Abstract
Although data show the importance of type I interferons (IFNs) in the regulation of the innate and adaptive immunity elicited in response to viral, bacterial and parasitic infections, the functional activities of these cytokines during fungal infections are poorly understood. We examined here the impact of IFN-β on the response of human monocyte-derived dendritic cells (DCs) infected in vitro with Aspergillus fumigatus. Having found that A. fumigatus-infected DCs do not express IFN-β, we evaluated the effect of the exogenous addition of IFN-β on the maturation of human DCs induced by the infection with A. fumigatus conidia. Although the phagocytosis of the fungus was not affected by IFN-β treatment, the expression of CD86 and CD83 induced upon A. fumigatus challenge was enhanced in IFN-β-conditioned DCs, which also showed an increased expression of IL-27 and IL-12p70, members of IL-12 family. Through these modifications, IFN-β improved the capacity of DCs to promote an anti-Aspergillus T helper type 1 response, as evaluated by mixed leucocyte reaction, which plays a crucial role in the control of invasive aspergillosis. Our results identified a novel effect of IFN-β on anti-Aspergillus immune responses which, in turn, might open new perspectives on the use of IFN-β in immunotherapy for fungal infections aimed at enhancing the immunological functions of DCs.
Keywords: Aspergillus fumigatus, dendritic cells, interferon-β, interleukin-12 family, T helper response
Introduction
Aspergillus fumigatus (A. fumigatus) conidia are ubiquitous in the environment. Although the inhaled spores are normally eliminated without causing disease in an immunocompetent host, they can elicit invasive aspergillosis (IA), a rapidly progressive infection in patients who are severely immunosuppressed.
Efficient responses to the fungus require a complex network of immunological mechanisms. Together with alveolar macrophages and neutrophils, which constitute a primary line of innate cellular defence against A. fumigatus,1,2 the crucial role of the adaptive immunity has been extensively demonstrated.3 Indeed, besides the well-characterized protective role of T helper type 1 (Th1) lymphocytes,4–7 the newly described regulatory T cells and interleukin-17 (IL-17) -producing cells (Th17) represent important mediators of the inflammatory and anti-inflammatory host responses against A. fumigatus.8 However, dendritic cells (DCs) also play a fundamental function in initiating and modulating the specific immune responses upon recognition of A. fumigatus.5,9,10 After internalization of A. fumigatus conidia, DCs mature and acquire the capacity to polarize naive T cells and, in turn, to promote a protective response.9 In keeping with these findings, in vivo results on the migration of lung DCs into lymphoid organs, where they drive an appropriate T-cell response to fungal antigens,11 have brought DCs centre stage as promising targets for intervention in immunotherapy and fungal vaccine development.12 In addition, it is important to consider several studies that have recently pointed to DCs and type I interferons (IFNs) as special players in the immune response tailored to combat tumours and infections.13–15 Indeed, although the anti-microbial properties of these cytokines have not been fully characterized yet, type I IFNs represent important immunomodulators of the innate, as well as the adaptive, arm of the immune system. Type I IFN can promote the differentiation of human blood monocytes into DCs and contribute to their maturation.16,17 This leads to the generation of DCs able to stimulate a primary human antibody response, a Th1 proliferation,18 and a cross-priming of CD8 T cells against viral antigens.19 In addition, one crucial outcome of type I IFN-induced effects is the ability to directly stimulate IFN-γ production in natural killer and T cells,20–22 which in turn promotes the development of a cell-mediated immune response.
Based on these immunoregulatory properties, in this work the expression and the capacity of type I IFN, namely IFN-β, to modulate the T-cell polarizing capacity of A. fumigatus-infected DCs was investigated in an attempt to evaluate the effects induced by this cytokine on anti-fungal immunity. Although the phagocytosis of the fungus was not affected by IFN-β treatment, the maturation induced by A. fumigatus infection was enhanced in IFN-β-primed DCs, as evaluated by analysing the immunophenotype and the release of pro-inflammatory and regulatory cytokines. Accordingly, IFN-β endowed DCs with potent Th1 polarizing capacity because an enhanced IFN-γ production in T cells co-cultured with A. fumigatus-infected DCs was observed in the presence of IFN-β. These results indicate that IFN-β potentiates DC immunological functions following A. fumigatus infection, which suggests that IFN-β is a possible adjuvant to elicit an appropriate Th reactivity to A. fumigatus.
Materials and methods
Preparation of DCs
Dendritic cells were prepared as previously described.9 CD14+ monocytes were cultured with 25 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; Schering-Plough, Levallois Perret, France) and 1000 U/ml IL-4 (R&D Systems, Minneapolis, MN) for 5 days. On day 5, about 90% of the cells express CD1a+ and 95% express CD14−. The DCs were starved from IL-4 and GM-CSF for 20 hr before infection or treatments.
Antibodies and other reagents
Monoclonal antibodies specific for CD1a, CD14, CD38, CD40, CD83, CD86, HLA-DR, CD3 and CD4 as well as immunoglobulin G1 (IgG1), IgG2a and IgG2b (BD Bioscience PharMingen, San Diego, CA) were used as direct conjugates to fluorescein isothiocyanate (FITC) or phycoerythrin. Lipopolysaccharide (LPS) from Escherichia coli 0111:B4 (Sigma-Aldrich, St Louis, MO) was used at a concentration of 100 ng/ml to stimulate DC maturation and IFN-β expression. The IFN-β (Avonex®; Biogen Inc., Cambridge, MA) was used at 200 pm.
Aspergillus fumigatus preparation and infection
A wild-type clinical isolate of A. fumigatus (CBS 144 89) was grown on Sabouraud–chloramphenicol agar for 3 days, at 37°, as previously described.23 Preparations of A. fumigatus were analysed for LPS contamination by the Limulus lysate assay (Biowhittaker, Verviers, Belgium) and were found to contain less than 10 pg/ml LPS. In all experiments, DCs were infected with live A. fumigatus conidia at a 1 : 1 ratio. Amphotericin B (0·75 μg/ml; Sigma-Aldrich) was added to the cell cultures to prevent fungal overgrowth 6 hr after infection when the internalization of A. fumigatus conidia was completed.9
For the adherence assay, A. fumigatus conidia were incubated with FITC at a final concentration of 3 mg/ml overnight at 4°, and then washed extensively with PBS. After a 6-hr incubation with FITC-labelled conidia (ratio 1 : 1), DCs were washed and the adherence was measured by flow cytometric analysis.
Flow cytometric analysis
The cells were incubated with purified monoclonal antibodies at 4° for 30 min. After washing, the cells were fixed with 2% paraformaldehyde before analysis on a FACScan using the cellquest software (BD Bioscience PharMingen). A total of 5000 cells were analysed per sample.
RNA isolation and real-time reverse transcription–polymerase chain reaction quantifications
RNA extraction, reverse transcription (RT) and real-time RT-polymerase chain reaction (PCR) assays were performed as previously described.24 Sequences of the primer pairs used for glyceraldehyde 3-phosphate dehydrogenase (GaPDH), IFN-β, IL-12p35, IL-23p19 and IL-27p28 quantification were previously described.24
Cytokine detection
Cytokine concentration in filtered supernatants was evaluated with the human inflammation cytometric bead array (CBA) [for IL-12p70, IL-10, tumour necrosis factor-α (TNF-α) and IL-6: BD Bioscience PharMingen] and enzyme-linked immunsorbent assay (ELISA; for IFN-β: PBL Biomedical Laboratories, Piscataway, NJ; for IL-23: eBioscience, San Diego, CA).
Mixed leucocyte reaction
Cord blood CD4+ T cells were purified by indirect magnetic sorting with CD4+ T Cell Isolation Kit (Miltenyi Biotec, Bergish Gladbach, Germany). The proliferative response was performed at various T-cells : DC ratios using a fixed number of T cells (3 × 104) and evaluated after 5 days by measuring thymidine incorporation (0·5 μCi/well of [3H]thymidine; Amersham, Little Chalfont, UK). The results were expressed as mean counts per minute of triplicate cultures. Supernatant of T-cell cultures was harvested at day 6 after infection and IFN-γ and IL-4 concentrations were measured by ELISA kits (R&D Systems).
Statistical analysis
Statistical analysis was performed by non-parametric two-tailed Mann–Whitney U-test using the graphpad prism 4 software (GraphPad Inc., San Diego, CA).
Results
Aspergillus fumigatus-infected DCs do not express IFN-β
We and other authors previously observed that DCs could release IFN-β following viral and bacterial infection,25–28 while no data have been published on the capacity of A. fumigatus to induce the expression of type I IFN in DCs. To investigate this aspect, DCs were infected with A. fumigatus and the expression of IFN-β was evaluated by real-time RT-PCR at various times after infection (2, 6 and 20 hr). To verify the capacity of DC culture to express IFN-β, a treatment with LPS was also included at each time-point. Interestingly, no induction of IFN-β expression was observed at early time-points, whereas only a slight increase was noted 20 hr after A. fumigatus infection (Fig. 1). The lack of IFN-β messenger RNA (mRNA) expression following A. fumigatus infection of DCs was confirmed by ELISA (data not shown). Conversely, IFN-β mRNA induction by LPS began 2 hr post-infection, the level remained elevated at 6 hr and declined rapidly as previously described.25
Figure 1.
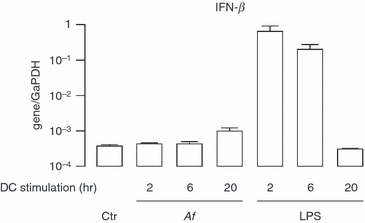
Lack of interferon-β (IFN-β) expression in Aspergillus fumigatus-infected dendritic cells (DCs). Total RNA was extracted at the indicated time-points following A. fumigatus conidia (Af) infection (1 : 1 ratio) and lipopolysaccharide (LPS) treatment (100 ng/ml). The expression of IFN-β was analysed by real-time reverse transcription–polymerase chain reaction (RT-PCR). The results are shown as a ratio to the GaPDH level and represent the mean ± SE of triplicate values. This is a representative real time RT-PCR experiment, which was repeated twice more with RNA extracted from different DC cultures.
Exogenous addition of IFN-β improves the maturation of A. fumigatus-infected DCs without affecting their capacity to uptake A. fumigatus conidia
After determining that A. fumigatus-infected DCs did not express IFN-β and knowing the potential immunoregulatory properties of this cytokine,16 we investigated whether the exogenous addition of IFN-β could modify DC responses to the fungal infection. Dendritic cells were pre-treated for 4 hr with IFN-β and then infected with A. fumigatus conidia for 24 hr. The immunophenotype of the DCs was evaluated by flow cytometry through the analysis of the molecules involved in T-cell activation, such as CD86, CD83, HLA-DR and CD38 (Fig. 2a). As previously shown, the treatment of immature DCs with IFN-β induced a selective increased expression of CD38 (an IFN-inducible marker) and CD86 but not of CD83.24 Interestingly, while the IFN-β-induced expression of CD38 was not further increased upon A. fumigatus challenge, a strong effect of IFN-β was instead observed on CD83 and CD86 expression in A. fumigatus-infected cells. Conversely, the constitutive expression of HLA-DR was not significantly modified by A. fumigatus or IFN-β treatment.
Figure 2.

Influence of interferon-β (IFN-β) pre-treatment on the in vitro maturation of Aspergillus fumigatus-infected dendritic cells (DCs). (a) DCs were pre-treated for 4 hr with IFN-β (200 pm) before the infection with A. fumigatus (Af). After 24 hr, cells were stained for CD83, CD86, HLA-DR and CD38 and analysed by flow cytometry. The results are the mean of mean fluorescence intensity (MFI; subtracted from the isotype value) ± SE values from six different blood donors. (b) After a treatment with IFN-β (200 pm) or with lipopolysaccharide (LPS; 100 ng/ml) for 24 hr, DCs were incubated with fluorescein isothiocyanate (FITC) -labelled conidia at a ratio of 1 : 1 for 6 hr at 37°. The percentage of DCs with bound FITC-labelled A. fumigatus conidia (Af-FITC-positive DC) was measured by flow cytometric analysis. The results represent the mean ± SE of four separate experiments.
The phagocytosis of A. fumigatus conidia was then evaluated in DCs treated with IFN-β for 24 hr to check whether the IFN-β effect on DC maturation was the result of an enhanced capacity to uptake A. fumigatus. The ability of IFN-β-treated DCs to internalize A. fumigatus conidia was compared with LPS-matured DCs (100 ng/ml for 24 hr). The percentage of DCs that have fixed FITC-A. fumigatus conidia was analysed by flow cytometry at 6 hr post-infection. Treatment with IFN-β did not alter the capacity of DCs to internalize the pathogen whereas, as expected, LPS-matured DCs showed a reduced capacity to uptake A. fumigatus conidia. Indeed, 55% of control and IFN-β-treated DCs phagocytosed A. fumigatus but only 35% of LPS-stimulated DCs fixed the conidia (Fig. 2b).
Overall, our results indicated that IFN-β treatment did not modify the capacity of immature DCs to phagocytose A. fumigatus conidia although it strongly modulated the A. fumigatus-induced expression of co-stimulatory and maturation molecules. These results prompted us to further investigate the impact of IFN-β on the response of DCs to A. fumigatus infection.
Interferon-β modulates the A. fumigatus-stimulated expression of pro-inflammatory and regulatory cytokines
Cytokine production critically impacts the ability of DCs to activate and prime T cells. For this reason, we assessed IFN-β capacity to modulate the profile of cytokines released by A. fumigatus-infected DCs. To this end, supernatants were harvested from DCs stimulated for 24 hr with A. fumigatus with or without 4 hr IFN-β pre-treatment, and the release of IL-12p70, IL-23, IL-10, TNF-α and IL-6 was analysed (Fig. 3). The presence of IFN-β significantly increased the secretion of IL-12p70 and IL-6 by A. fumigatus-infected DCs but it did not modify the release of IL-10, IL-23 and TNF-α.
Figure 3.

Effects of interferon-β (IFN-β) pre-treatment on cytokine production by Aspergillus fumigatus-infected dendritic cells (DCs). DC were stimulated as described in Fig. 2 and cell culture supernatants were collected. The amounts of interleukin-12 p70 (IL-12p70), IL-23, IL-10, tumour necrosis factor-α (TNF-α), and IL-6 were measured in the supernatants by cytometric bead assay and enzyme-linked immunosorbent assay. The results represent the mean ± SE of four separate experiments performed with different donors.
To investigate whether the robust induction of IL-12p70 in IFN-β-primed DCs was determined by an increased transcription of the IL-12p35 subunit and to extend our study also to IL-27, a critical cytokine involved in regulating T cell differentiation and functions, we investigated in IFN-β-primed DC the expression of inducible subunits composing the IL-12 family members following A. fumigatus infection. Total RNA was extracted 20 hr after A. fumigatus infection with or without a 4-hr IFN-β priming and the transcripts encoding IL-12p35, IL-23p19, and IL-27p28 subunits were analysed by real-time RT-PCR (Fig. 4). The weak expression of IL-12p35 in A. fumigatus-infected cells was increased by IFN-β pre-treatment explaining the synergistic effect on IL-12p70 secretion observed by CBA analysis (compare Figs 3 and 4). Conversely, no effect of IFN-β treatment was observed on the expression of IL-23p19, confirming the ELISA results presented in Fig. 3. Interestingly, IFN-β pre-treatment induced a robust expression of IL-27p28, which was further increased in A. fumigatus-stimulated DCs. The lack of IL-27p28 expression, in DCs stimulated with A. fumigatus alone, correlated well with the incapacity of this fungus to stimulate IFN-β gene transcription (Fig. 1) and further highlighted the type I IFN-dependent expression of IL-27, as previously demonstrated.24 All these data suggest that IFN-β can profoundly modify the response of DC to A. fumigatus infection acting on their ability to release pro-inflammatory and regulatory cytokines involved in orchestrating the Th response.
Figure 4.

Expression of interleukin-12 (IL-12) family members in interferon-β (IFN-β) pre-treated dendritic cells (DCs) upon stimulation with Aspergillus fumigatus. RNA was extracted from immature DCs, DCs treated for 20 hr with A. fumigatus (Af) with or without a 4-hr pre-treatment with IFN-β, or were stimulated with IFN-β alone. Real time reverse transcription–polymerase chain reaction was performed to measure the expression of IL-12p35, IL-23p19 and IL-27p28. The results are shown as a ratio to the GaPDH level and represent the mean ± SE of triplicate values. The results shown are from one of three experiments performed with different donors that yielded similar results.
Interferon-β pre-treatment strengthens the capacity of A. fumigatus-infected DCs to expand Th1-oriented CD4+ T cells
Next, T-cell proliferation and polarization were investigated by mixed leucocyte reaction to determine whether the effect induced by IFN-β on A. fumigatus-infected DC maturation resulted in an enhanced capacity in promoting the expansion of Th1-oriented CD4+ T cells. As shown in Fig. 5(a), A. fumigatus-infected DCs induced the proliferation of naive allogeneic cord blood CD4+ T cells, which was not significantly modified when infected DCs were primed with IFN-β. Interestingly, IFN-β priming of A. fumigatus-infected DCs highly enhanced the production of IFN-γ, as observed by the analysis of supernatants obtained from mixed leucocyte reaction cultures (Fig. 5b). Conversely, no induction of IL-4 was found when T cells were co-cultured with A. fumigatus-stimulated DCs in the presence or absence of IFN-β (data not shown).
Figure 5.

Immunoregulatory effect of interferon-β (IFN-β) on the priming of naive T-cell responses by Aspergillus fumigatus-infected dendritic cells (DCs). The DCs were either left untreated or were stimulated with IFN-β, or were infected for 24 hr with A. fumigatus (Af) with or without IFN-β pre-treatment. (a) A mixed leucocyte reaction assay was set up with DCs cultured at various cell numbers with 3 × 104 purified allogeneic naive CD4+ T cells isolated from cord blood. The proliferative response was measured after 5 days and expressed as mean counts per minute (cpm). The results represent the mean ± SE of three separate experiments performed with different donors. (b) DCs were cultured at 1 : 10 ratio (stimulator : effector cells) with allogeneic naive T cells and after 5 days the supernatants were collected. IFN-γ production means ± SE were obtained in three independent experiments performed with different donors.
Discussion
Type I IFNs, originally identified for their ability to induce cellular resistance to viral infections, are key immunomodulators of the innate and adaptive immune responses.29 By acting on DC differentiation and maturation, these cytokines can induce cross-priming of CD8 T cells19 and stimulate a Th1-oriented T-cell response.21,22 Accordingly, our recent findings showed that IFN-β potentiates DC immunological functions following bacillus Calmette–Guérin infection, pointing to the importance of IFN-β in promoting a protective Th1 immune response against Mycobacterium tuberculosis.30 Based on this evidence, the use of type I IFN constitutes a promising immunotherapy for infectious diseases.13,15
Invasive aspergillosis is a serious opportunistic fungal infection in immunocompromised hosts. Advances in more potent and less toxic antifungal agents have reduced the mortality rate of IA and represent a promising area of research and development to cure invasive fungal infections. Moreover, novel strategies for immunotherapy and vaccine are also currently designed on the knowledge of the immunopathogenesis of fungal infections.31 Although clinical evidence points to a crucial role for the Th1 reactivity in the control of IA, more recently regulatory T cells and Th17 cells could display important functions in the scenario of the immune response against A. fumigatus.32 However, if the role of IL-17-producing T cells in protection versus pathology in fungal infections is still controversial,33–35 it is generally accepted that a defective differentiation of regulatory T cells may cause an unacceptable level of tissue damage.3 Several studies in human and murine models have, however, confirmed the central role of IFN-γ released by interstitial lung lymphocytes in controlling IA through the stimulation of phagocytosis and intracellular antifungal killing mechanisms of neutrophils and macrophages.4,36 In line with these data, an elegant demonstration of the importance of specific adjuvants in fungal vaccination development to selectively induce Th1-dominated immune responses was published by Romani et al.,37 who demonstrated that thymosin-α1 stimulates the Th1-polarizing capacity of DC. CpG was also shown to act as a potent adjuvant for the vaccine-induced protection against the fungus by promoting a dominant Th1 response to Aspergillus antigens and allergens.38,39 Given the capacity of CpG to stimulate the release of type I IFN by plasmacytoid DCs, it is tempting to speculate that these cytokines can act as immuno-adjuvants in fungal defence. In addition, the capacity of type I IFN to exert an anti-fungal activity by promoting natural killer cell activation in mice40,41 suggests that these cytokines could represent a promising adjuvant able to reinforce also the innate defence mechanisms such as those involving natural killer cells.
Based on these data, we investigated whether IFN-β could also modulate the T-cell polarizing capacity of A. fumigatus-stimulated DCs, which fail to secrete this cytokine when challenged with A. fumigatus conidia. Interestingly, we observed that the exogenous addition of IFN-β to A. fumigatus-stimulated DCs reinforced the expression of CD86, CD83 and IL-12p70 and, in turn, the capacity to stimulate a Th1 response. Moreover, in the presence of IFN-β, DCs may also express IL-27, a key cytokine involved in controlling excessive inflammation by suppressing Th17 differentiation.42 In addition to that, in this scenario IFN-β could also limit the development of a Th17 inflammatory response acting directly on T cells as recently proposed in patients with multiple sclerosis undergoing IFN-β therapy.43,44 Collectively these data identified a novel effect of IFN-β on the anti-Aspergillus immune response which, in turn, might open new perspectives on the use of IFN-β as a candidate adjuvant in immunotherapy for fungal infections aimed at potentiating DC immunological functions.
Acknowledgments
We would like to thank Eugenio Morassi for preparing the drawings and Pierre-Emmanuel Colle and Claudine Pinel for invaluable discussions. This work was supported by an Italian Public Health Ministry grant (Ricerca Finalizzata 2006; #8ABF).
Glossary
Abbreviations:
- CBA
cytometric bead array
- DCs
dendritic cells
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- GaPDH
glyceraldehyde 3-phosphate dehydrogenase
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IA
invasive aspergillosis
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- LPS
lipopolysaccharide
- MFI
mean fluorescence intensity
- RT-PCR
reverse transcription–polymerase chain reaction
- Th1
T helper type 1
- TNF
tumour necrosis factor
Disclosures
The authors declare no conflict of interest.
References
- 1.Balloy V, Chignard M. The innate immune response to Aspergillus fumigatus. Microbes Infect. 2009;11:919–27. doi: 10.1016/j.micinf.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–50. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romani L. Cell mediated immunity to fungi: a reassessment. Med Mycol. 2008;46:515–29. doi: 10.1080/13693780801971450. [DOI] [PubMed] [Google Scholar]
- 4.Beck O, Topp MS, Koehl U, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107:2562–9. doi: 10.1182/blood-2005-04-1660. [DOI] [PubMed] [Google Scholar]
- 5.Bozza S, Gaziano R, Spreca A, Bacci A, Montagnoli C, di Francesco P, Romani L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168:1362–71. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- 6.Grazziutti M, Przepiorka D, Rex JH, Braunschweig I, Vadhan-Raj S, Savary CA. Dendritic cell-mediated stimulation of the in vitro lymphocyte response to Aspergillus. Bone Marrow Transplant. 2001;27:647–52. doi: 10.1038/sj.bmt.1702832. [DOI] [PubMed] [Google Scholar]
- 7.Hebart H, Bollinger C, Fisch P, et al. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100:4521–8. doi: 10.1182/blood-2002-01-0265. [DOI] [PubMed] [Google Scholar]
- 8.Zelante T, De Luca A, D’Angelo C, Moretti S, Romani L. IL-17/Th17 in anti-fungal immunity: what's new? Eur J Immunol. 2009;39:645–8. doi: 10.1002/eji.200839102. [DOI] [PubMed] [Google Scholar]
- 9.Gafa V, Lande R, Gagliardi MC, et al. Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response. Infect Immun. 2006;74:1480–9. doi: 10.1128/IAI.74.3.1480-1489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrano-Gomez D, Dominguez-Soto A, Ancochea J, Jimenez-Heffernan JA, Leal JA, Corbi AL. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. J Immunol. 2004;173:5635–43. doi: 10.4049/jimmunol.173.9.5635. [DOI] [PubMed] [Google Scholar]
- 11.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 12.Bozza S, Clavaud C, Giovannini G, et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol. 2009;183:2407–14. doi: 10.4049/jimmunol.0900961. [DOI] [PubMed] [Google Scholar]
- 13.Bracci L, La Sorsa V, Belardelli F, Proietti E. Type I interferons as vaccine adjuvants against infectious diseases and cancer. Expert Rev Vaccines. 2008;7:373–81. doi: 10.1586/14760584.7.3.373. [DOI] [PubMed] [Google Scholar]
- 14.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–23. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 15.Nelson DR, Marousis CG, Ohno T, Davis GL, Lau JY. Intrahepatic hepatitis C virus-specific cytotoxic T lymphocyte activity and response to interferon α therapy in chronic hepatitis C. Hepatology. 1998;28:225–30. doi: 10.1002/hep.510280129. [DOI] [PubMed] [Google Scholar]
- 16.Coccia EM. IFN regulation and functions in myeloid dendritic cells. Cytokine Growth Factor Rev. 2008;19:21–32. doi: 10.1016/j.cytogfr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Ferrantini M, Capone I, Belardelli F. Dendritic cells and cytokines in immune rejection of cancer. Cytokine Growth Factor Rev. 2008;19:93–107. doi: 10.1016/j.cytogfr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–15. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 20.Matikainen S, Paananen A, Miettinen M, Kurimoto M, Timonen T, Julkunen I, Sareneva T. IFN-α and IL-18 synergistically enhance IFN-γ production in human NK cells: differential regulation of Stat4 activation and IFN-γ gene expression by IFN-α and IL-12. Eur J Immunol. 2001;31:2236–45. [PubMed] [Google Scholar]
- 21.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza A virus-induced IFN-α/β and IL-18 synergistically enhance IFN-γ gene expression in human T cells. J Immunol. 1998;160:6032–8. [PubMed] [Google Scholar]
- 23.Gafa V, Remoli ME, Giacomini E, Gagliardi MC, Lande R, Severa M, Grillot R, Coccia EM. In vitro infection of human dendritic cells by Aspergillus fumigatus conidia triggers the secretion of chemokines for neutrophil and Th1 lymphocyte recruitment. Microbes Infect. 2007;9:971–80. doi: 10.1016/j.micinf.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN-β modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol. 2007;37:3499–508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- 25.Coccia EM, Severa M, Giacomini E, et al. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 26.Remoli ME, Giacomini E, Lutfalla G, et al. Selective expression of type I IFN genes in human dendritic cells infected with Mycobacterium tuberculosis. J Immunol. 2002;169:366–74. doi: 10.4049/jimmunol.169.1.366. [DOI] [PubMed] [Google Scholar]
- 27.Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000;12:419–24. doi: 10.1016/s0952-7915(00)00111-4. [DOI] [PubMed] [Google Scholar]
- 28.Feng H, Zhang D, Palliser D, Zhu P, Cai S, Schlesinger A, Maliszewski L, Lieberman J. Listeria-infected myeloid dendritic cells produce IFN-β, priming T cell activation. J Immunol. 2005;175:421–32. doi: 10.4049/jimmunol.175.1.421. [DOI] [PubMed] [Google Scholar]
- 29.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–36. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 30.Giacomini E, Remoli ME, Gafa V, Pardini M, Fattorini L, Coccia EM. IFN-β improves BCG immunogenicity by acting on DC maturation. J Leukoc Biol. 2009;85:462–8. doi: 10.1189/jlb.0908583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnan-Natesan S, Chandrasekar PH. Current and future therapeutic options in the management of invasive aspergillosis. Drugs. 2008;68:265–82. doi: 10.2165/00003495-200868030-00002. [DOI] [PubMed] [Google Scholar]
- 32.Zelante T, Bozza S, De Luca A, et al. Th17 cells in the setting of Aspergillus infection and pathology. Med Mycol. 2009;47(Suppl 1):S162–9. doi: 10.1080/13693780802140766. [DOI] [PubMed] [Google Scholar]
- 33.Werner JL, Metz AE, Horn D, et al. Requisite role for the dectin-1 β-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–46. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zelante T, De Luca A, Bonifazi P, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 35.Chai LYA, van de Veerdonk F, Marijnissen RJ, et al. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology. 2010;130:46–54. doi: 10.1111/j.1365-2567.2009.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roilides E, Holmes A, Blake C, Venzon D, Pizzo PA, Walsh TJ. Antifungal activity of elutriated human monocytes against Aspergillus fumigatus hyphae: enhancement by granulocyte-macrophage colony-stimulating factor and interferon-γ. J Infect Dis. 1994;170:894–9. doi: 10.1093/infdis/170.4.894. [DOI] [PubMed] [Google Scholar]
- 37.Romani L, Bistoni F, Gaziano R, et al. Thymosin α1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood. 2004;103:4232–9. doi: 10.1182/blood-2003-11-4036. [DOI] [PubMed] [Google Scholar]
- 38.Bozza S, Gaziano R, Lipford GB, et al. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 2002;4:1281–90. doi: 10.1016/s1286-4579(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee B, Kelly KJ, Fink JN, Henderson JD, Jr, Bansal NK, Kurup VP. Modulation of airway inflammation by immunostimulatory CpG oligodeoxynucleotides in a murine model of allergic aspergillosis. Infect Immun. 2004;72:6087–94. doi: 10.1128/IAI.72.10.6087-6094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tandon RN, Feuillette AR, Mahouy G, Badillet G, Friedman RM, Maheshwari RK. Interferon protects mice against an extracellular infection of Aspergillus fumigatus. Ann N Y Acad Sci. 1988;544:409–11. doi: 10.1111/j.1749-6632.1988.tb40437.x. [DOI] [PubMed] [Google Scholar]
- 41.Maheshwari RK, Tandon RN, Feuillette AR, Mahouy G, Badillet G, Friedman RM. Interferon inhibits Aspergillus fumigatus growth in mice: an activity against an extracellular infection. J Interferon Res. 1988;8:35–44. doi: 10.1089/jir.1988.8.35. [DOI] [PubMed] [Google Scholar]
- 42.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–56. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 43.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-β inhibits human Th17 cell differentiation. J Immunol. 2009;183:5418–27. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 44.Durelli L, Conti L, Clerico M, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-β. Ann Neurol. 2009;65:499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]


