Abstract
Rapid prototyping of polydimethylsiloxane (PDMS) is often used to build microfluidic devices. However, the inherent hydrophobic nature of the material limits the use of PDMS in many applications. While different methods have been developed to transform the hydrophobic PDMS surface to a hydrophilic surface, the actual implementation proved to be time consuming due to differences in equipment and the need for characterization. This paper reports a simple and easy protocol combining a second extended oxygen plasma treatments and proper storage to produce usable hydrophilic PDMS devices. The results show that at a plasma power of 70 W, an extended treatment of over 5 min would allow the PDMS surface to remain hydrophilic for more than 6 h. Storing the treated PDMS devices in de-ionized water would allow them to maintain their hydrophilicity for weeks. Atomic force microscopy analysis shows that a longer oxygen plasma time produces a smoother surface.
INTRODUCTION
Polydimethylsiloxane (PDMS) is one of the most widely used silicone-based polymers in microfluidics.1 The ease of fabrication, low cost, biocompatibility, elastomeric property, and optical transparency are attractive reasons for its wide usage in rapid prototyping.2 However despite these advantages, the intrinsic hydrophobicity of PDMS restricts its use in certain applications. For example, analysis of biological samples and chemical synthesis require hydrophilic surfaces3 in order to work expeditiously. Electrophoretic separation4 also requires adequate surface wettability. Stable droplet5 and bubble6 formations require the continuous phase fluid to preferentially wet the channel walls. From the above mentioned applications, surface properties of PDMS are to be considered in the design and fabrication of microfluidic devices.
In the past, the surface properties of PDMS has been modified using different methods such as coating of the inner walls,7 attachment of active groups,8 plasma oxidation,9 thermal aging,10 and chemical coating.11 However, most methods require hours to process and to characterize before one can effectively use the devices. In addition, the varieties in technologies and equipment of different laboratories cause further delay in the implementation of the technology. Recognizing this predicament, this paper reports a simple protocol to produce PDMS with hydrophilic channel walls without the use of additional chemicals or equipment. The characterization of this method also ensures that the device can be stored for days before use while maintaining the hydrophilicity of the channel walls within a time span reasonable for experimentation.
Oxygen plasma treatment has been used extensively by many in the fabrication of PDMS microfluidic devices. The treatment of oxygen plasma on PDMS introduces polar functional groups12 which is mainly the silanol group (SiOH). This group changes the surface properties of PDMS from being hydrophobic to hydrophilic. On the one hand, normal plasma treated surfaces undergo hydrophobic recovery within minutes13 after bonding and thermal treatment. On the other hand, extended plasma treatment induces undesirable surface cracks,12 which affect the bonding integrity of the device. Hydrophobic recovery of PDMS has been well studied by sessile water contact angle measurement,14 scanning electron microscopy,15 and x-ray photoelectron spectroscopy.16 The main reasons for hydrophobic recovery are the reorientation of the polar groups from the surface to the bulk,16 diffusion of pre-existing low-molecular-weight species from the bulk to the surface,15 and condensation of the hydroxyl groups.17 The recovery rate is also affected by storage condition such as temperature,10 humidity,18 aqueous fluid,19 and surfactants20 used to store the PDMS device. To our knowledge, limited or very few studies report on surface modification using a bonded and sealed PDMS device. Most studies are conducted using thin PDMS membranes which are not bonded together to form a typical concealed microfluidic device. The main purpose of the technique reported in this paper is to perform surface modification on a fully bonded and functional microfluidic device.
MATERIALS AND METHODS
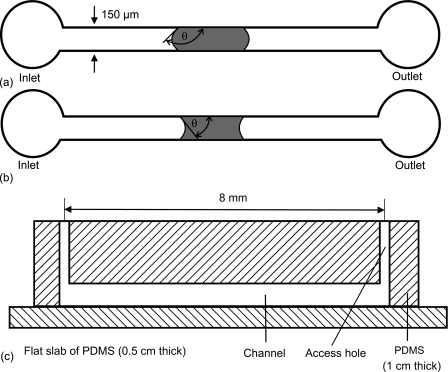
The test devices used in this work are fabricated using standard soft lithography.21 To summarize, we casted the PDMS substrate (Sylgard 184, Dow Corning) on a master mold made of the thick-film resist SU-8. The thickness of the SU-8 layer is 100 μm. The PDMS substrate with the microchannel was first peeled off from the master mold. A manual puncher (Harris Uni-Core, World Precision Instruments, Inc., Florida, USA) of 0.75 mm diameter was then used to create the fluidic access into the device. Oxygen plasma created by a plasma reactor (790 Series, Plasma-Therm, Inc., Florida, USA) was then used to bond the former PDMS substrate to a slab of blank PDMS. The bonded PDMS devices were then placed in an oven at 150 °C for 2 h to ensure good bonding between both surfaces. Figure 1 shows the schematic sketch of the microchannel used in the test. The straight channel has a width of 150 μm and a height of 100 μm. Contact angle measurements are carried out for a stationary water plug in the channel as depicted in Fig. 1(a,b).
Figure 1.
Schematic sketch of the test device used in the experiment (not to scale, the channel has a length of 8 mm): (a) water plug in a hydrophobic channel (θ>90°), (b) water plug in a hydrophilic channel (θ<90°), and (c) cross section.
The fully bonded and leak free devices subsequently underwent a second oxygen plasma treatment. A total of six different treatment times was used to study the effectiveness of the protocol proposed. While the plasma power was kept constant at 70 W, the treatment times were varied as 100, 200, 300, 400, and 500 s. Immediately after the plasma treatment, the devices were immersed in a container filled with de-ionized (DI) water. Air bubbles are removed by injecting DI water through the access holes and storing in a vacuum chamber for 7 days. This duration provides a realistic time scale in commercial context, where the storage and delivery of the devices may take longer than in the laboratory environment. The vacuum ensures that all air bubbles trapped in the devices were removed and devices are stored at a constant low temperature.
For the characterization experiments, the devices were removed from the vacuum chamber and DI water within the microchannels was removed manually using syringes. In order to ensure a minimal amount of DI water remaining in the channel, manual pumping of the syringes was repeated at least 20 times. Thereafter, DI water is pumped into the device at a very slow rate of 1 μl∕h using a precision syringe pump (KDS 250, KD Scientific). The flow is then stopped when the fluid just entered the inlet port. A stabilization time of 5 min is then used to obtain a near static image of the clear meniscus of the water∕air interface. An epifluorescent inverted microscope (Nikon, Eclipse TE2000-S) was used to observe the meniscus of the liquid inside the channel. A sensitive interline transfer CCD camera (HiSense MKII, Dantec Dynamics, Denmark) was employed for recording the meniscus image. Images of the interfaces were then taken at periods of 0, 2, 4, and 6 h from the time the devices are exposed to the ambient air. In addition, to ensure fair experimental conditions, all the fluids are pumped out manually after each recording.
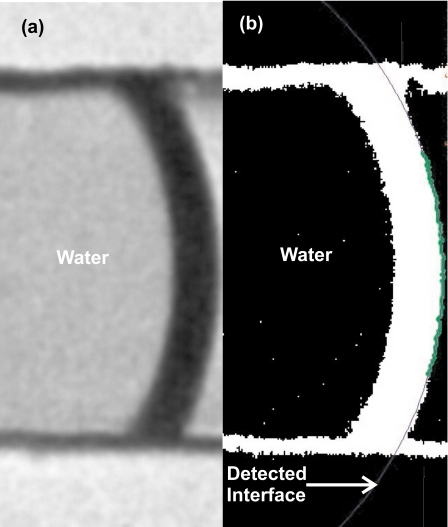
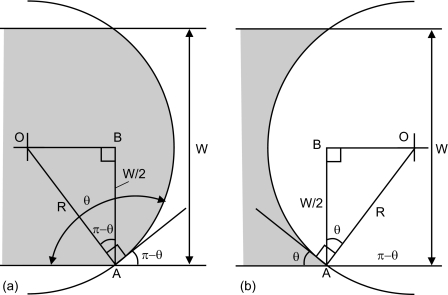
Figure 2 shows an example of the meniscus image taken at the interface for a bonded pristine PDMS microfluidic device. A total of 20 pictures using ten similar devices for each plasma parameter are then evaluated and averaged using a customized MATLAB program. The program first imports the bitmap image and converts it into a binary form. Subsequently, the water∕air interface was detected by the contrast of the interface. The radius of curvature R was detected by curve fitting. With the known channel width W of 150 μm, the contact angle is determined in degree for hydrophobic case as [Fig. 3a],
| (1) |
For the hydrophilic case, the contact angle is [Fig. 3b]
| (2) |
The measured results for the case of a bonded pristine PDMS microfluidic device (without the second plasma treatment) agree well with the reported result measured using conventional contact angle measurement with a sessile water droplet. For our case, the contact angle measured was about 120° while the reported case was about 112°.22 Therefore, it is reasonable to assume that our measured results also correlate fairly well with measurement using a sessile droplet.
Figure 2.
Evaluation of contact angle using the customized MATLAB program (the image is taken using a bonded pristine PDMS device): (a) original image and (b) processed image.
Figure 3.
Geometry of the water∕air meniscus (gray shaded area represents water) (a): hydrophobic case (θ>90°) and (b) hydrophilic case (θ<90°).
To further ascertain the surface properties of the proposed protocol, atomic force microscopy (AFM) analysis (Veeco, D5000) was performed on blank PDMS slabs measuring 1×1×1 cm3 before and after oxygen plasma treatments. The AFM images were obtained using the tapping mode. The scanning area used was 10×10 μm2. All AFM tests were carried out within 1 h after exposure to the oxygen plasma. The root mean square (rms) roughness was measured on the area without the nanocracks,23 which was formed due to the oxygen plasma treatment. However, these nanocracks can be useful. Recent studies have shown that these nanocracks can be decorated with adhesive proteins, which support the growth and modulation of biological cells.24
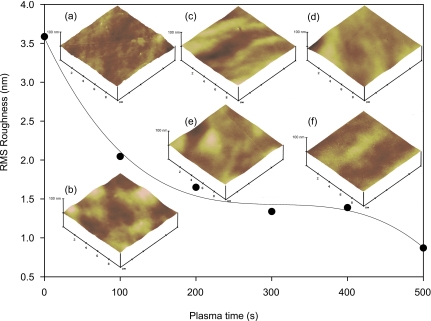
Figure 4 depicts the rms roughness at different oxygen plasma exposure times. The result distinctively shows that exposure to oxygen plasma treatment decreases the roughness of the PDMS surface. For instance, without any oxygen plasma treatment, the rms roughness measures 3.6 nm. However, the rms roughness decreases to 0.9 nm after a treatment of 500 s.
Figure 4.
Root mean square roughness as a function of treatment time with oxygen plasma. Respective AFM images are taken of specimen with oxygen plasma exposure of (a) 0 s, (b) 100 s, (c) 200 s, (d) 300 s, (e) 400 s, and (f) 500 s.
RESULTS AND DISCUSSION
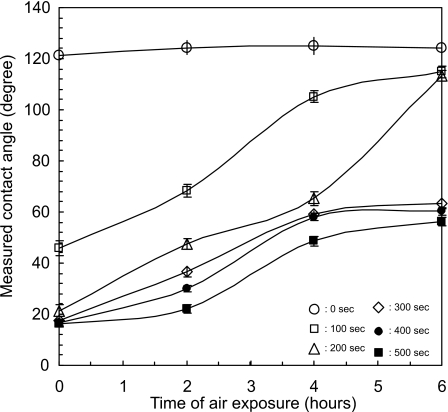
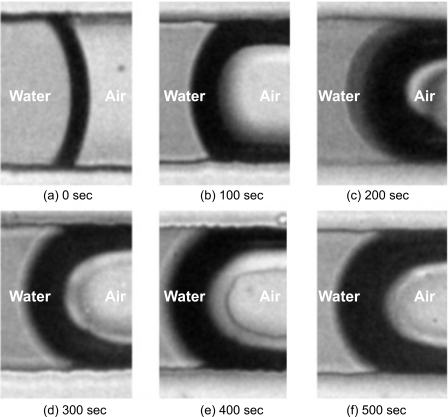
Figure 5 shows the contact angle as a function of exposure time to air. Generally, a longer plasma treatment results in a smaller contact angle. A PDMS microchannel without additional plasma treatment (circles in Fig. 5) has an almost constant contact angle of 120°. The contact angle of a fresh surface treated for 100 s is 46° and increases to about 115° after 6 h exposure to air. A treatment time of 200 s decreases the contact angle of the fresh surface further to 21°. The contact angle again approaches 115° after 6 h exposure to air. PDMS channels with treatment time longer than 300 s have a fresh contact angle of 17° and can maintain a contact angle between 50° and 60° after 6 h exposure to air. The representative images of the meniscus of the water plug in microchannels with different treatment times are depicted in Fig. 6.
Figure 5.
Contact angle as a function of exposure time in air.
Figure 6.
Images of air∕water interfaces in a PDMS microchannel treated with second oxygen plasma after bonding: (a) not treated, (b) treated for 100 s, (c) treated for 200 s, (d) treated for 300 s, (e) treated for 400 s, and (f) treated for 500 s.
From the obtained results, the concept of producing hydrophilic microchannels in PDMS, which can be stored and used when needed for experiments, appears very promising. Devices treated with the second oxygen plasma of 100 and 200 s can only retain the hydrophilicity (contact angle of less than 90°) for about 3 h after exposure to air. However, with a treatment time from 300 to 500 s the devices remain hydrophilic and never fully regain their hydrophobicity during the time period of our investigation. This difference also suggests that a longer plasma time of 300 s or more may provide an efficacious remedy to the production of bonded and sealed hydrophilic PDMS microfluidic devices.
A possible explanation for the above difference is that as the PDMS devices are bonded, the ionized gas is only able to enter into the inner channel walls via the fluidic access holes. As result, a limited amount of ionized gas diffused into the inner channel walls and does not result in the formation of brittle silica layers, which facilitate the migration of low molar mass molecules from the bulk of the structure to the inner walls.25 According to one-dimensional diffusion approximation of t=x2∕2D, where t is the diffusion time, x is the characteristic length and D is the diffusion coefficient of oxygen plasma ions, the exposure time is well sufficient to cover the entire channel length. For instance, with a diffusion coefficient on the order of 10−3,26 the plasma takes only about 0.032 s to diffuse along a channel length of 8 mm. Devices with longer treatment time show minimum changes to the hydrophobic recovery rate when the plasma time increases from 300 to 500 s. This could be due to a saturation of the ionized gas which is able to diffuse into the microchannel. The second plasma treatment done after the devices are well bonded ensures that minimum damage is incurred at the inner channel walls despite prolonged plasma treatment time.
The results also ascertain that storage of PDMS devices in water and under vacuum is able to prolong the hydrophilicity of the PDMS device for at least 7 days or more. The high surface energy of water prevents the reorganization of the silanol group (SiOH) on the inner channel walls.27 This hypothesis suggests that changing the surface energy of the fluid may affect the storage time of hydrophilic PDMS devices.
As shown in Fig. 4, a longer exposure time creates a smoother surface which also affects the hydrophobic recovery kinetics of the PDMS surfaces. The hydrophobic recovery rate decreases with increasing plasma exposure time12 and this agrees well with measured contact angles reported above. However, as the confinement of the channel influences the result of the plasma treatment, the in-channel surface analysis cannot effectively be compared with the case of a planar surface. For example, the measured minimum contact angle reached in our study was 17°, which is well above the complete wetting condition of less than 10° obtained for a planar surface. This difference could well be due to the nature of exposure. In our case, limited oxygen ions can only enter the channel through the inlets access, while a planar surface has a direct exposure to the oxygen ions.
CONCLUSIONS
In summary, the concept of producing hydrophilic PDMS devices through the use of a second extended oxygen plasma treatment after bonding and the storage in DI water under vacuum condition proves to be feasible. The method reported in this paper can maintain the hydrophilicity of the devices for several weeks. At 70 W plasma power and treatment time longer than 300 s, the PDMS surface can remain hydrophilic for more than 6 h after the exposure to ambient air. The reasonably low contact angle between 50° and 60° is sufficient for most microfluidic applications. The time span of 6 h is also enough for most experiments in microfluidics. Surface analysis reveals that a longer plasma treatment time produces a much smoother surface.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support from the Agency of Science, Technology and Research (A*STAR), Singapore (Grant No. SERC 052 101 0108 “Droplet-based micro∕nanofluidics”).
References
- McDonald J. C., Duffy D. C., Anderson J. R., Chiu D. T., Wu H., Schueller O. J. A., and Whitesides G. M., Electrophoresis 21, 27 (2000). [DOI] [PubMed] [Google Scholar]
- McDonald J. C. and Whitesides G. M., Acc. Chem. Res. 35, 491 (2002). 10.1021/ar010110q [DOI] [PubMed] [Google Scholar]
- Kakuta M., Bessoth F. G., and Manz A., Chem. Rec. 1, 395 (2001). 10.1002/tcr.1023 [DOI] [PubMed] [Google Scholar]
- Duffy D. C., McDonald J. C., Schueller O. J. A., and Whitesides G. M., Anal. Chem. 70, 4974 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- Christopher G. F. and Anna S. L., J. Phys. D: Appl. Phys. 40, R319 (2007). 10.1088/0022-3727/40/19/R01 [DOI] [Google Scholar]
- Garstecki P., Gañán-Calvo A. M., and Whitesides G. M., Bull. Pol. Acad. Sci.: Tech. Sci. 53, 361 (2005). [Google Scholar]
- Dou Y. H., Bao N., Xu J. J., and Chen H. Y., Electrophoresis 23, 3558 (2002). [DOI] [PubMed] [Google Scholar]
- Alcantar N. A., Aydil E. S., and Israelachvili J. N., J. Biomed. Mater. Res. 51, 343 (2000). [DOI] [PubMed] [Google Scholar]
- Ginn B. T. and Steinbock O., Langmuir 19, 8117 (2003). 10.1021/la034138h [DOI] [Google Scholar]
- Eddington D. T., Puccinelli J. P., and Beebe D. J., Sens. Actuators B 114, 170 (2006). 10.1016/j.snb.2005.04.037 [DOI] [Google Scholar]
- Xiao D., Zhang H., and Wirth M., Langmuir 18, 9971 (2002). 10.1021/la0205553 [DOI] [Google Scholar]
- Hillborg H., Ankner J. F., Gedde U. W., Smith G. D., Yasuda H. K., and Wikström K., Polymer 41, 6851 (2000). 10.1016/S0032-3861(00)00039-2 [DOI] [Google Scholar]
- Bhattacharya S., Datta A., Berg J. M., and Gangopadhyay S., J. Microelectromech. Syst. 14, 590 (2005). 10.1109/JMEMS.2005.844746 [DOI] [Google Scholar]
- Lawton R. A., Price C. R., Runge A. F., Doherty Iii W. J., and Saavedra S. S., Colloids Surf., A 253, 213 (2005). 10.1016/j.colsurfa.2004.11.010 [DOI] [Google Scholar]
- Bodas D. and Khan-Malek C., Sens. Actuators B 123, 368 (2007). 10.1016/j.snb.2006.08.037 [DOI] [Google Scholar]
- Morra M., Occhiello E., Marola R., Garbassi F., Humphrey P., and Johnson D., J. Colloid Interface Sci. 137, 11 (1990). 10.1016/0021-9797(90)90038-P [DOI] [Google Scholar]
- Tóth A., Bertóti I., Blazsó M., Bánhegyi G., Bognar A., and Szaplonczay P., J. Appl. Polym. Sci. 52, 1293 (1994). 10.1002/app.1994.070520914 [DOI] [Google Scholar]
- Kim J., Chaudhury M. K., Owen M. J., and Orbeck T., J. Colloid Interface Sci. 244, 200 (2001). 10.1006/jcis.2001.7909 [DOI] [Google Scholar]
- Chen I. J. and Lindner E., Langmuir 23, 3118 (2007). 10.1021/la0627720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Shevkoplyas S. S., Zasońska B., Szymborski T., Garstecki P., and Whitesides G. M., Small 4, 1795 (2008). 10.1002/smll.200800591 [DOI] [PubMed] [Google Scholar]
- Friend J. and Yeo L., Biomicrofluidics 4, 026502 (2010). 10.1063/1.3259624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Ellis A. V., and Voelcker N. H., Electrophoresis 31, 2 (2010). 10.1002/elps.200900475 [DOI] [PubMed] [Google Scholar]
- Hui A. Y. N., Wang G., Lin B. C., and Chan W. T., Lab Chip 5, 1173 (2005). 10.1039/b504271b [DOI] [PubMed] [Google Scholar]
- Zhu X., Mills K. L., Peters P. R., Bahng J. H., Liu E. H., Shim J., Naruse K., Csete M. E., Thouless M. D., and Takayama S., Nature Mater. 4, 403 (2005). 10.1038/nmat1365 [DOI] [PubMed] [Google Scholar]
- Owen M. J. and Smith P. J., J. Adhes. Sci. Technol. 8, 1063 (1994). 10.1163/156856194X00942 [DOI] [Google Scholar]
- Murphy A. B. and Arundell C. J., Plasma Chem. Plasma Process. 14, 451 (1994). 10.1007/BF01570207 [DOI] [Google Scholar]
- Lavielle L. and Schultz J., J. Colloid Interface Sci. 106, 438 (1985). 10.1016/S0021-9797(85)80017-5 [DOI] [Google Scholar]