Abstract
Background
Docosahexaenoic acid (DHA) is required for normal brain function. The concentration of DHA in the brain depends on both diet and liver metabolism.
Objective
To determine rat brain DHA concentration and consumption in relation to dietary n-3 (omega-3) polyunsaturated fatty acid (PUFA) content and liver secretion of DHA derived from circulating α-linolenic acid (α-LNA).
Design
Following weaning, male rats were fed for 15 weeks either: (1) a diet with a high DHA and α-LNA content, (2) an n-3 PUFA “adequate” diet containing 4.6% α-LNA but no DHA, or (3) an n-3 PUFA “deficient” diet containing 0.2% α-LNA and no DHA. Brain DHA consumption rates were measured following intravenous infusion in unanesthetized rats of [1-14C]DHA, whereas liver and brain DHA synthesis rates were measured by infusing [1-14C]α-LNA.
Results
Brain DHA concentrations equaled 17.6 μm/g, 11.4 μm/g and 7.14 μm/g in rats on diets 1, 2 and 3, respectively. With each diet, the rate of brain DHA synthesis from α-LNA was much less than the brain DHA consumption rate, whereas the liver synthesis-secretion rate was 5-10 fold higher. Higher elongase 2 and 5 and desaturase Δ5 and Δ6 activities in liver than in brain accounted for the higher liver DHA synthesis rates; these enzymes were transcriptionally upregulated in liver but not in brain of rats fed the deficient diet.
Conclusions
While DHA is essential to normal brain function, this need might be covered by dietary α-LNA when liver metabolic conversion machinery is intact and the diet has a high α-LNA content.
Keywords: docosahexaenoic acid, liver, brain, rat, n-3, omega-3, PUFA, imaging, metabolism, diet, synthesis, α-linolenic acid
Introduction
Studies of animals fed diets with different proportions of polyunsaturated fatty acids (PUFAs) have identified dietary requirements for maintaining optimal brain function and PUFA composition (1, 2), and have shown that metabolic, functional and behavioral changes can arise from long-term n-3 PUFA dietary deprivation. Clinical studies also have indicated that a low dietary consumption of n-3 PUFAs or a low plasma docosahexaenoic acid (DHA, 22:6n-3) concentration is correlated with a number of brain diseases, and with cognitive or behavioral defects during early development and aging (3-6). Dietary n-3 PUFA supplementation has been suggested to be beneficial in some of these conditions (6, 7).
Nevertheless, controversy exists about the optimal dietary n-3 PUFA composition to maintain normal human brain function, in different stages of the life span (6-11). One issue relevant to this controversy regards the liver’s capacity to convert circulating α-linolenic acid (α-LNA, 18:3n-3) or eicosapentaenoic acid (EPA, 20:5n-3) to DHA (12). Changes in this capacity with development, aging or disease, and in relation to diet, would likely impact brain PUFA content, metabolism and function (13-16). This controversy has been addressed by examining the relation of brain DHA consumption to the ability of the liver and brain to synthesize DHA from circulating α-LNA, in rats fed each of three diets differing in their n-3 PUFA composition.
Methods and Models
Figure 1 illustrates the brain DHA cascade at the synapse (12). It shows DHA esterified at the stereospecifically numbered (sn)-2 position of synaptic membrane phospholipids, from where it can be released by a phospholipase A2 (PLA2), particularly Ca2+-independent iPLA2 Type VI (17-20). Most of the released DHA will be rapidly reincorporated into an available lysophospholipid after being converted to DHA-CoA, through the action of an acyl-CoA synthetase, with the consumption of two molecules of ATP (21). A smaller fraction will be converted to docosanoids (including neuroprotectins and neuroregulins) by cyclooxygenase (COX), lipoxygenase, or cytochrome P450 enzymes, or lost by β-oxidation within mitochondria or by other pathways (22-25).
Figure 1.
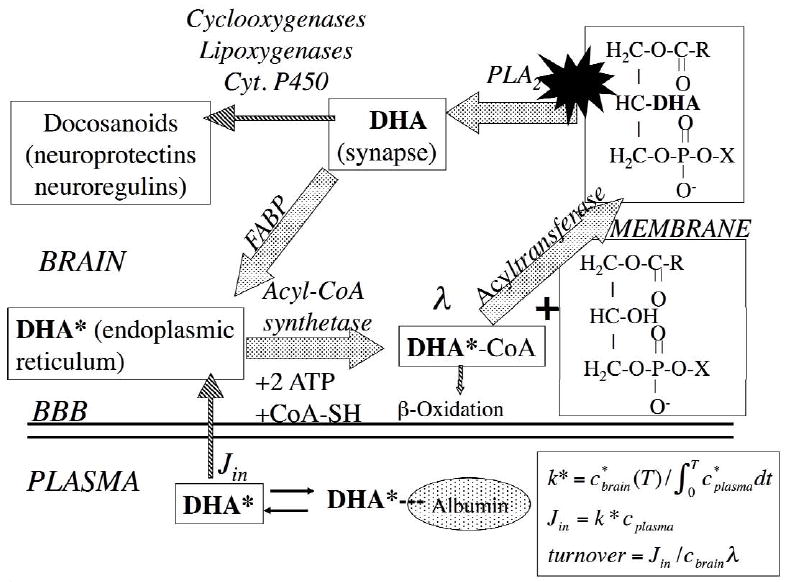
Brain docosahexaenoic acid (DHA) recycling at the synapse. DHA at the sn-2 position of a phospholipid is liberated by activation (star) of synaptic PLA2 at the synapse, secondary to neuroreceptor activation. A fraction of the unesterified DHA is converted to docosanoids, whereas the remainder is transported by a fatty acid binding protein (FABP) to the endoplasmic reticulum, where it is converted to DHA-CoA by an acyl-CoA synthetase, with the consumption of 2 ATPs. It then is esterified into an available lysophospholipid by an acyltransferase, or lost by β-oxidation in mitochondria or peroxisomes, or through other pathways (not shown). The brain unesterified DHA pool (not shown) rapidly exchanges with unesterified plasma DHA and labeled DHA*, which have dissociated from albumin. The DHA incorporation rate Jin equals the amount metabolized within brain and is calculated by equations in the right corner, where λ represents the ratio of DHA-CoA to plasma DHA specific activity. Modified from (12).
DHA recycling within brain phospholipids, as well DHA loss due to brain metabolism, can be experimentally accessed by infusing radiolabeled DHA intravenously for 5 min in awake rats, and using analytical procedures to follow passage of the label (DHA* in Figure 1) through the acyl-CoA and phospholipid pools of high-energy microwaved brain (26, 27). In this way, several kinetic parameters can be determined. One is the coefficient of incorporation k* (ml/sec/g wet wt brain) of plasma unesterified DHA into stable brain lipids,
| (Eq. 1) |
where t is time after beginning tracer infusion, (T) (nCi/g)is brain radioactivity at time T of sampling (5 min), and (nCi/ml) is plasma radioactivity due to DHA* (metabolites do not appear in plasma within 5-min).
The rate of DHA incorporation from plasma into brain, Jin (nmol/sec/g brain), equals the product of k* and the unlabeled unesterified plasma DHA concentration, cplasma (nmol/ml),
| (Eq. 2) |
Jin also is the rate of brain DHA metabolic consumption, because circulating α-LNA does not contribute significantly (< 1% converted) to brain DHA, and DHA cannot be synthesized de novo from 2-carbon fragments in vertebrate tissue (28-31). k* and Jin are independent of changes in cerebral blood flow, and thus they reflect only brain DHA metabolism (24, 32, 33). Eqs. 1 and 2 can be used to determine k* and Jin for α-LNA or other PUFAs, in brain or other organs.
To estimate organ rates of DHA synthesis from α-LNA in an unanesthetized rat, radiolabeled α-LNA is infused intravenously for 5 min, and its distribution and that of its elongated products are measured in different lipid compartments of the high-energy microwaved organ. A synthesis (conversion) coefficient (ml/sec/g) is defined as,
| (Eq. 3) |
where (nCi/g) is the labeled DHA concentration in stable organ lipid i. The synthesis-conversion rate (nmol/s/g) then equals,
| (Eq. 4) |
With regard to the liver, we assume that the DHA formed from α-LNA eventually will be secreted when esterified in phospholipids and triglycerides within very low-density lipoproteins (34). The rate of DHA secretion (nmol/s/g) then is,
| (Eq. 5) |
λα−LNA−CoA is liver α-LNA-CoA specific activity divided by plasma α-LNA specific activity at the steady state. λα−LNA−CoA corrects for contributions of other than unesterified plasma α-LNA to the liver precursor α-LNA-CoA pool.
Kinetic parameters were determined using Eqs. 1-5 in unanesthetized male rats that had been fed, for 15 weeks post-weaning (starting at 21 days of age), one of three diets: (1) a high DHA containing diet (DHA 2.3% of total fatty acids, 5.1% α-LNA, 4% fat); (2) a DHA-free diet containing 4.6% α-LNA (of total fatty acids), 10% fat; or (3) a DHA-free diet containing 0.2% α-LNA, 10% fat. The compositions of each of the diets can be found in the original publications (12, 35-37).
The latter two diets are termed n-3 PUFA “adequate” and “deficient,” respectively (35, 36, 38), following the convention of Bourre (1, 2). This convention was based on measuring a number of endpoints in brain and retina in relation to α-LNA dietary content, including brain accumulation of docosapentaenoic acid (DPA, 22:5n-6 (omega-6)), performance on learning tasks, enzyme activities and electrophysiological parameters.
Results
DHA consumption and synthesis rates in unanesthetized rats
An organ’s ability to convert (synthesize) circulating α-LNA to DHA is given in terms of a coefficient ki(a−LNA→DHA) (Eq. 3), which represents serial steps involving elongation and desaturation by elongases, desaturases and other enzymes within the organ (39). Table 1 gives experimental values for this coefficient in the brain and liver of unanesthetized rats that had been fed each of the three diets, with regard to i = phospholipid and triacylglycerol (37). ki(a−LNA→DHA) in brain was not evidently related to diet, whereas in liver the coefficient was increased in relation to reduced dietary and plasma levels of n-3 PUFAs. The liver coefficients in rats fed the high DHA and n-3 PUFA adequate diets were 10 fold the respective brain coefficients, and 100 fold in rats fed the n-3 PUFA deficient diet.
Table 1.
Brain and liver synthesis (conversion) coefficients of DHA derived from α-linolenic acid in rats fed different n-3 PUFA containing diets. Data from (16, 29, 36, 37).
| Diet during 15 weeks post-waning | BRAIN | LIVER |
|---|---|---|
| ml/s/g × 10-4 (i = PL, TG) | ||
| High DHA, fishmeal containing NIH-31-18-4 diet (2.3% FA) | 0.0055, 0.00040 | 0.03, 0.1 |
| High α-LNA diet (4.6% FA); no DHA | 0.0063, 0.00077 | 0.053, 0.219 |
| Low α-LNA diet (0.2% FA); no DHA | 0.0051, 0.00089 | 0.444, 1.45 |
FA = fatty acid; PL, phospholipids: TG, triacylglycerol; DHA, docosahexaenoic acid
The differences between the brain and liver conversion-synthesis coefficients in relation to diet (Table 1) correlated with organ expression of enzymes involved in the conversion (40, 41). Thus, activity levels of the Δ5 and Δ6 desaturases, and of elongases 2 and 5, were much higher in liver than brain in animals on the n-3 PUFA “adequate” diet, and their mRNA levels were upregulated in liver but not brain in the animals fed the n-3 PUFA deficient compared with adequate diet (39).
Table 2 summarizes additional data from the three dietary experiments. It provides whole brain and unesterified plasma concentrations of DHA and α-LNA, rates of DHA consumption by the 1.5 g rat brain (Jin (Eq. 2) × 1.5 g), rates of DHA synthesis from α-LNA within brain (Eq. 4 applied to brain), and rates of secretion of DHA derived from circulating α-LNA by the 11.5 g rat liver (Jsecretion(liver) (Eq. 5) multiplied by 11.5 g).
Table 2.
DHA synthesis rates from α-LNA by liver but not by brain exceed brain consumption rates with each of three diets. Data from (16, 29, 36, 37, 52).
| Diet during 15 weeks post-wearing | Cbrain (DHA) [Cbrian (α-LNA)] | cplasma(DHA) [cplasma(α-LNA)] | Estimated rate DHA consumption by 1.5 g brain, Jin | Estimated rate DHA formation from α-LNA by 1.5 g brain, | Estimated rate DHA secretion by 11.5 g liver |
|---|---|---|---|---|---|
| μmol/g | nmol/ml | μmol/day | μmol/day | μmol/day | |
| High DHA, fishmeal containing NIH-31-18-4 diet (2.3% FA) | 17.6 ± 0.3#[0.010 ± 0.002] | 26 ± 12 [14 ± 13] | 0.23 | 0.002 | 1.57 |
| High α-LNA diet (4.6% FA); no DHA | 11.4 ± 0.8 [0.16 ± 0.003] | 6.5 ± 2.6 [27 ± 6] | 0.29 | 0.0016 | 2.19 |
| Low α-LNA diet (0.2% FA); no DHA | 7.14 ± 0.24 [ND] | 0.23 ± 0.10 [1.0 ± 0.45] | 0.06 | 0.0000006 | 0.82 |
Mean ± SD; FA = fatty acid; ND, not detected.
Unesterified plasma DHA declined in relation to reduced dietary n-3 PUFA content, as did brain DHA. Brain DHA equaled 17.6 μm/g wet wt in rats fed the high DHA diet, 11.4 μm/g in rats fed the n-3 PUFA adequate diet, and 7.14 μm/g in rats fed the deficient n-3 PUFA diet. In the latter case, brain DHA consumption, Jin, was markedly reduced due to the low plasma DHA concentration. For each diet, the rate of DHA synthesis from α-LNA by brain was less than the brain’s DHA consumption rate, whereas the rate of liver DHA secretion exceeded the brain’s DHA consumption rate 5-10 fold.
PUFA incorporation and consumption rates of the human brain
Jin also has been determined for DHA and arachidonic acid (AA, 20:4n-6) in healthy human volunteers on uncontrolled diets, using positron emission tomography and the intravenous infusion of the positron-emitting radiotracers, [1-11C]DHA and [1-11C]AA, respectively, and applying Eqs. 1 and 2. Whole-brain Jin equaled 3.8 mg/day (11.6 μm/day) for DHA compared with 17.8 mg/day for AA (42, 43).
Discussion
In unanesthetized rats fed for 15 weeks post-weaning each of three diets containing different n-3 PUFA contents, the rate of brain DHA synthesis from circulating α-LNA was markedly less than the rate of brain DHA consumption, whereas the rate of liver synthesis-secretion of DHA, calculated following 5 min of intravenous [1-14C]α-LNA infusion, was 5-10 fold greater. With the diet containing 4.6% α-LNA but no DHA, the brain DHA concentration was 11.4 μm/g. While this concentration was less than the 17.6 μm/g in rats on the high DHA diet, earlier studies (1, 2, 36, 38) concluded that it was sufficient to maintain normal brain function and metabolism, and thus that the 4.6% α-LNA diet should be considered “adequate.” A parsimonious conclusion from these dietary studies is that normal brain function and metabolism can be maintained in the adult rat by hepatic conversion and secretion of DHA derived from circulating α-LNA, provided sufficient α-LNA is in the diet. This conclusion is further supported by our recent report, based on infusing rats fed a high-DHA containing diet (Diet 1) intravenously with [U-13C]α-LNA for more than 1 hour so as to produce a steady-state liver DHA synthesis-secretion rate. At the steady state, this synthesis-secretion rate exceeded the brain DHA consumption rate 30-fold (44). As this rate was more than was calculated using the 5-min [1-14C]α-LNA infusion method (Table 2), the latter method underestimates the liver synthesis-secretion rate because measurements are made before steady-state liver metabolism is established.
The different values of conversion-synthesis coefficients ki(a−LNA→DHA) (Eq. 3) in liver and brain, and the 10-fold increased liver coefficient in rats fed the n-3 PUFA deficient compared with adequate diet (Table 1), reflect differences in transcription of the Δ5 and Δ6 desaturases and elongases 2 and 5 that mediate conversion of α-LNA to DHA (39-41). The increased expression of the liver enzymes in rats on the deficient diet was not found to be related to changes in the nuclear transcription factors, peroxisomal proliferator-activated receptor α and sterol-regulatory element binding protein-1 (39).
In contrast to our data suggesting that the rat liver can synthesize sufficient DHA for the brain, a number of clinical and animal studies involving ingestion of isotopic [U-13C]α-LNA and measuring its subsequent whole body oxidation and pharmacokinetics have led to the conclusion that DHA synthesis from α-LNA by the human liver, whatever the dietary α-LNA content, is insufficient to maintain brain DHA integrity (45-47). Clearly, this issue remains controversial and deserves additional study.
We do not know as yet to what extent our data as well as animal data of others (1, 2, 36, 38), which suggest that the adult rat liver is capable of synthesizing sufficient DHA from dietary or circulating α-LNA to maintain a normal brain DHA content, apply to the human condition. One calculation suggests that they do. For example, using an average ingestion of 1400 mg/day of α-LNA (48,49), and evidence that 1-10% of ingested α-LNA is converted to DHA (45-47), gives calculated liver synthesis rates of 14 to 140 mg/day, 3.7 to 36-fold, respectively, the human brain DHA consumption rate of 3.8 mg/day (see Results) (43). In the future, the liver synthesis might be determined directly by infusing [U-13C]α-LNA intravenously for an extended period of time and measuring the rate of appearance of esterified [13C]DHA in plasma (44).
Additional clinical data also argue that liver synthesis of DHA from α-LNA in humans not eating high amounts DHA or EPA, or even having no dietary DHA or EPA, is sufficient to maintain normal brain function and a normal life expectancy. Thus, mortality due to different causes and mortality in general did not differ significantly between vegetarians and omnivores in one study, despite lower plasma DHA levels in vegetarians (12 mg/L in vegans, 29 mg/L in vegetarians, and 50 mg/L in omnivores) (50, 51).
An issue that has yet to be resolved concerns the range of brain DHA concentrations that produce optimal outcomes in life span, cognition and behavior. In rats, some of these outcomes have been worked out by Bourre when he established the n-3 PUFA adequate diet (1, 2). Another issue is whether dietary DHA requirements for brain are increased in certain disease states such as liver disease, which is associated with low plasma PUFA levels (14), and in brain conditions that involve excitotoxicity and neuroinflammation, where excess dietary DHA might be neuroprotective (25). Phase III clinical trials could test whether DHA is protective in these conditions, in which case DHA might be considered a “therapeutic” agent rather than an essential nutrient.
Acknowledgments
This work was entirely supported by the intramural program of the National Institute on Aging. We thank Dr. Richard P. Bazinet for his helpful suggestions.
Footnotes
Supported entirely by the Intramural Program of the National Institute on Aging, NIH
Presented at “Workshop on DHA as a Required Nutrient,” June 20-21, 2008, Baltimore, MD.
M. Igarashi was responsible for data analysis and writing. S. Rapoport was responsible for data analysis and writing. Neither author has a conflict of interest with regard to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bourre JM, Francois M, Youyou A, et al. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–92. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 2.Bourre JM, Dumont O, Pascal G, Durand G. Dietary alpha-linolenic acid at 1.3 g/kg maintains maximal docosahexaenoic acid concentration in brain, heart and liver of adult rats. J Nutr. 1993;123:1313–9. doi: 10.1093/jn/123.7.1313. [DOI] [PubMed] [Google Scholar]
- 3.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–12. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 4.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–7. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 5.Pawlosky RJ, Salem N., Jr Alcohol consumption in rhesus monkeys depletes tissues of polyunsaturated fatty acids and alters essential fatty acid metabolism. Alcohol Clin Exp Res. 1999;23:311–317. [PubMed] [Google Scholar]
- 6.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci. 2000;22:474–80. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- 7.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: A preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 8.Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–88S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 9.British Nutrition Foundation. Unsaturated fatty acids nutritional and physiological significance: the report of the British Nutrition Foundation’s task force. New York: Chapman and Hall; 1992. [Google Scholar]
- 10.Simopoulos AP. Commentary on the workshop statement. Essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2000;63:123–4. doi: 10.1054/plef.2000.0177. [DOI] [PubMed] [Google Scholar]
- 11.Scientific Review Committee. Report. Ottawa, Canada: Minister of National Health and Welfare, Canadian Government Publishing Centre; 1990. Nutritional recommendations; p. 208. [Google Scholar]
- 12.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–61. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourre JM, Piciotti M. Delta-6 desaturation of alpha-linolenic acid in brain and liver during development and aging in the mouse. Neurosci Lett. 1992;141:65–8. doi: 10.1016/0304-3940(92)90335-5. [DOI] [PubMed] [Google Scholar]
- 14.Burke PA, Ling PR, Forse RA, Lewis DW, Jenkins R, Bistrian BR. Sites of conditional essential fatty acid deficiency in end stage liver disease. JPEN J Parenter Enteral Nutr. 2001;25:188–93. doi: 10.1177/0148607101025004188. [DOI] [PubMed] [Google Scholar]
- 15.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Upregulated liver conversion of alpha-linolenic acid to docosahexaenoic acid in rats on a 15-week n-3 PUFA-deficient diet. J Lipid Res. 2007;48:152–64. doi: 10.1194/jlr.M600396-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Strokin M, Sergeeva M, Reiser G. Prostaglandin synthesis in rat brain astrocytes is under the control of the n-3 docosahexaenoic acid, released by group VIB calcium-independent phospholipase A2. J Neurochem. 2007;102:1771–1782. doi: 10.1111/j.1471-4159.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- 18.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 19.Ahmad S, Fowler LJ, Whitton PS. Effects of combined lamotrigine and valproate on basal and stimulated extracellular amino acids and monoamines in the hippocampus of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:1–8. doi: 10.1007/s00210-004-1008-4. [DOI] [PubMed] [Google Scholar]
- 20.Jones CR, Arai T, Rapoport SI. Evidence for the involvement of docosahexaenoic acid in cholinergic stimulated signal transduction at the synapse. Neurochem Res. 1997;22:663–70. doi: 10.1023/a:1027341707837. [DOI] [PubMed] [Google Scholar]
- 21.Purdon AD, Rapoport SI. Energy consumption by phospholipid metabolism in mammalian brain. In: Gibson G, Dienel G, editors. Neural Energy Utilization: Handbook of Neurochemistry and Molecular Biology. 3. New York: Springer; 2007. pp. 401–427. [Google Scholar]
- 22.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–72. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Sun GY, MacQuarrie RA. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann N Y Acad Sci. 1989;559:37–55. doi: 10.1111/j.1749-6632.1989.tb22597.x. [DOI] [PubMed] [Google Scholar]
- 24.Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: Review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- 25.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acidderived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapoport SI. In vivo fatty acid incorporation into brain phospholipids in relation to plasma availability, signal transduction and membrane remodeling. J Mol Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. [DOI] [PubMed] [Google Scholar]
- 27.Rapoport SI. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J Pediatr. 2003;143:S26–34. doi: 10.1067/s0022-3476(03)00399-8. [DOI] [PubMed] [Google Scholar]
- 28.Holman RT. Control of polyunsaturated acids in tissue lipids. J Am Coll Nutr. 1986;5:183–211. doi: 10.1080/07315724.1986.10720125. [DOI] [PubMed] [Google Scholar]
- 29.Demar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem. 2005;94:1063–1076. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- 30.DeMar JC, Jr, Lee HJ, Ma K, et al. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006;1761:1050–9. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Rapoport SI, Chang MC, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–85. [PubMed] [Google Scholar]
- 32.Purdon D, Arai T, Rapoport S. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. J Lipid Res. 1997;38:526–30. [PubMed] [Google Scholar]
- 33.Chang MC, Arai T, Freed LM, et al. Brain incorporation of [1-14C]-arachidonate in normocapnic and hypercapnic monkeys, measured with positron emission tomography. Brain Res. 1997;755:74–83. doi: 10.1016/s0006-8993(97)00088-7. [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Ginsberg HN. Microsomal triglyceride transfer protein binding and lipid transfer activities are independent of each other, but both are required for secretion of apolipoprotein B lipoproteins from liver cells. J Biol Chem. 2001;276:28606–12. doi: 10.1074/jbc.M100294200. [DOI] [PubMed] [Google Scholar]
- 35.Demar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–80. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–37. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 37.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–8. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Stinson AM, Wiegand RD, Anderson RE. Recycling of docosahexaenoic acid in rat retinas during n-3 fatty acid deficiency. J Lipid Res. 1991;32:2009–17. [PubMed] [Google Scholar]
- 39.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–70. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura MT, Nara TY. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot Essent Fatty Acids. 2003;68:145–50. doi: 10.1016/s0952-3278(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 41.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–6. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 42.Giovacchini G, Lerner A, Toczek MT, et al. Brain incorporation of 11Carachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med. 2004;45:1471–9. [PubMed] [Google Scholar]
- 43.Umhau JC, Zhou W, Polozova A, et al. Human brain incorporation of docosahexaenoic acid measured using PET. J Lipid Res. 2008 Dec [Google Scholar]
- 44.Gao F, Kiesewetter D, Chang L, et al. Whole body synthesis-secretion rates of long-chain n-3 polyunsaturated fatty acids from circulating unesterified alpha -linolenic acid in unanesthetized rats. J Lipid Res. 2008 doi: 10.1194/jlr.D800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. 2002;5:127–32. doi: 10.1097/00075197-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Sinclair AJ, Attar-Bashi NM, Li D. What is the role of alpha-linolenic acid for mammals? Lipids. 2002;37:1113–23. doi: 10.1007/s11745-002-1008-x. [DOI] [PubMed] [Google Scholar]
- 47.Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ. Longchain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 2005;46:269–80. doi: 10.1194/jlr.M400225-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Albert CM, Oh K, Whang W, et al. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112:3232–8. doi: 10.1161/CIRCULATIONAHA.105.572008. [DOI] [PubMed] [Google Scholar]
- 49.Denomme J, Stark KD, Holub BJ. Directly quantitated dietary (n-3) fatty acid intakes of pregnant Canadian women are lower than current dietary recommendations. J Nutr. 2005;135:206–11. doi: 10.1093/jn/135.2.206. [DOI] [PubMed] [Google Scholar]
- 50.Rosell MS, Lloyd-Wright Z, Appleby PN, Sanders TA, Allen NE, Key TJ. Longchain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am J Clin Nutr. 2005;82:327–34. doi: 10.1093/ajcn.82.2.327. [DOI] [PubMed] [Google Scholar]
- 51.Key TJ, Appleby PN, Davey GK, Allen NE, Spencer EA, Travis RC. Mortality in British vegetarians. Am J Clin Nutr. 2003;78:533S–538S. doi: 10.1093/ajcn/78.3.533S. [DOI] [PubMed] [Google Scholar]
- 52.Contreras MA, Chang MC, Rosenberger TA, et al. Chronic nutritional deprivation of n-3 alpha-linolenic acid does not affect n-6 arachidonic acid recycling within brain phospholipids of awake rats. J Neurochem. 2001;79:1090–9. doi: 10.1046/j.1471-4159.2001.00658.x. [DOI] [PubMed] [Google Scholar]


