Abstract
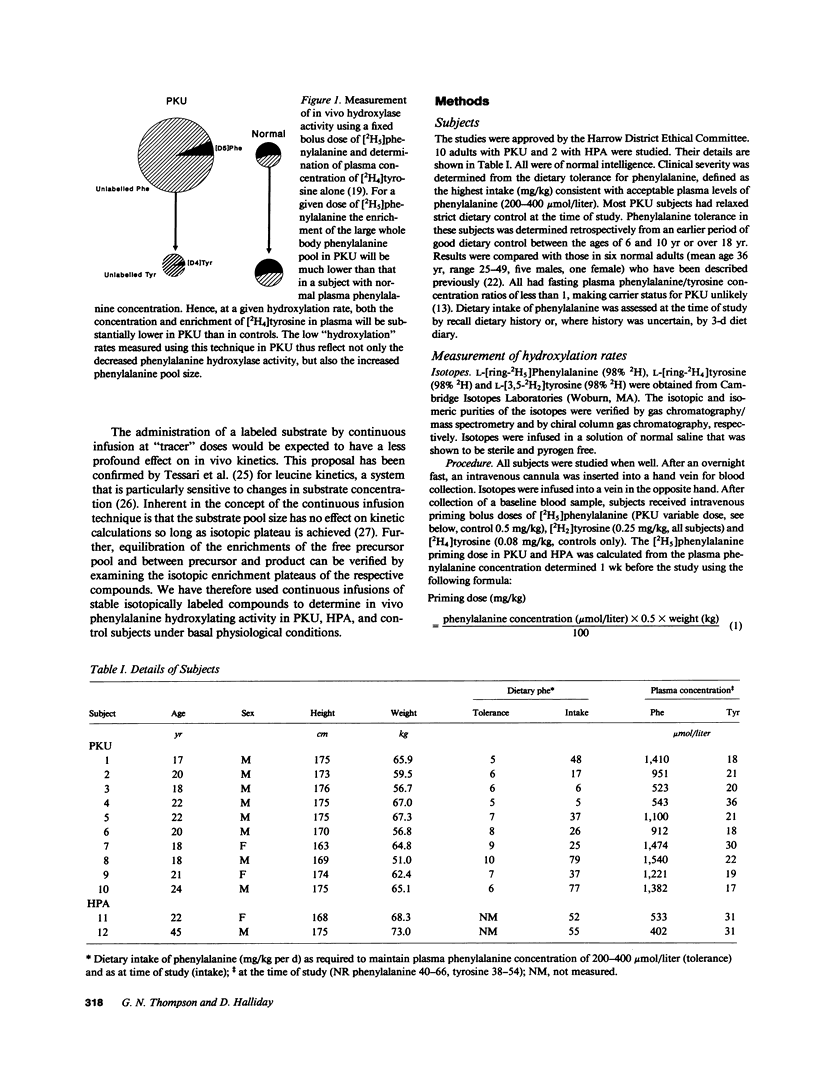
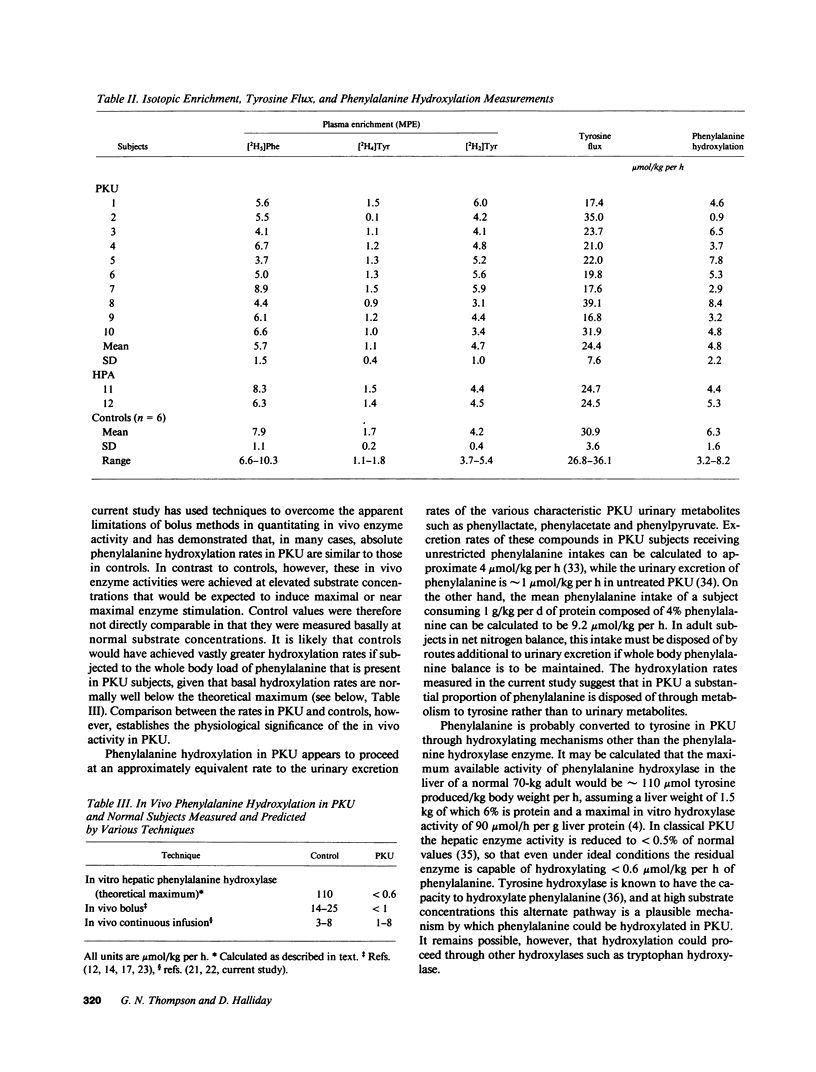
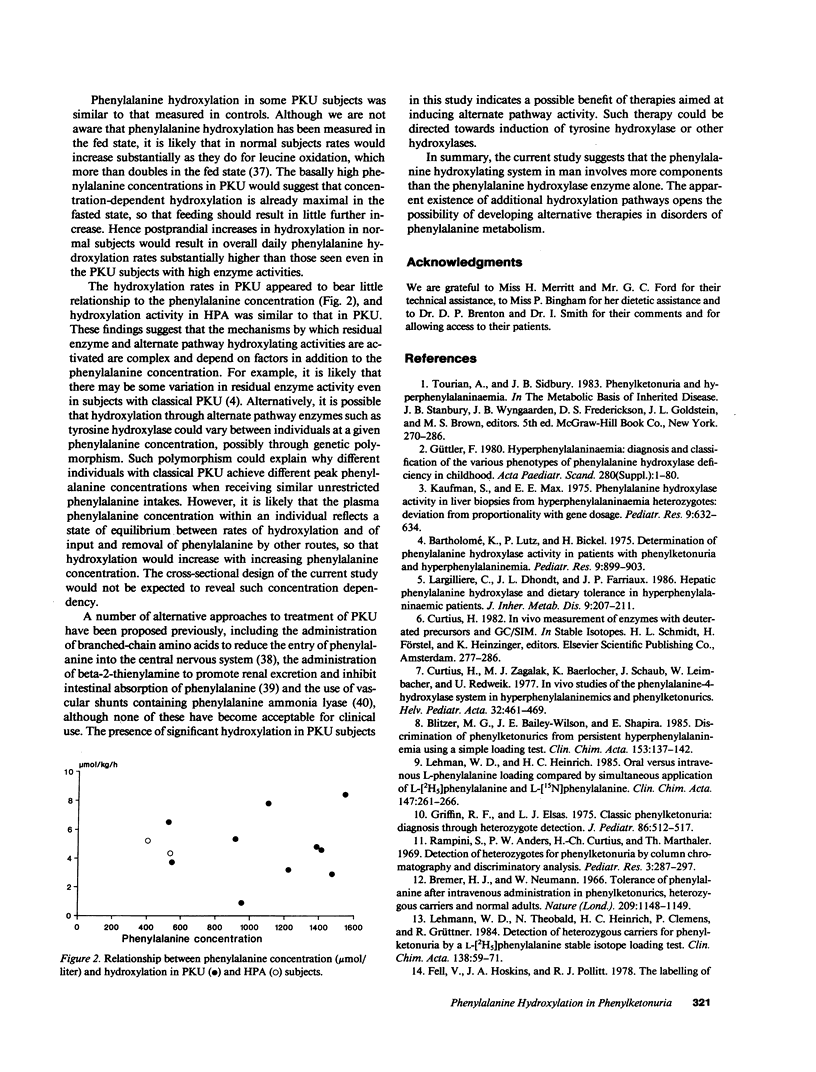
Indirect measurements have previously suggested that patients with classical phenylketonuria (PKU) do not convert significant amounts of phenylalanine to tyrosine. Low-dose continuous infusion techniques employing [2H5]phenylalanine and [2H2]tyrosine were used to quantitate in vivo phenylalanine hydroxylation in 10 subjects with classical phenylketonuria, 2 with hyperphenylalaninemia (HPA), and 7 controls. Plasma phenylalanine concentration ranged from 523 to 1,540 mumols/liter in PKU, 402 to 533 in HPA, and 49 to 54 in controls. Subjects with classical PKU hydroxylated mean +/- SD 4.8 +/- 2.2 mumols/kg per h (range 0.9-8.4) of phenylalanine to tyrosine and those with HPA 4.4 and 5.3, respectively. These rates were substantial in comparison with those in controls (6.3 +/- 1.6, 3.2-8.2). The significant hydroxylation in PKU and HPA subjects is likely to result from induction of activity of tyrosine hydroxylase towards phenylalanine by the greatly elevated phenylalanine concentration. The presence of such activity in PKU suggests that therapy aimed at promotion of this usually latent hydroxylating capacity may be a future alternative to dietary treatment of PKU.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus C. M., Sharma S. D., Horváth C., Kalghatgi K., Anthone S., Ambrus J. L., Cooley C., Mirand E. A. In vivo safety of hollow fiber enzyme-reactors with immobilized phenylalanine ammonia-lyase in a large animal model for phenylketonuria. J Pharmacol Exp Ther. 1983 Mar;224(3):598–602. [PubMed] [Google Scholar]
- Bartholomé K., Lutz P., Bickel H. Determination of phenylalanine hydroxylase activity in patients with phenylketonuria and hyperphenylalaninemia. Pediatr Res. 1975 Dec;9(12):899–903. doi: 10.1203/00006450-197512000-00006. [DOI] [PubMed] [Google Scholar]
- Berry H. K., Bofinger M. K., Hunt M. M., Phillips P. J., Guilfoile M. B. Reduction of cerebrospinal fluid phenylalanine after oral administration of valine, isoleucine, and leucine. Pediatr Res. 1982 Sep;16(9):751–755. doi: 10.1203/00006450-198209000-00009. [DOI] [PubMed] [Google Scholar]
- Blitzer M. G., Bailey-Wilson J. E., Shapira E. Discrimination of phenylketonurics from persistent hyperphenylalaninemia patients using a simple phenylalanine loading test. Clin Chim Acta. 1985 Dec 13;153(2):137–142. doi: 10.1016/0009-8981(85)90164-0. [DOI] [PubMed] [Google Scholar]
- Bremer H. J., Neumann W. Tolerance of phenylalanine after ntravenous administration in phenylketonurics, heterozygous carriers, and normal adults. Nature. 1966 Mar 12;209(5028):1148–1149. doi: 10.1038/2091148a0. [DOI] [PubMed] [Google Scholar]
- Chalmers R. A., Watts R. W. Quantitative studies on the urinary excretion of unconjugated aromatic acids in phenylketonuria. Clin Chim Acta. 1974 Sep 30;55(3):281–294. doi: 10.1016/0009-8981(74)90002-3. [DOI] [PubMed] [Google Scholar]
- Clarke J. T., Bier D. M. The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of L-[ring-2H5]phenylalanine and L-[1-13C] tyrosine in the postabsorptive state. Metabolism. 1982 Oct;31(10):999–1005. doi: 10.1016/0026-0495(82)90142-1. [DOI] [PubMed] [Google Scholar]
- Curtius H. C., Zagalak M. J., Baerlocher K., Schaub J., Leimbacher W., Redweik U. In vivo studies of the phenylalanine-4-hydroxylase system in hyperphenylalaninemics and phenylketonurics. Helv Paediatr Acta. 1978 Feb;32(6):461–469. [PubMed] [Google Scholar]
- Fell V., Hoskins J. A., Pollitt R. J. The labelling of urinary acids after oral doses of deuterated L-phenylalanine and L-tyrosine in normal subjects. Quantitative studies with implications for the deuterated phenylalanine load test in phenylketonuria. Clin Chim Acta. 1978 Feb 15;83(3):259–269. doi: 10.1016/0009-8981(78)90114-6. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Fisher D. B., Kang E. S., Kaufman S. Detection of hepatic phenylalanine 4-hydroxylase in classical phenylketonuria. Proc Natl Acad Sci U S A. 1973 Feb;70(2):552–556. doi: 10.1073/pnas.70.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R. F., Elsas L. J. Classic phenylketonuria: diagnosis through heterozygote detection. J Pediatr. 1975 Apr;86(4):512–517. doi: 10.1016/s0022-3476(75)80139-9. [DOI] [PubMed] [Google Scholar]
- Hsieh M. C., Berry H. K., Bofinger M. K., Phillips P. J., Guilfoile M. B., Hunt M. M. Comparative diagnostic value of phenylalanine challenge and phenylalanine hydroxylase activity in phenylketonuria. Clin Genet. 1983 Jun;23(6):415–421. doi: 10.1111/j.1399-0004.1983.tb01975.x. [DOI] [PubMed] [Google Scholar]
- Jagenburg R., Regårdh C. G., Rödjer S. Detection of heterozygotes for phenylketonuria. Total body phenylalanine clearance and concentrations of phenylalanine and tyrosine in the plasms of fasting subjects compared. Clin Chem. 1977 Sep;23(9):1654–1660. [PubMed] [Google Scholar]
- Kaufman S., Max E. E., Kang E. S. Phenylalanine hydroxylase activity in liver biopsies from hyperphenylalaninemia heterozygotes: deviation from proportionality with gene dosage. Pediatr Res. 1975 Aug;9(8):632–634. doi: 10.1203/00006450-197508000-00004. [DOI] [PubMed] [Google Scholar]
- Kindt E., Lunde H. A., Gjessing L. R., Halvorsen S., Lie S. O. Fasting plasma amino acid concentrations in PKU children on two different levels of protein intake. Acta Paediatr Scand. 1988 Jan;77(1):60–66. doi: 10.1111/j.1651-2227.1988.tb10598.x. [DOI] [PubMed] [Google Scholar]
- Krips C., Lines D. R. Phenylketonuria: reduction of serum levels of phenylalanine following oral administration of B-2 thienylalanine. Aust Paediatr J. 1972 Dec;8(6):318–321. doi: 10.1111/j.1440-1754.1972.tb01845.x. [DOI] [PubMed] [Google Scholar]
- Lehmann W. D., Heinrich H. C. Oral versus intravenous L-phenylalanine loading compared by simultaneous application of L-[2H5] and L-[15N]phenylalanine. Clin Chim Acta. 1985 Apr 30;147(3):261–266. doi: 10.1016/0009-8981(85)90208-6. [DOI] [PubMed] [Google Scholar]
- Lehmann W. D., Theobald N., Heinrich H. C., Clemens P., Grüttner R. Detection of heterozygous carriers for phenylketonuria by a L-[2H5]phenylalanine stable isotope loading test. Clin Chim Acta. 1984 Mar 27;138(1):59–71. doi: 10.1016/0009-8981(84)90354-1. [DOI] [PubMed] [Google Scholar]
- Lines D. R., Waisman H. A. Urinary amino acid excretion in phenylketonuric, hyperphenylalaninemic, and normal patients. J Pediatr. 1971 Mar;78(3):474–480. doi: 10.1016/s0022-3476(71)80230-5. [DOI] [PubMed] [Google Scholar]
- Matalon R., Matthews D. E., Michals K., Bier D. The use of deuterated phenylalanine for the in vivo assay of phenylalanine hydroxylase activity in children. J Inherit Metab Dis. 1982;5(1):17–19. doi: 10.1007/BF01799749. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Schwarz H. P., Yang R. D., Motil K. J., Young V. R., Bier D. M. Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982 Nov;31(11):1105–1112. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Motil K. J., Matthews D. E., Bier D. M., Burke J. F., Munro H. N., Young V. R. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol. 1981 Jun;240(6):E712–E721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- Okano Y., Chow I. Z., Isshiki G., Inoue A., Oura T. Effects of phenylalanine loading on protein synthesis in the fetal heart and brain of rat: an experimental approach to maternal phenylketonuria. J Inherit Metab Dis. 1986;9(1):15–24. doi: 10.1007/BF01813896. [DOI] [PubMed] [Google Scholar]
- Rampini S., Anders P. W., Curtius H. C., Marthaler T. Detection of heterozygotes for phenylketonuria by column chromatography and discriminatory analysis. Pediatr Res. 1969 Jul;3(4):287–297. doi: 10.1203/00006450-196907000-00004. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Edwards R. H., Halliday D., Matthews D. E., Wolman S. L., Millward D. J. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982 Dec;63(6):519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Rey F., Munnich A., Lyonnet S., Rey J. Classification et hétérogénéité des hyperphénylalaninémies liées à un déficit en phénylalanine hydroxylase. Arch Fr Pediatr. 1987;44 (Suppl 1):639–642. [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- Shiman R., Gray D. W. Substrate activation of phenylalanine hydroxylase. A kinetic characterization. J Biol Chem. 1980 May 25;255(10):4793–4800. [PubMed] [Google Scholar]
- Thompson G. N., Pacy P. J., Ford G. C., Merritt H., Halliday D. Relationships between plasma isotope enrichments of leucine and alpha-ketoisocaproic acid during continuous infusion of labelled leucine. Eur J Clin Invest. 1988 Dec;18(6):639–643. doi: 10.1111/j.1365-2362.1988.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Thompson G. N., Pacy P. J., Merritt H., Ford G. C., Read M. A., Cheng K. N., Halliday D. Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol. 1989 May;256(5 Pt 1):E631–E639. doi: 10.1152/ajpendo.1989.256.5.E631. [DOI] [PubMed] [Google Scholar]
- Trefz F. K., Byrd D. J., Blaskovics M. E., Kochen W., Lutz P. Determination of deuterium-labeled phenylalanine and tyrosine in human plasma with high pressure liquid chromatography and mass spectrometry. Clin Chim Acta. 1976 Dec;73(3):431–438. doi: 10.1016/0009-8981(76)90144-3. [DOI] [PubMed] [Google Scholar]
- Trefz F. K., Erlenmaier T., Hunneman D. H., Bartholomé K., Lutz P. Sensitive in vivo assay of the phenylalanine hydroxylating system with a small intravenous dose of heptadeutero L-phenylalanine using high pressure liquid chromatography and capillary gas chromatography/mass fragmentography. Clin Chim Acta. 1979 Dec 17;99(3):211–230. doi: 10.1016/0009-8981(79)90264-x. [DOI] [PubMed] [Google Scholar]
- Westwood A., Raine D. N. Heterozygote detection in phenylketonuria. Measurement of discriminatory ability and interpretation of the phenylalanine loading test by determination of the heterozygote likelihood ratio. J Med Genet. 1975 Dec;12(4):327–333. doi: 10.1136/jmg.12.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. R. Tracers in metabolic research: radioisotope and stable isotope/mass spectrometry methods. Lab Res Methods Biol Med. 1984;9:1–287. [PubMed] [Google Scholar]



