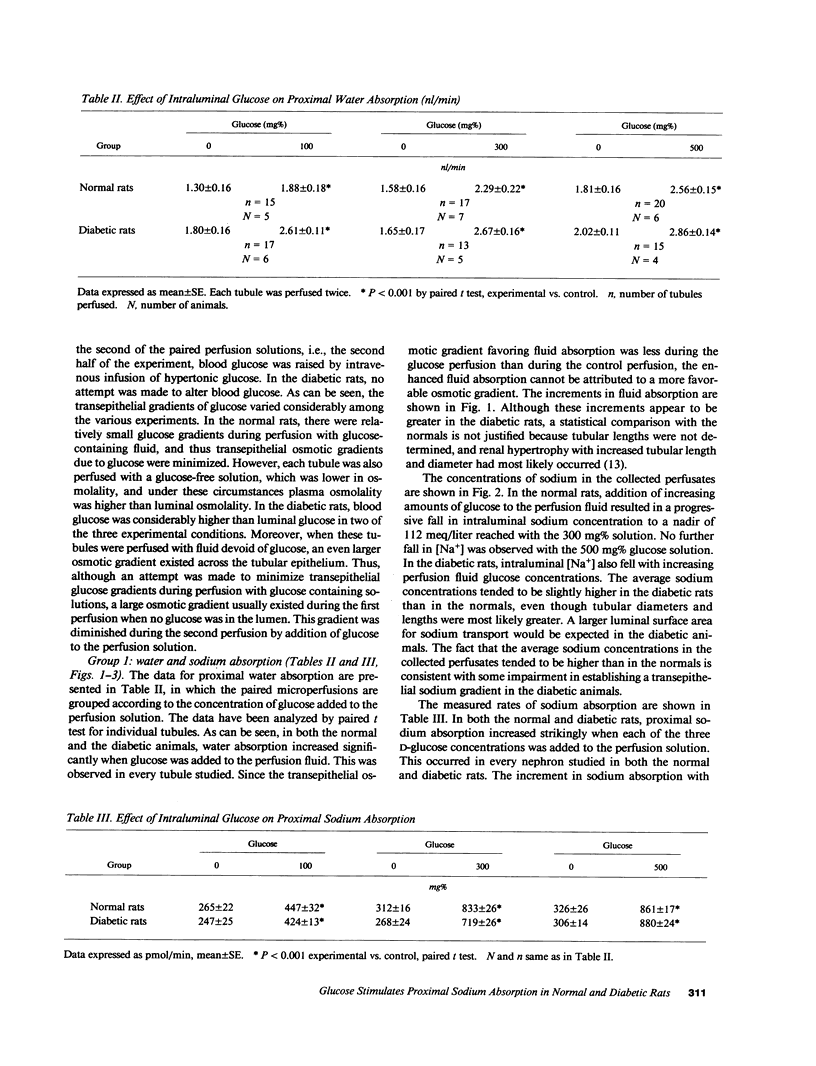
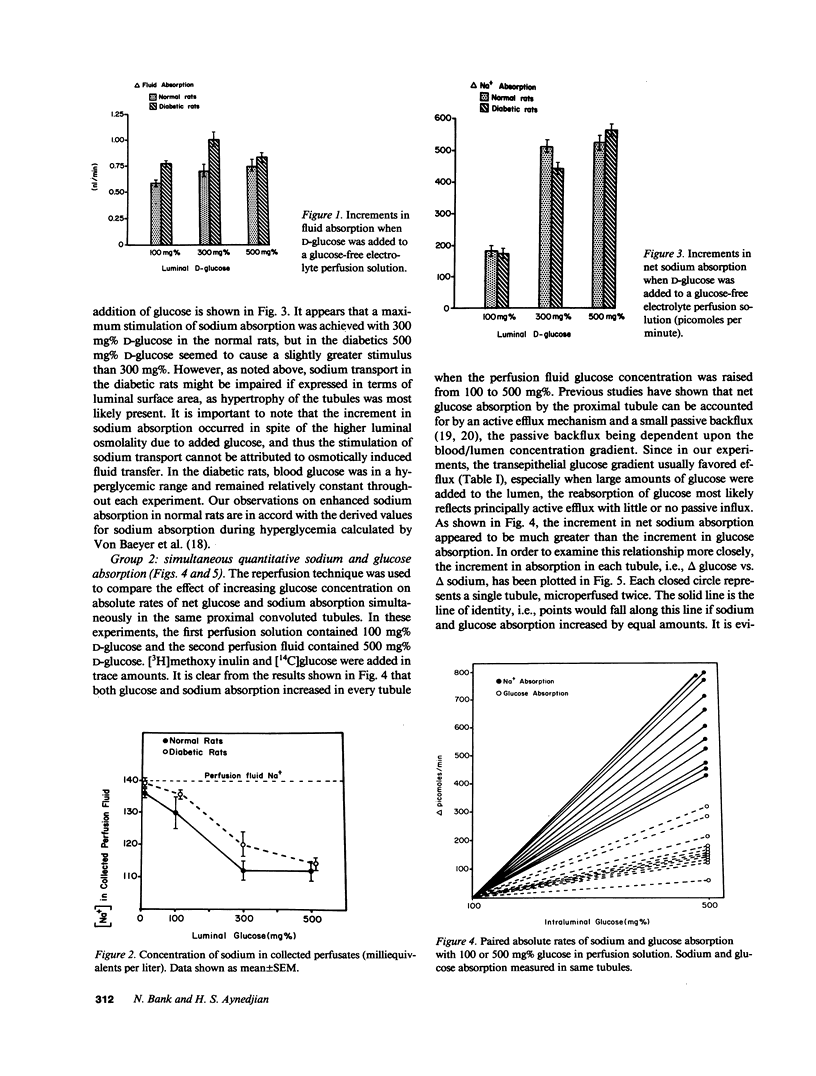
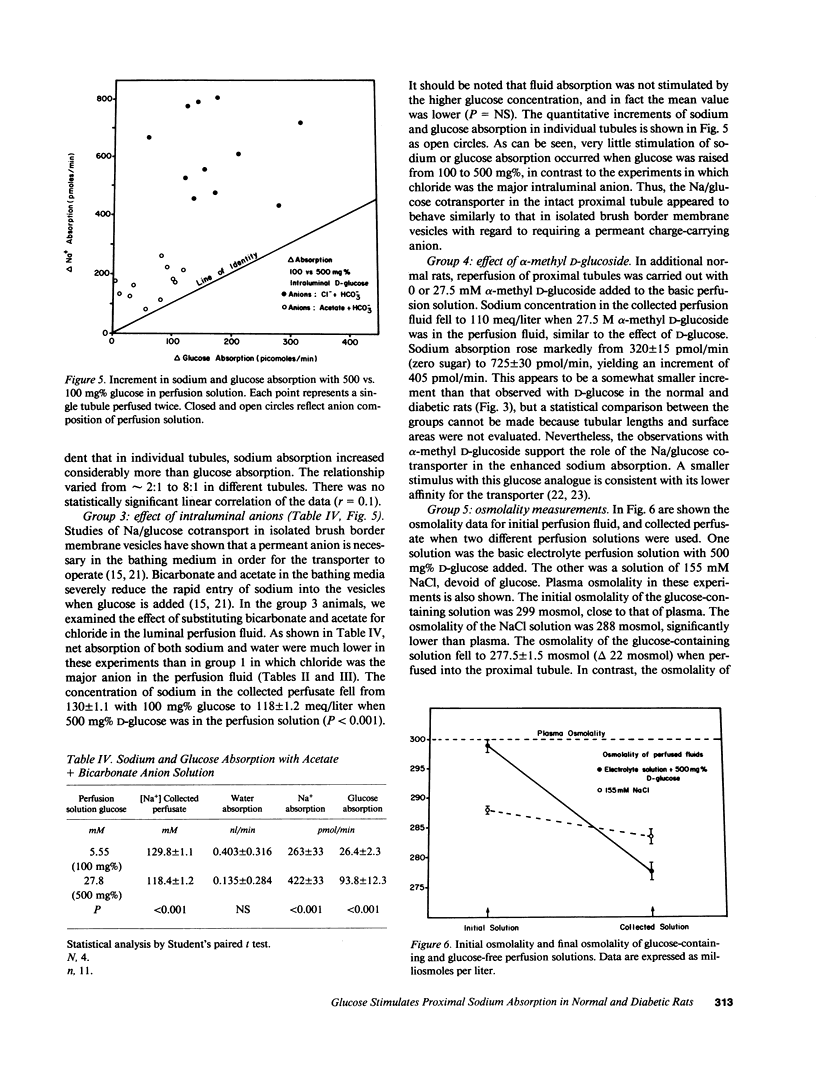
The effect of progressive increases in intraluminal glucose concentration on proximal tubule sodium absorption was studied in normal and streptozotocin diabetic rats by microperfusion. Each tubule was perfused twice, with and without glucose added to the perfusion fluid. Net sodium and water absorption were markedly enhanced by 300-500 mg% intraluminal glucose in both normal and diabetic rats. Substituting the transported but nonmetabolized glucose analogue, alpha-methyl D-glucoside for glucose also resulted in marked stimulation of sodium absorption, whereas substituting bicarbonate and acetate for chloride in the perfusion solution inhibited the effect of glucose. These observations suggest that the stimulation of sodium absorption by glucose was mediated by the brush border Na/glucose cotransporter. Sodium concentration and osmolality were found to fall markedly to hypotonic levels when high glucose concentrations were in the perfusion fluid. This luminal hypotonicity may be an important driving force for proximal fluid absorption. In poorly controlled diabetes, high filtered glucose concentrations may lead to enhanced proximal sodium and water absorption, which could in turn contribute to volume expansion, hypertension, and renal hypertrophy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Sacktor B. The Na+ gradient-dependent transport of D-glucose in renal brush border membranes. J Biol Chem. 1975 Aug 10;250(15):6032–6039. [PubMed] [Google Scholar]
- BANK N., AYNEDJIAN H. S. A MICROPUNCTURE STUDY OF THE RENAL CONCENTRATING DEFECT OF POTASSIUM DEPLETION. Am J Physiol. 1964 Jun;206:1347–1354. doi: 10.1152/ajplegacy.1964.206.6.1347. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S., Mutz B. F. Evidence for a DCCD-sensitive component of proximal bicarbonate reabsorption. Am J Physiol. 1985 Nov;249(5 Pt 2):F636–F644. doi: 10.1152/ajprenal.1985.249.5.F636. [DOI] [PubMed] [Google Scholar]
- Bank N., Lahorra G., Aynedjian H. S., Wilkes B. M. Sodium restriction corrects hyperfiltration of diabetes. Am J Physiol. 1988 May;254(5 Pt 2):F668–F676. doi: 10.1152/ajprenal.1988.254.5.F668. [DOI] [PubMed] [Google Scholar]
- Bank N., Mower P., Aynedjian H. S., Wilkes B. M., Silverman S. Sorbinil prevents glomerular hyperperfusion in diabetic rats. Am J Physiol. 1989 Jun;256(6 Pt 2):F1000–F1006. doi: 10.1152/ajprenal.1989.256.6.F1000. [DOI] [PubMed] [Google Scholar]
- Barfuss D. W., Schafer J. A. Differences in active and passive glucose transport along the proximal nephron. Am J Physiol. 1981 Sep;241(3):F322–F332. doi: 10.1152/ajprenal.1981.241.3.F322. [DOI] [PubMed] [Google Scholar]
- Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest. 1987 Apr;79(4):1104–1109. doi: 10.1172/JCI112925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. Energetics of the Na+-dependent transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1975 Nov 25;250(22):8674–8680. [PubMed] [Google Scholar]
- Bishop J. H., Green R., Thomas S. Effects of glucose on water and sodium reabsorption in the proximal convoluted tubule of rat kidney. J Physiol. 1978 Feb;275:481–493. doi: 10.1113/jphysiol.1978.sp012202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. H., Green R., Thomas S. Free-flow reabsorption of glucose, sodium, osmoles and water in rat proximal convoluted tubule. J Physiol. 1979 Mar;288:331–351. [PMC free article] [PubMed] [Google Scholar]
- Burg M., Patlak C., Green N., Villey D. Organic solutes in fluid absorption by renal proximal convoluted tubules. Am J Physiol. 1976 Aug;231(2):627–637. doi: 10.1152/ajplegacy.1976.231.2.627. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frega N. S., Weinberg J. M., Ross B. D., Leaf A. Stimulation of sodium transport by glucose in the perfused rat kidney. Am J Physiol. 1977 Sep;233(3):F235–F240. doi: 10.1152/ajprenal.1977.233.3.F235. [DOI] [PubMed] [Google Scholar]
- Green R., Giebisch G. Luminal hypotonicity: a driving force for fluid absorption from the proximal tubule. Am J Physiol. 1984 Feb;246(2 Pt 2):F167–F174. doi: 10.1152/ajprenal.1984.246.2.F167. [DOI] [PubMed] [Google Scholar]
- Gregg C. M., Cohen J. J., Black A. J., Espeland M. A., Feldstein M. L. Effects of glucose and insulin on metabolism and function of perfused rat kidney. Am J Physiol. 1978 Jul;235(1):F52–F61. doi: 10.1152/ajprenal.1978.235.1.F52. [DOI] [PubMed] [Google Scholar]
- Hilden S. A., Sacktor B. D-Glucose-dependent sodium transport in renal brush border membrane vesicles. J Biol Chem. 1979 Aug 10;254(15):7090–7096. [PubMed] [Google Scholar]
- Häberle D. A., Müller U., Nagel W. Glomerular tubular balance: mediation by luminal hypotonicity. Miner Electrolyte Metab. 1989;15(3):108–113. [PubMed] [Google Scholar]
- Knight T. F., Senekjian H. O., Sansom S. C., Weinman E. J. Proximal tubule glucose efflux in the rat as a function of delivered load. Am J Physiol. 1980 Jun;238(6):F499–F503. doi: 10.1152/ajprenal.1980.238.6.F499. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G., Rector F. C., Jr Axial heterogeneity in the rat proximal convoluted tubule. II. Osmolality and osmotic water permeability. Am J Physiol. 1984 Nov;247(5 Pt 2):F822–F826. doi: 10.1152/ajprenal.1984.247.5.F822. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Hoshi T. The effect of D-glucose on the electrical potential profile across the proximal tubule of newt kidney. Biochim Biophys Acta. 1972 Sep 1;282(1):214–225. doi: 10.1016/0005-2736(72)90327-6. [DOI] [PubMed] [Google Scholar]
- Ortola F. V., Ballermann B. J., Anderson S., Mendez R. E., Brenner B. M. Elevated plasma atrial natriuretic peptide levels in diabetic rats. Potential mediator of hyperfiltration. J Clin Invest. 1987 Sep;80(3):670–674. doi: 10.1172/JCI113120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostand S. G., Watkins J. B., Clements R. S., Jr The effect of insulin and of anti-insulin serum on handling of sodium by the isolated, perfused kidney of the streptozotocin-diabetic rat. Diabetes. 1980 Sep;29(9):679–685. doi: 10.2337/diab.29.9.679. [DOI] [PubMed] [Google Scholar]
- Stanton B. A., Kaissling B. Regulation of renal ion transport and cell growth by sodium. Am J Physiol. 1989 Jul;257(1 Pt 2):F1–10. doi: 10.1152/ajprenal.1989.257.1.F1. [DOI] [PubMed] [Google Scholar]
- Trimble M. E., Bowman R. H. Renal Na+ and K+ transport: effects of glucose, palmitate, and alpha-bromopalmitate. Am J Physiol. 1973 Nov;225(5):1057–1062. doi: 10.1152/ajplegacy.1973.225.5.1057. [DOI] [PubMed] [Google Scholar]
- Trimble M. E. Effects of L-glucose on sodium reabsorption in the isolated perfused rat kidney. Life Sci. 1975 Dec 15;17(12):1799–1806. doi: 10.1016/0024-3205(75)90462-2. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol. 1982;70(1):37–45. doi: 10.1007/BF01871587. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Stoichiometric studies of the renal outer cortical brush border membrane D-glucose transporter. J Membr Biol. 1982;67(1):73–80. doi: 10.1007/BF01868649. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Specificity and sodium dependence of the active sugar transport in the proximal convolution of the rat kidney. Pflugers Arch. 1974;351(1):35–48. doi: 10.1007/BF00603509. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J. Sugar, amino acid, and Na+ cotransport in the proximal tubule. Annu Rev Physiol. 1979;41:181–195. doi: 10.1146/annurev.ph.41.030179.001145. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Patlak C. S., Burg M. B. Contribution of leaked load to solute transport by renal tubules. Am J Physiol. 1978 Jun;234(6):F480–F484. doi: 10.1152/ajprenal.1978.234.6.F480. [DOI] [PubMed] [Google Scholar]
- Weinman E. J., Suki W. N., Eknoyan G. D-Glucose enhancement of water reabsorption in proximal tubule of the rat kidney. Am J Physiol. 1976 Sep;231(3):777–780. doi: 10.1152/ajplegacy.1976.231.3.777. [DOI] [PubMed] [Google Scholar]
- von Baeyer H., Haeberle D. A., van Liew J. B., Hare D. Glomerular tubular balance of renal D-glucose transport during hyperglycemia: clearance and micropuncture studies on its characterisation at saturated transport conditions. Pflugers Arch. 1980 Mar;384(1):39–47. doi: 10.1007/BF00589512. [DOI] [PubMed] [Google Scholar]
- von Baeyer H., von Conta C., Haeberle D., Deetjen P. Determination of transport constants for glucose in proximal tubules of the rat kidney. Pflugers Arch. 1973 Nov 8;343(4):273–286. doi: 10.1007/BF00595815. [DOI] [PubMed] [Google Scholar]



