Abstract
Background
This study evaluates differences in smoking abstinence between white and minority smokers using pharmaceutical aids.
Methods
This is an analysis of data from a multi-center, randomized, clinical trial conducted in the United States. Of the 1,684 subjects randomized to one of three medications (nicotine inhaler, bupropion, or a combination of both), 60% were women and 10% were minority races.
Results
Factors associated with a decreased likelihood of smoking at 12 weeks were older age (OR = 0.971, p < 0.0001), being married (OR = 0.678, p = 0.0029), using bupropion SR (OR = 0.480, p < 0.0001), and using combination therapy (OR = 0.328, p < 0.0001). Factors associated with an increased likelihood of smoking were higher tobacco dependence scores (OR = 1.244, p < 0.0001), prior quit attempts (OR = 1.812, p = 0.004), and being a minority (OR = 1.849, p = 0.0083). Compared to white smokers, minority smokers were significantly older at time of study entry (46 vs. 42 years, p < 0.0001), less likely to be married (35% vs. 59%, p < 0.0001), older at smoking initiation (21 vs. 19 years of age, p < 0.0001), and had a lower abstinence rate (16% vs. 26%, p = 0.0065).
Conclusion
Regardless of the treatment used, minority smokers in the US have lower smoking abstinence after treatment for tobacco dependence. Future research should focus on the improvement in treatment strategies for minority smokers.
Keywords: Smoking abstinence, Minority smokers, Tobacco
Introduction
Globally, about one in three men smoke, and one in ten adults die annually from tobacco-related diseases. As the smoking rates in the developed countries continue to reduce, the rate in developing countries continues to rise (World Health Organization 2002). Indeed, part of the preamble to the WHO Framework Convention on Tobacco Control (FCTC) discusses the “deep concern about the escalation in smoking and other forms of tobacco consumption by indigenous populations and other minority groups worldwide.” The FCTC also mentions the “need to take measures to promote the participation of indigenous individuals and communities in the development, implementation and evaluation of tobacco control programs that are socially and culturally appropriate to their needs and perspectives” (World Health Organization 2000).
Currently, 19.8% of Americans or one out of every four Americans is a current smoker (Centers for Disease Control and Prevention 2008). Unfortunately this statistic does not apply equally to all races living in the USA. The smoking rate in the USA can vary from 12% to 33% depending on the race of the smoker. This translates to one out of every four white Americans, one out of every four African Americans, two out of every three American Indians/Alaskan Natives, and one out of eight Asian Americans being smokers (American Lung Association et al. January 2006; Center for Disease Control and Prevention 2006). Although over 70% of all smokers indicate a desire to stop smoking, it is noteworthy that African-American adults, who smoke fewer cigarettes and have more frequent quit attempts than white smokers (49% vs. 40%, respectively), are less successful (8% vs. 14%, respectively) in achieving smoking abstinence than white smokers (Center for Disease Control and Prevention 1993). Whether this is attributable to differences in nicotine metabolism (Murray et al. 2001) or environmental circumstances is unknown, but the fact remains that despite smoking fewer cigarettes per day, African Americans are 50% more likely to die from lung cancer than their white counterpart (US Department of Health and Human Services 1998).
An earlier study that offered a nicotine inhaler, bupropion SR, or a combination of both for 12 weeks to smokers wishing to stop smoking (Croghan et al. 2007) observed that smokers assigned to the combination therapy were more likely to be abstinent than those assigned to individual therapies. However, this study also showed that the smoking abstinence rate for minority smokers was significantly lower than that of the white smokers. The purpose of this current report is to analyze the study data as they relate to minority smokers enrolled in the open-label phase of this study.
Methods
Study subjects
This study recruited smokers from 19 different cities of the North Central Cancer Treatment Group (NCCTG). The NCCTG is a large cooperative group of oncology practices throughout the USA that performs multiple clinical trials in oncology patients. A total of 1,708 smokers were recruited from the general population, and 1,700 were randomized to the study.
Among these 1,700 smokers, 16 were of Hispanic ethnicity, and 1,684 were non-Hispanic. Of the 1,684 non-Hispanic smokers, 1,512 were white and 172 were minority races (138 were African American and 34 were other races, including Asian-American, American Indian, and Hispanic-American).
Study design
This report is a subanalysis of a larger study involving three study phases. The first phase included 12 weeks of open-label medication to measure efficacy; the second phase assessed blinded relapse prevention or blinded re-treatment; finally, a third phase was the post-medication follow-up. Methodology and results of this larger study have been reported previously (Croghan et al. 2007).
This current report focuses only on the 1,684 non-Hispanic participants who received the first 12 weeks of medication. Study participants were randomized to receive one of three treatment assignments for 12 weeks: 300 mg of bupropion SR per day (150 mg bid), ad libitum use of a nicotine inhaler (up to 16 cartridges per day), or a combination of both the nicotine inhaler and bupropion. The primary endpoint for the study was the smoking status at the end of the treatment period.
Study entry criteria
Potential subjects were eligible for enrollment if they were at least 18 years of age, had smoked at least ten cigarettes/day for at least 12 months, were motivated to use the study medication according to the study protocol, were in general good health, had the ability to participate in all aspects of the study, and provided written informed consent. Potential subjects were excluded if they were pregnant/breast-feeding, using concurrent behavioral/pharmacologic treatments to stop smoking, using tobacco products other than cigarettes, or taking an investigational drug. Subjects were also excluded if they had a known hypersensitivity/allergy to nicotine, bupropion, or menthol, unstable angina/myocardial infarction, a history of bulimia/anorexia nervosa, seizure disorders, serious head trauma or other predisposing factors to seizure, or chemical dependence on any drug other than nicotine in the past year. Subjects were also excluded if they were using antipsychotics, antidepressants, theophylline, systemic steroids, antiepileptic medications, or a monoamine oxidase inhibitor (MAO-I).
Recruitment and enrollment
Recruitment for this large study included smokers contacting their regional North Central Cancer Treatment Group site and undergoing a telephone pre-screen, consent visit and a screening visit. If the smoker was found to be eligible, they were randomized to one of three medications. After randomization, participants returned for study visits at week 4, week 8, and week 12 post-randomization. The subjects were instructed to abstain from smoking upon awakening on the target quit date. All subjects received the NCI “Clearing the Air” booklet and individual counseling based on the “Smoke Free and Living It” manual© (Mayo Clinic Nicotine Research Program 2000).
Study measures
The National Institutes of Health (NIH) define ethnicity as the sociological constructs that emphasize the cultural aspect of a group of people, and race is defined as a biological aspect of a group of people (Crews and Bindon 1991; O'Neil 2008). This study uses this definition, which distinguishes between someone’s ethnic origins ("Hispanic origin" and "Not of Hispanic origin") and race (“American Indian or Alaskan Native,” “Asian or Pacific Islander,” “Black or African American,” and “White”) (Executive Office of the President et al. 2007).
Severity of nicotine dependence was assessed at baseline using the Fagerström Test of Nicotine Dependence (FTND) (Heatherton et al. 1991). Scores for this measure can range from 0 to 10, with a score of 6 or higher indicating severe nicotine dependence (Fagerström 1978). The Health Status Questionnaire (HSQ) (Ware and Sherbourne 1992) is a 39-item instrument comprised of the original SF-36 items plus three additional items used to screen for depression (Radosevich et al. 1994). The SF-36 is a global assessment of health concepts that represent basic human values relevant to functional status and well-being (Ware et al. 1993). Alcohol dependence was assessed using the Self-Administered Alcoholism Screening Test (SAAST) (Hurt et al. 1980).
Abstinence from smoking was defined as a self-report of nonsmoking for the previous 7 days and was considered to be biochemically confirmed with an expired air carbon monoxide value below eight parts per million (ppm). If the participant failed either of these two conditions (reported any smoking in the previous 7 days or had a CO greater than 8 ppm) then the participant was categorized as a smoker. In order to produce a conservative estimate of smoking abstinence, study outcome data were analyzed in an intent-to-treat model. If a participant failed to complete a visit for whatever reason (missed a visit or terminated early from the study), the participant was classified as a smoker for that incomplete visit.
Randomization and statistical analysis
Randomization was performed using the Pocok-Simon approach, which is a dynamic allocation procedure that balanced the marginal distributions of the stratification factors between treatment groups (Pocock and Simon 1975). Stratification factors were: site, gender, number of cigarettes smoked per day (10–39 vs. 40 or more), and number of years smoked (<5, 5–9, >9). Randomization assigned subjects to one of three medications.
Demographics and smoking abstinence rates at 12 weeks were compared using chi-square tests for categorical variables and ANOVA for continuous variables. Comparisons were done between blacks and other minorities to determine if minorities were similar enough to be combined in the main analyses. The main analyses were comparisons between whites and the combined minorities.
The primary endpoint of this study was the week 12, 7-day point-prevalence, biochemically confirmed, smoking abstinence rate. These cessation rates were tested using two-sided chi-squared tests. With about 172 minorities and over 1,500 whites, this study had 80% power to detect a clinically significant 10% point difference in smoking abstinence rates between the two groups (assuming a baseline success rate of 25% for each of the treatments).
Logistic regression
Smoking cessation rates at week 12 were modeled using logistic regression. First, bivariate logistic regression models were fit for each explanatory variable. Then stepwise logistic regression models were used to identify variables prognostic for smoking cessation. Variables were added to the model one at a time in a stepwise method with an inclusion significance level of 0.05. Variables included for selection in the logistic models were minority, treatment arm, age, age started smoking, years smoked, gender, education, marital status, number of cigarettes smoked per day, depression status, FTND, body mass index (BMI), previous quit attempts, previous use of nicotine replacement, and the longest duration of smoking abstinence. To assess whether differences between race groups were dependent on treatment, the race-by-treatment interaction effect was added to the final model determined using the stepwise approach.
Results
Of the 1,684 smokers analyzed for this report, 172 (10%) were of the four minority races, and 1,512 (90%) were white. A comparison of demographics and abstinence rates between black and other minorities showed that no significant difference existed between the diverse minorities enrolled in this study. The results in Table 1 show that blacks and other minorities were not significantly different on any of the baseline characteristics or in smoking abstinence rates. The smoking abstinence rates at end of medication were 25% (2/8) for the Asians, 16% (22/138) for the African Americans, 11% (2/19) for the Native Americans, and 29% (2/7) for the other participants. For this reason and in order to increase our study power, we combined the four minority populations and made a larger comparison of the minority population with the white smokers.
Table 1.
Demographics of blacks versus other minorities
| Black (N = 138) | Other (N = 34) | Total (N = 172) | p value | |
|---|---|---|---|---|
| Age | 0.0688 | |||
| Mean (±SD) | 47.1 (10.37) | 43.2 (14.38) | 46.3 (11.33) | |
| Gender | 0.2738 | |||
| Female | 91 (65.9%) | 19 (55.9%) | 110 (64%) | |
| Male | 47 (34.1%) | 15 (44.1%) | 62 (36%) | |
| Marital status | 0.0846 | |||
| Missing | 1 | 0 | 1 | |
| Never married | 31 (22.6%) | 5 (14.7%) | 36 (21.1%) | |
| Married | 42 (30.7%) | 18 (52.9%) | 60 (35.1%) | |
| Separated/divorced/widowed | 62 (45.3%) | 10 (29.4%) | 72 (42.1%) | |
| Other | 2 (1.5%) | 1 (2.9%) | 3 (1.8%) | |
| Education | 0.2989 | |||
| Missing | 1 | 0 | 1 | |
| Less than HS | 6 (4.4%) | 3 (8.8%) | 9 (5.3%) | |
| HS or greater | 131 (95.6%) | 31 (91.2%) | 162 (94.7%) | |
| Age started smoking | 0.1071 | |||
| Mean (±SD) | 22.0 (±7.00) | 19.8 (±7.51) | 21.5 (±7.14) | |
| Month 0: CPD | 0.1355 | |||
| Mean (±SD) | 18.5 (±8.90) | 21.1 (±9.40) | 19.0 (±9.03) | |
| Years smoking cigarettes | 0.4394 | |||
| Mean (±SD) | 25.1 (±10.42) | 23.4 (±14.87) | 24.8 (±11.42) | |
| Ever tried to stop smoking | 0.7615 | |||
| Missing | 1 | 1 | 2 | |
| 1: Yes | 119 (86.9%) | 28 (84.8%) | 147 (86.5%) | |
| 2: No | 18 (13.1%) | 5 (15.2%) | 23 (13.5%) | |
| Number of times tried to stop | 0.4487 | |||
| Mean (±SD) | 3.9 (±3.76) | 3.3 (±3.66) | 3.8 (±3.74) | |
| Overall Fagerström score | 0.5900 | |||
| Mean (±SD) | 5.8 (±1.88) | 5.6 (±2.12) | 5.7 (±1.93) | |
| Fagerström groups | 0.2584 | |||
| Missing | 5 | 0 | 5 | |
| 1–4 (low) | 34 (25.6%) | 12 (35.3%) | 46 (27.5%) | |
| 5–6 (med) | 55 (41.4%) | 9 (26.5%) | 64 (38.3%) | |
| ≥7 (high) | 44 (33.1%) | 13 (38.2%) | 57 (34.1%) | |
| HSQ major depression | 0.3641 | |||
| Missing | 3 | 2 | 5 | |
| No | 104 (77%) | 27 (84.4%) | 131 (78.4%) | |
| Yes | 31 (23%) | 5 (15.6%) | 36 (21.6%) | |
| HSQ mental composite month 3 minus baseline | 0.5341 | |||
| Mean (±SD) | 1.8 (±7.97) | -0.3 (±7.15) | 1.3 (±7.72) | |
| HSQ physical composite month 3 minus baseline | 0.0984 | |||
| Mean (±SD) | 3.3 (±6.64) | -1.9 (±8.52) | 2.1 (±7.31) | |
| Week 12: confirmed nonsmoker | 0.8094 | |||
| No | 116 (84.1%) | 28 (82.4%) | 144 (83.7%) | |
| Yes | 22 (15.9%) | 6 (17.6%) | 28 (16.3%) | |
| Arm | 0.5034 | |||
| Nicotine inhaler | 42 (30.4%) | 8 (23.5%) | 50 (29.1%) | |
| Bupropion | 54 (39.1%) | 17 (50%) | 71 (41.3%) | |
| Combination treatment | 42 (30.4%) | 9 (26.5%) | 51 (29.7%) |
Table 2 presents the demographics of this study population. In comparison to whites, minority smokers were significantly older in age at the time of study entry (46 years of age versus 42 years of age, minorities and white, respectively, p < 0.0001), less likely to be married (35% versus 59%, minorities and white, respectively, p < 0.0001), older when they began smoking (22 years of age versus 19 years of age, minorities and white, respectively, p < 0.0001), smoked fewer cigarettes per day at baseline (19 versus 24, minorities and white, respectively, p < 0.0001), and were less likely to have stopped smoking at the end of the 3 months of medication treatment (16% abstinent versus 26% abstinent, minorities and white, respectively, p = 0.0065). Figure 1 shows abstinence rates by race within each treatment arm.
Table 2.
Demographics of study population*
| Minority (N = 172) | White (N = 1,512) | Total (N = 1,684) | p value | |
|---|---|---|---|---|
| Age at randomization (mean ± SD) | 46.3 (±11.33) | 42.4 (±11.49) | 42.8 (±11.53) | <0.0001 |
| Gender | 0.2278 | |||
| Female | 110 (64.0%) | 895 (59.2%) | 1005 (59.7%) | |
| Male | 62 (36.0%) | 617 (40.8%) | 679 (40.3%) | |
| Marital status | <0.0001 | |||
| Never married | 36 (21.1%) | 211 (14%) | 247 (14.7%) | |
| Married | 60 (35.1%) | 894 (59.3%) | 954 (56.9%) | |
| Separated/divorced/widowed | 72 (42.1%) | 381 (25.3%) | 453 (27%) | |
| Other | 3 (1.8%) | 21 (1.4%) | 24 (1.4%) | |
| Education | 0.1747 | |||
| Less than HS | 9 (5.3%) | 63 (4.2%) | 72 (4.3%) | |
| HS or greater | 162 (94.7%) | 1,410 (94%) | 1,572 (94.1%) | |
| Other | 0 (0%) | 27 (1.8%) | 27 (1.6%) | |
| Age started smoking (mean ± SD) | 21.5 (±7.14) | 18.7 (±5.85) | 19.0 (±6.05) | <0.0001 |
| Cigarettes per day at baseline (mean ± SD) | 19.0 (±9.03) | 23.6 (±9.84) | 23.2 (±9.86) | <0.0001 |
| Years of regular smoking (mean ± SD) | 24.8 (±11.42) | 23.7 (±11.20) | 23.8 (±11.22) | 0.2414 |
| Any prior quit attempts | 147 (86.5%) | 1,299 (86.3%) | 1,446 (86.3%) | 0.9383 |
| Prior quit attempts (mean ± SD) | 3.8 (±3.74) | 3.4 (±3.87) | 3.4 (±3.86) | 0.2271 |
| Overall Fagerström score (mean ±SD) | 5.7 (±1.93) | 5.8 (±2.16) | 5.8 (±2.14) | 0.5157 |
| Fagerström score | 0.2341 | |||
| 1–4 (low) | 46 (27.5%) | 408 (27.6%) | 454 (27.6%) | |
| 5–6 (med) | 64 (38.3%) | 478 (32.4%) | 542 (33.0%) | |
| ≥7 (high | 57 (34.1%) | 590 (40%) | 647 (39.4%) | |
| HSQ: major depression | 36 (21.6%) | 296 (20.3%) | 332 (20.5%) | 0.7096 |
| HSQ mental composite: month 3–baseline (mean ± SD) | 1.3 (±7.72) | -1.2 (±8.46) | -1.1 (±8.43) | 0.1144 |
| HSQ physical composite: month 3–baseline (mean ± SD) | 2.1 (±7.31) | 1.2 (±6.93) | 1.3 (±6.95) | 0.5015 |
| Smoking abstinence at month 3 (week 12) | 28 (16.3%) | 389 (25.7%) | 417 (24.8%) | 0.0065 |
| Treatment arm | 0.0672 | |||
| Nicotine inhaler | 50 (29.1%) | 512 (33.9%) | 562 (33.3%) | |
| Buproprion | 71 (41.3%) | 491 (32.5%) | 562 (33.4%) | |
| Nicotine inhaler + bupropion | 51 (29.7%) | 509 (33.7%) | 560 (33.3%) |
*Study population for this report is limited to those not of Hispanic origin
Fig. 1.
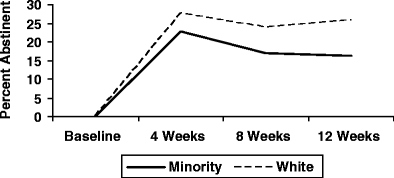
Smoking abstinence by races for weeks 4, 8, and 12
Bivariate logistic model results
Smoking abstinence rates at week 12 were modeled using bivariate logistic models as shown in Table 3. Variables independently associated with lower abstinence rates were: smoking 40 or more cigarettes per day at baseline (OR = 1.498, p = 0.0396), an FTND score (OR = 1.197, p < 0.0001), major depression (OR = 1.357, p = 0.0408), ever attempted to stop smoking before this study (OR = 1.853, p = 0.0011), and being a minority race (OR = 1.781, p = 0.0072). Variables associated with higher abstinence rates were treatment (bupropion OR = 0.489, combination OR = 0.323, p < 0.0001), age (OR = 0.976, p < 0.0001), age started smoking (OR = 0.976, p = 0.0070), being married (OR = 0.623, p < 0.0001), and having quit smoking before this study for at least 1 day (OR = 0.601, p = 0.0083).
Table 3.
Bivariate logistic regression models for smoking rates at 12 weeks*
| Variable | Odds ratio (smoking) | 95% confidence intervals for the odds ratio | p-value |
|---|---|---|---|
| Age† | 0.976 | 0.967 to 0.986 | <0.0001 |
| Age started smoking† | 0.976 | 0.960 to 0.993 | 0.0070 |
| Marital status | <0.0001 | ||
| Not married | 1.000 | ||
| Married | 0.623 | 0.494 to 0.784 | |
| Fagerström score† | 1.197 | 1.134 to 1.263 | <0.0001 |
| Prior quit attempt | 0.0011 | ||
| No | 1.000 | ||
| Yes | 1.853 | 1.279 to 2.684 | |
| Quit before for at least 1 day | 0.0083 | ||
| No | 1.000 | ||
| Yes | 0.601 | 0.412 to 0.877 | |
| 40 or more cigarettes per day at study entry | 0.0396 | ||
| No | 1.000 | ||
| Yes | 1.498 | 1.019 to 2.202 | |
| Major depression | 0.0408 | ||
| No | 1.000 | ||
| Yes | 1.357 | 1.013 to 1.819 | |
| Treatment | <0.0001 | ||
| Nicotine inhaler | 1.000 | ||
| Bupropion | 0.489 | 0.361 to 0.661 | |
| Combination treatment | 0.323 | 0.241 to 0.433 | |
| Gender | 0.0681 | ||
| Female | 1.000 | ||
| Male | 0.812 | 0.649 to 1.016 | |
| Education beyond high school | 0.8938 | ||
| No | 1.000 | ||
| Yes | 0.984 | 0.771 to 1.255 | |
| BMI† | 0.990 | 0.973 to 1.008 | 0.2858 |
| Tried prior nicotine | 0.8026 | ||
| No | 1.000 | ||
| Yes | 0.972 | 0.778 to 1.214 | |
| Number of years smoked | 0.4885 | ||
| Less than 5 Years | 1.047 | 0.600 to 1.827 | |
| At least 5 years but less than 10 years | 1.000 | ||
| 10 years or more | 0.825 | 0.581 to 1.172 | |
| Race | 0.0072 | ||
| White | 1.000 | ||
| Minority | 1.781 | 1.169 to 2.714 |
†Age, age started smoking, BMI, and Fagerstrom score were treated as continuous variables. For these variables the odds ratio presented is for a one-unit increase (i.e., per 1-year increase in age). All other characteristics were treated as categorical variables. For these characteristics an odds ratio of 1.000 is used to indicate the reference group. Odds ratios >1.000 indicate an increased likelihood of smoking at 12 weeks
Multivariate logistic model results
A multivariate logistic regression model (Table 4) showed that when controlling for all other significant factors, the following were found to be predictive of smoking abstinence after 12 weeks of pharmacotherapy: the younger the age, the greater was the likelihood to be abstinent (OR = 0.971, p < 0.0001); those who were married were more likely to be abstinent from smoking (OR = 0.678, p = 0.0029); using bupropion was associated with greater abstinence from smoking (OR = 0.480, p < 0.0001); using a combination of bupropion and nicotine inhaler was also associated with greater abstinence from smoking (OR = 0.328, p < 0.0001); conversely, the higher the FTND score, the greater the likelihood to be a continuing smoker (OR 1.244, p < 0.0001); having any prior quit attempts indicated greater likelihood to be a continuing smoker (OR = 1.812, p = 0.004); being a minority was associated with continued smoking (OR = 1.849, p = 0.0083).
Table 4.
Multivariate logistic regression model for smoking rates at 12 weeks*
| Variable† | Odds ratio (smoking) | 95% Confidence intervals for the odds ratio | p-value |
|---|---|---|---|
| Age | 0.971 | 0.960 to 0.982 | <0.0001 |
| Marital status | 0.0029 | ||
| Not married | 1.00 | ||
| Married | 0.678 | 0.525 to 0.876 | |
| Fagerström score | 1.244 | 1.172 to 1.320 | <0.0001 |
| Prior quit attempt | 0.0044 | ||
| No | 1.000 | ||
| Yes | 1.812 | 1.203 to 2.728 | |
| Treatment† | <0.0001 | ||
| Nicotine inhaler | 1.000 | ||
| Bupropion | 0.480 | 0.348 to 0.663 | |
| Combination treatment | 0.328 | 0.240 to 0.450 | |
| Race† | 0.0083 | ||
| White | 1.000 | ||
| Minority | 1.849 | 1.171 to 2.919 |
*In order to assess whether differences between race groups was dependent on treatment, an initial model was fit that included all main effect terms along with the race-by-treatment interaction effect. From this analysis the race-by-treatment interaction was not found to be statistically significant (p = 0.8113). Therefore, the findings presented here are from the model that includes only main effect terms
†Age and Fagerstrom score were treated as continuous variables. For these variables the odds ratio presented is for a one-unit increase (i.e., per 1-year increase in age). All other characteristics were treated as categorical variables. For these characteristics an odds ratio of 1.000 is used to indicate the reference group. Odds ratios >1.000 indicate an increased likelihood of smoking at 12 weeks
In order to assess whether differences between race groups was dependent on treatment, an initial model was fit that included all main effect terms along with the race-by-treatment interaction effect. From this analysis the race-by-treatment interaction was not found to be statistically significant (p = 0.8113). Therefore, the findings presented here are from the model that includes only main effect terms.
Discussion
Main findings
Our study compared the nicotine inhaler and bupropion versus the combination of the two in a large number of smokers, of which 10% were minority smokers. We observed that minority smokers were less likely to stop smoking at the end of 12 weeks of treatment when compared to white smokers. This is consistent with current US data indicating that overall smoking abstinence rates among white smokers is higher than that of the minority smokers who are trying to quit (Croghan et al. 2008a; U.S. Department of Health and Human Services 1998).
Predictors of smoking abstinence at end of treatment (12 weeks) include younger age at the time of the quit attempt, being married at the time of study participation (and quit attempt), less severe tobacco dependence based on lower FTND scores, fewer past smoking quit attempts, using bupropion (either alone or in combination with inhaler), and being white. Demographics of the study population indicate that at the time of enrollment, minority subjects enrolled in this study were more likely to be older and less likely to be married. Minority smokers enrolled in our program also reported being older when they started smoking and smoking fewer cigarettes per day at time of study enrollment.
In our study we did not find any evidence to suggest that treatment efficacy differed according to race (i.e., no treatment-by-race interaction). However, minority smokers may be less accepting of nicotine replacement products than white smokers (Fu et al. 2005). Latino smokers have been observed to use pharmaceutical aids less often than white smokers (Levinson et al. 2004). Because drug accountability was not collected in our study, we cannot assess for this. However, we did demonstrate that the two treatments were predictive of smoking abstinence in minority smokers. This is similar to another report where bupropion with active nicotine gum or bupropion with placebo nicotine gum was more effective among minority smokers than placebo bupropion and placebo nicotine gum (Piper et al. 2007). Furthermore, bupropion has been shown to help African-American smokers to stop smoking at higher rates compared to placebo (Ahluwalia et al. 2002; Robles et al. 2008).
Conclusions
Because minority groups have disproportionately greater health problems related to smoking than do whites, some have hypothesized that greater availability and individualization of health-care options may help reduce some of this disproportion (Dundas et al. 2001; Houston et al. 2005; Shah and Cook 2008). In a study by White et al., it was found that despite the fact that British ethnic minorities (Bangladeshi and Pakistani) had high motivation to quit smoking, the barriers were too many to overcome (White et al. 2006). The many barriers named include: perceived barriers such as peer pressure, stresses, withdrawal, lack of accessibility to health care for smoking cessation and lack of pharmaceutical aids; the health-care community believed that language, religion, and culture contributed to reduced smoking cessation health care among this population. Despite the fact that this study by White et al. (2006) took place in Britain and our study was in the USA, the issues with health care among minorities in our respective nations is still the same. It can be hypothesized that the lower rates of smoking abstinence among minority smokers may be explained by reduced health care, possibly due to economics, misconceptions, lack of communication, and/or lack of cultural understanding by health-care providers (Fu et al. 2007; King et al. 1997). The various options of tobacco dependence treatment should be explored with the individual smokers for acceptability as well as to meet individual needs (White et al. 2006). Currently, treatment for a general smoker who is considered a “light” smoker (<15 cigarettes per day) is not the same as that for a “heavy” smoker (>25 cigarettes per day), but as is evident in this study, the number of cigarettes per day should not be the sole deciding factor in determining the type and intensity of treatment a smoker receives. A more targeted approach to the role of behavioral interventions in the treatment of smokers has been evaluated in the past (Fernander et al. 2006), but a targeted approach that considers racial and cultural differences that can lead to acceptability of and compliance with treatment for medical interventions has yet to be examined (Lillard et al. 2007; White et al. 2006). In a current study from a clinical treatment program, it was found that whereas gender differences existed in those enrolling in a formal treatment program, gender did not ultimately affect smoking abstinence at 6 months. It was hypothesized that this could possibly be due to individualization of the treatment program for each patient (Croghan et al. 2008b). An overall targeted approach in the medical management of tobacco dependence (behavioral and medical intervention) may also be the preferred approach in minority smokers.
Limitations
This study was designed to mimic a “real-life” situation as much as possible. Although this reasoning was noteworthy, it limited the investigation results. Limitations to this study include the facts that all study sites were within the USA and that study subjects were all volunteers and may not be representative of minority smokers in the general global population. In addition, no data were collected regarding the frequency and patterns of medication use. Whereas all subjects were instructed on the proper use and dosing of all study medication, no tracking of their actual use was conducted, nor was their acceptability of the medication assigned to them or their comprehension of the instructions documented. In addition, no follow-up or data capture took place concerning any additional medications or behavioral counseling the subject may have used outside the study.
Acknowledgments
We wish to acknowledge the fine work and dedication provided by Kathleen Harding and the personnel of the Mayo Medical Center Oncology Pharmacy, the North Central Cancer Treatment Group Randomization Center, and the Protocol Development and Communications Office from the Mayo Clinic. In addition the authors wish to acknowledge Darrell Schroeder, MS, for his statistical counsel and foresight. Finally, we are also indebted to the Clinical Research Associates and Institutional Review Boards at each contributing site for their hard work and assistance in completing this project.
The NCCTG participating institutions included: Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882; Carle Cancer Center CCOP, Urbana, IL 61801; Geisinger Clinic & Medical Center CCOP, Danville, PA 17822; Iowa Oncology Research Association CCOP, Des Moines, IA 50309-1014; Illinois Oncology Research Assn. CCOP, Peoria, IL 61602; Sioux Community Cancer Consortium, Sioux Falls, SD 57105; Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403; Ann Arbor Regional CCOP, Ann Arbor, MI 48106; Rapid City Regional Oncology Group, Rapid City, SD 57709; Siouxland Hematology-Oncology Associates, Sioux City, IA 51105. Medication was provided by Pfizer, Inc., and Glaxo SmithKline. We are grateful for their contributions.
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-15083, CA-63826, CA-35195, CA-35448, CA-35431, CA-35101, CA-35113, CA-35103, CA-52352, and CA-63848.
Conflict of Interest
The authors confirm that there are no relevant associations that might pose a conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- BMI
Body Mass Index
- CO
carbon monoxide (expired air)
- COPD
chronic obstructive pulmonary disease
- FCTC
WHO Framework Convention on Tobacco Control
- FTND
Fagerström test of nicotine dependence
- HSQ
Health Status Questionnaire
- NCCTG
North Central Cancer Treatment Group
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- MAOI
monoamine oxidase inhibitor
- PPM
parts per million
- TQD
target quit day
- USPHS
United States Public Health System Service
References
- Ahluwalia JS, Harris KJ, Catley D, et al. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288(4):468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- American Lung Association (2006) Trends in tobacco use. http://www.lungusa.org/site/c.dvLUK9O0E/b.33347/k.AC09/Data__Statistics.htm
- Center for Disease Control and Prevention Smoking cessation during previous year among adults—United States 1990 and 1991. MMWR. 1993;42:504–507. [PubMed] [Google Scholar]
- Center for Disease Control and Prevention Tobacco use among adults—United States 2005. MMWR. 2006;55(42):1145–1148. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Cigarette smoking among adults—United States 2007. MMWR. 2008;57(45):1221–1226. [PubMed] [Google Scholar]
- Crews DE, Bindon JR. Ethnicity as a taxonomic tool in biomedical and biosocial research. Ethn Dis. 1991;1(1):42–9. [PubMed] [Google Scholar]
- Croghan IT, Hurt RD, Dakhil SR, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clin Proc. 2007;82(2):186–95. doi: 10.4065/82.2.186. [DOI] [PubMed] [Google Scholar]
- Croghan GA, Croghan IT, Hurt RD et al (2008a) A comparison by ethnicity of a 15 mg/16 hour nicotine patch alone versus nicotine nasal spray alone versus both. Presented at “10th European SRNT Europe Conference” in Rome, Italy
- Croghan IT, Ebbert JO, Hurt RD, et al. Gender differences among smokers receiving tobacco use interventions. Addict Behav. 2008;34(1):61–7. doi: 10.1016/j.addbeh.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Dundas R, Morgan M, Redfern J, et al. Ethnic differences in behavioural risk factors for stroke: implications for health promotion. Ethn Health. 2001;6(2):95–103. doi: 10.1080/13557850120068423. [DOI] [PubMed] [Google Scholar]
- Executive Office of the President, Office Management Budget, and Office of Information and Regulatory Affairs (2007) Recommendations from the Interagency Committee for the Review of the Racial and Ethnic Standards to the Office of Management and Budget Concerning Changes to the Standards for the Classification of Federal Data on Race and Ethnicity. Directive 15. Part ii. Federal Register Notices Pages 36873–36946
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fernander AF, Patten CA, Schroeder DR, et al. Characteristics of 6-month tobacco use outcomes of Black patients seeking smoking cessation intervention. J Health Care Poor Underserved. 2006;17(2):413–24. doi: 10.1353/hpu.2006.0059. [DOI] [PubMed] [Google Scholar]
- Fu SS, Sherman SE, Yano EM, et al. Ethnic disparities in the use of nicotine replacement therapy for smoking cessation in an equal access health care system. Am J Health Promot. 2005;20(2):108–16. doi: 10.4278/0890-1171-20.2.108. [DOI] [PubMed] [Google Scholar]
- Fu SS, Burgess D, van Ryn M, et al. Views on smoking cessation methods in ethnic minority communities: a qualitative investigation. Prev Med. 2007;44(3):235–40. doi: 10.1016/j.ypmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. British Journal of Addiction. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Houston TK, Scarinci IC, Person SD, et al. Patient smoking cessation advice by health care providers: the role of ethnicity, socioeconomic status, and health. Am J Public Health. 2005;95(6):1056–1061. doi: 10.2105/AJPH.2004.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Morse RM, Swenson WM. Diagnosis of alcoholism with a self-administered alcoholism screening test: results with 1,002 consecutive patients receiving general examinations. Mayo Clin Proc. 1980;55(6):365–70. [PubMed] [Google Scholar]
- King TK, Borrelli B, Black C, et al. Minority women and tobacco: implications for smoking cessation interventions. Ann Behav Med. 1997;19(3):301–13. doi: 10.1007/BF02892295. [DOI] [PubMed] [Google Scholar]
- Levinson AH, Perez-Stable EJ, Espinoza P, et al. Latinos report less use of pharmaceutical aids when trying to quit smoking. Am J Prev Med. 2004;26(2):105–11. doi: 10.1016/j.amepre.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Lillard DR, Plassmann V, Kenkel D, et al. Who kicks the habit and how they do it: socioeconomic differences across methods of quitting smoking in the USA. Soc Sci Med. 2007;64(12):2504–19. doi: 10.1016/j.socscimed.2007.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Clinic Nicotine Research Program (2000) Smoke-free and living it. Mayo Foundation for Medical Education and Research—Nicotine Research Program
- Murray RP, Connett JE, Buist AS, et al. Experience of black participants in the lung health study smoking cessation intervention program. Nicotine Tob Res. 2001;3(4):375–82. doi: 10.1080/14622200110081435. [DOI] [PubMed] [Google Scholar]
- O'Neil D (2008) Ethnicity and race: an introduction to the nature of social group differentiation and inequality. http://anthro.palomar.edu/ethnicity/Default.htm
- Piper ME, Federman EB, McCarthy DE, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine Tob Res. 2007;9(9):947–54. doi: 10.1080/14622200701540820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. doi: 10.2307/2529712. [DOI] [PubMed] [Google Scholar]
- Radosevich D M, Wetzler H, Wilson SM (1994) Health Status Questionnaire (HSQ) 2.0: scoring comparisons and reference data. Health Outcomes Institute
- Robles GI, Singh-Franco D, Ghin HL. A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations. Clin Ther. 2008;30(5):800–12. doi: 10.1016/j.clinthera.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Shah SM, Cook DG. Socio-economic determinants of casualty and NHS Direct use. J Public Health (Oxf) 2008;30(1):75–81. doi: 10.1093/pubmed/fdn001. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services . Tobacco use among U.S. racial/ethnic minority groups—African Americans, American Indians and Alaskan Natives, Asian Americans and the Pacific Islanders, and Hispanics. Atlanta: Centers for Disease Control and Prevention; 1998. [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Evans RW et al (1993) SF-36 health survey: manual and interpretation guide. The Health Institute
- White M, Bush J, Kai J, et al. Quitting smoking and experience of smoking cessation interventions among UK Bangladeshi and Pakistani adults: the views of community members and health professionals. J Epidemiol Community Health. 2006;60(5):405–11. doi: 10.1136/jech.2005.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2000) Second meeting of the working group on the WHO framework convention on tobacco control. Provisional Agenda Item #5. World Health Organization
- World Health Organization (2002) Fact sheets: smoking statistics http://www.wpro.who.int/media_centre/fact_sheets/fs_20020528.htm


