Abstract
Invariant natural killer T (iNKT) cells are known to constitutively express the high affinity interlukin-2 receptor α chain (CD25) in neonates, but the functional consequence of this phenotype is unknown. Here, we show that high numbers of CD25-expressing iNKT cells are present early in gestation and represent a significant proportion of the developing immune system. Despite their activated phenotype, neonatal iNKT cells express high levels of the Krüppel-like factor-2, a transcription factor associated with quiescent T cells, and require de novo T-cell receptor and CD28 co-stimulation to proliferate. In contrast to bona fide CD4/CD25-expressing regulatory T cells, neonatal iNKT cells do not suppress T-cell responses, indicating that they do not represent an immunosuppressive cell subset. Evidence that neonatal iNKT cells respond to dramatically reduced amounts of CD1d-restricted antigen compared with adult iNKT cells or T cells, and that their proliferation can be induced in the absence of early interleukin-2 suggest that constitutive expression of CD25 ‘primes’ neonatal iNKT cells to respond rapidly to low amounts of antigen. This unique phenotype, which is distinct from adult iNKT cells, as well as other CD25-expressing activated T or regulatory T cells, may be important to ensure stability of a structurally limited peripheral iNKT-cell repertoire early in life.
Keywords: cord blood, interleukin-2 receptor α chain, invariant natural killer T cells, T-cell proliferation
Introduction
Invariant CD1d-restricted natural killer T (i.e. iNKT) cells are phenotypically distinct from αβ T lymphocytes because of the expression of structurally limited T-cell receptors (TCRs) specific to non-classical CD1d-bound glycolipid antigens.1 Most notably, iNKT cells dramatically impact adaptive immune responses through specialized interactions with innate and CD4/CD25-expressing regulatory T (Treg) cells, suggesting that they may be instrumental for therapeutic modulation in autoimmune diseases and human malignancies.2
Much remains to be known about the development of human iNKT cells early in life. In mice, iNKT cells develop after birth whereas in humans, iNKT cells can be detected earlier, during gestation, and already comprise about 0·1% of blood CD3-positive (CD3+) lymphocytes at birth.3–5 Human peripheral iNKT cells display two main CD4+ and CD4– subsets functionally differing in their helper cytokine expression profiles and homeostatic requirements.6,7 The CD4+ iNKT cells dominate in fetal and neonatal blood (> 90% iNKT population) and their proportion decreases with age. Evidence also suggests that CD4+ iNKT cells directly expand from the thymus, whereas peripheral expansion of CD4– iNKT cells may be mainly driven through homeostatic proliferation.8 The relatively stringent antigenic receptor combinatorial rearrangement, resulting in a bottleneck effect on thymic output, implies the potential existence of mechanisms to maintain iNKT-cell repertoire diversity upon repeated antigen-driven cell proliferation.1,3,9
Unlike the vast majority of fetal or neonatal T cells, both neonatal and adult iNKT cells predominantly express the CD45RO isoform memory T-cell marker.10–12 However, neonatal iNKT cells display a specific and unique phenotype compared with their adult counterpart. Indeed, freshly isolated ex vivo human neonatal iNKT cells constitutively express CD25, the high affinity interleukin-2 (IL-2) receptor α chain. This characteristic was initially interpreted to be the result of antenatal recognition of a yet unidentified endogenous CD1d-ligand.10,11 However, constitutive CD25 expression may also reflect a discrete developmental stage that plays an important role in immunity in early life. The notion that neonatal CD25-expressing iNKT cells are not simply activated cells, but rather represent a developmentally distinct subset is supported by the absence of other markers of recent T-cell activation including CD69 and HLA-DR.10,13 Furthermore, even though it has long been recognized that neonatal iNKT cells express CD25, the functional consequence of this constitutive CD25 expression and its precise significance in neonatal NKT cell physiology remain unclear. In normal circumstances, CD25 is only expressed by resting or activated regulatory T (Treg) cells, or transiently following activation of conventional T cells.10,11 CD25 is necessary for signalling through the IL-2 receptor complex, which also includes the β and common γ chains.14 In activated T cells, TCR and CD28 co-stimulation triggers the expression of both IL-2 and CD25,15,16 CD25 expression is further driven by an IL-2-mediated positive regulatory loop. As a result, sustained interaction between IL-2 and CD25 is important to drive T cells through cell cycle progression following activation.17 Whether the constitutive expression of CD25 confers any proliferative advantage on neonatal iNKT cells is unknown.
In this study, we investigated the functional consequences of constitutive expression of CD25 on neonatal iNKT-cell responses. We demonstrate that neonatal iNKT cells have a substantially reduced proliferation threshold compared with adult iNKT, conventional neonatal T or adult T cells. This lower proliferation threshold is independent of CD4 expression, intrinsic to neonatal iNKT cells and is most dramatic upon stimulation with low potency CD1d ligation, implying that this heightened proliferative capacity may critically help to maintain TCR diversity in the iNKT-cell compartment in limiting antigenic conditions. Finally, IL-2 blocking experiments suggest an important role for de novo IL-2 receptor α chain expression in bypassing the initial requirement for IL-2 in driving CD25 expression and lowering the proliferation threshold following activation. In light of these data, we propose a potential role for the constitutive expression of the high-affinity IL-2 receptor α chain in ensuring survival, stability and expansion of a structurally diverse antigenic receptor iNKT-cell repertoire early in life.
Materials and methods
Cells, reagents and antibodies
Blood samples were obtained following written informed consent from cord blood of neonates delivered at Children's & Women's Health Centre of BC (Vancouver, Canada) or from healthy adult peripheral control subjects. All cord or peripheral adult blood samples were collected in sodium heparin anti-coagulated Vacutainers™ (Becton Dickinson, Mississauga, ON, Canada) and processed within 1 hr of collection. Placental histology was reviewed for clinical signs of chorioamnionitis by our institutional clinical pathologist. This study protocol was approved by the University of British Columbia Clinical Research Ethic Board.
Flow cytometry staining antibodies against human CD3, CD19, CD45RO, CD62L, HLA-DR, CD25, CD69, CD122, CD127, CD4 and CD161 were purchased from BD Biosciences (Mississauga, ON, Canada). Flow cytometry antibodies against human FOXP3 and CD132 were purchased from eBioscience (San Diego, CA). OCH, and allophycocyanin- (APC) or phycoerythrin-conjugated, PBS57-loaded or unloaded CD1d MHC tetramers were supplied through a non-commercial contractual agreement with the National Institute of Health Tetramer Facility. α-Galactosylceramide (alpha-GC) was provided by Dr Peter van den Elzen (Child & Family Research Institute, Canada) and OKT3 (stimulating anti-CD3 antibody) was produced from hybridoma cells by the University of British Columbia antibody core facility. The concentration of PBS57-loaded CD1d-tetramers used for staining of iNKT cells was regularly determined to obtain maximal specific mean fluorescence intensity signal over background when comparing with an unloaded CD1d-tetramer molecule and was stable throughout the study period. The CD1d-transfected K562 myelogenous leukaemia cell line was generously supplied by Dr D. Branch Moody (Brigham and Women's Hospital, Boston, MA; unpublished data).
Mononuclear, T-cell and iNKT-cell purification
Cord blood or peripheral blood mononuclear cells were extracted from whole blood by Ficoll–Paque (Amersham, Baie d'Urfe, QC, Canada) gradient centrifugation. When excessive, the higher density cord blood reticulocytes remaining following the Ficoll–Paque gradient centrifugation were negatively depleted using CD235a (glycophorin A) microbeads (Miltenyi Biotech). Purified T cells were obtained by cell sorting on CD3+ cells from adult peripheral or cord blood mononuclear cells, using flow cytometry. The iNKT cells were obtained by magnetic bead separation (positive extraction on magnetic antibody cell sorting columns; Miltenyi Biotech, Auburn, CA) of APC-CD1d-PBS57-loaded tetramer-labelled mononuclear cells using anti-APC microbeads and subsequent cell sorting according to a CD3+PBS57-loaded CD1d-tetramer+ surface expression using flow cytometry. All cultures were carried out in RPMI-1640 medium (Invitrogen, Burlington, ON, Canada) containing 10% heat-inactivated fetal bovine serum (FBS; Fisher Scientific, Ottawa, ON, Canada), 100 units/ml penicillin and 100 mg/ml streptomycin (Invitrogen).
Activation and proliferation experiments
For CFSE proliferation experiments, mononuclear cells were stained with 2–10 μm carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich, Oakville, ON, Canada) for 5 min in phosphate-buffered saline (PBS)/5% FBS at 37° as described elsewhere.18 For antigen-presenting cell-free stimulation experiments, 1 × 105 T cells were cultured with (plate-bound) stimulating anti-CD3 (OKT3) ± anti-CD28 (BD Biosciences) ± recombinant IL-2 (eBioscience) ± anti-human IL-2 (clone MQ1-17H12; BD Biosciences) ± anti-CD25 (clone M-A251; BD Biosciences) as specified. Unless otherwise mentioned, the concentration of OKT3 used for these experiments was sub-saturating, as determined by proliferation among several neonatal and human donors, and to avoid excessive TCR down-regulation interfering with detection of cells (not shown). For T-cell suppression experiments, 5 × 104 T cells were stimulated with anti-CD3 (1 μg/ml) in the presence of 5 × 104 irradiated allogeneic feeder cells ± purified iNKT cells ± specified ratios of sorted CD19– CD25high CD4+CD3+ Treg cells as specified. Stimulation with CD1d-transfected K562 cells was carried out using 2·5 × 104 K562 cells cultured with 1 × 105 purified T cells in the presence of anti-human CD28 (2 μg/ml). For IL-2 inhibition experiments, 1 × 105 T cells were cultured with an excess of anti-human IL-2 antibody (corresponding to a fivefold greater concentration than required to completely inhibit detectable T-cell and neonatal iNKT-cell proliferation using CFSE) in the presence of anti-CD3, anti-CD28 ± recombinant IL-2 or anti-human IL-2 for the specified period of time. Following stimulation, cells were washed three times in Dulbecco's Phosphate Buffered Saline/2% FBS to remove excess antibody and re-cultured in the presence of anti-CD3, anti-CD28 and recombinant IL-2 at the same concentrations. After stimulation, cell viability was assessed by propidium iodide (PI) uptake in parallel experiments. Flow cytometry data acquisition was carried out on LSRII and FACSCalibur. Cell sorting was carried out on a FACSAria instruments (Becton Dickinson). Data were analysed using FlowJo (Tree Star Inc., Ashland, OR). CFSE time–courses were analysed as described.19 [3H]Thymidine incorporation was measured in similar proliferation conditions and was added in the final 12–18 hr of a 72-hr culture.
Polymerase chain reaction quantification of messenger RNA expression
For messenger RNA (mRNA) expression procedures were carried out at 4° and cells were collected in RNAprotect (Qiagen, Mississauga, ON, Canada) to minimize RNA degradation. Total mRNA was extracted using TRIzol (Invitrogen), from adult (resting) CD19– CD14– CD3+ CD25 T cells, neonatal or adult (CD19– CD14– CD3+ CD1d-PBS57-tetramer+) iNKT cells purified by cell sorting (> 98% purity), adult CD45RO+ CD25– memory T cells or sorted adult T cells activated using phytohaemagglutinin for 72 hr or using staphylococcal enterotoxin B for 5 days. Complementary DNA reverse transcription was carried out using the Superscript VILO cDNA synthesis kit (Invitrogen). Quantitative polymerase chain reaction (PCR) analyses were performed in triplicates using Express SYBR GreenER qPCR supermix (Invitrogen) according to the manufacturer's protocol on a 7300 real-time PCR instrument (Applied Biosystems Inc., Carlsbad, CA). Gene expression was normalized over expression of the housekeeping β-actin gene using the Livak method.20 The oligonucleotide sequences used for PCR amplification are provided in supplementary Table S1.
Results
Neonatal iNKT cells are highly abundant early in gestation and display a quiescent CD25+ memory T-cell phenotype
As previous studies addressing the phenotype and abundance of iNKT cells in cord blood were based on a limited number of human subjects,10,11 we first confirmed the frequency of iNKT cells at term, as well as earlier in gestation in mononuclear cells ex vivo (Fig. 1a). Invariant NKT cells were identified by flow cytometry using fluorescent antigen-loaded PBS57-CD1d-tetramers to detect cells within CD19– CD3+ cells. The proportion of neonatal iNKT cells in human neonatal (cord blood) mononuclear cells was 0·12% (95% confidence interval 0·10–0·15%; geometric mean; n = 53). Furthermore, by comparing a large number of donors in each age group, we found that this proportion was significantly higher in cord blood obtained from infants born after < 28 weeks of gestation and decreased towards the term of gestation and in adulthood, even more so in male compared with female subjects (P < 0·0004 by Kruskal–Wallis). CD25 expression was also detectable on iNKT cells much earlier in gestation, as indicated by staining cord blood mononuclear cells from preterm infants born early in the third trimester of pregnancy (< 28 weeks of gestation; Fig. 1b).
Figure 1.

Phenotype of invariant natural killer T (iNKT) cells in adults and in neonates born at term and before the term of gestation. (a) Proportion of CD1d-restricted iNKT cells in preterm (born below 28 weeks of gestation), term cord blood or adult (F: female; M: male) peripheral mononuclear percentages are, based on the number of CD19– CD1d-tetramer+ cells among CD3+ cells. Decrease with aging was statistically significant (P < 0.001 by Kruskal–Wallis test). (b) Expression of CD25 in neonatal iNKT cells from preterm neonates (representative from three donors tested). (c) Expression of interleukin-2 (IL-2) receptor components (CD122: β chain and CD132: common γ chain) (clear area) on neonatal iNKT cells from infants born at term. Fluorescence-minus-one negative staining controls (grey area) yielded results similar to isotype controls (not shown). (d) Krüppel-like factor 2 (KLF-2) and CD25 messenger RNA expression (real-time polymerase chain reaction) in T cells activated either with phytohaemagglutinin (PHA) or staphylococcal enterotoxin B (SEB) and sorted for positive CD25 expression, neonatal iNKT, adult resting memory (CD45RO+ CD25–) T cells and adult iNKT cells (error bars indicate SEM among up to three different donors tested per condition). Messenger RNA expression was normalized on levels measured in adult naive (CD25–) T cells.
Previous investigators showed that both neonatal and adult iNKT cells express the CD45RO isoform, indicating a memory T-cell phenotype.10,11 Data with regard to the expression of CD62 ligand (CD62L), which is also generally expressed on naive T cells, however, were conflicting.10–12,21 As the identification of iNKT cells requires at least three phenotypic surface markers in flow cytometry, potential for spectral cross-interference between fluorescent detectors may confound the determination of a positive surface expression. To address the question of CD62L expression on neonatal iNKT cells, cord blood cells were stained fresh ex vivo using antibodies against CD62L and CD45RO labelled with fluorescent markers having minimal emission overlap with one another (i.e. fluorescein isothiocyanate and phycoerythrin–Cy7, respectively) and no emission overlap with identification markers of other iNKT cells (i.e. APC-labelled CD1d-tetramer and Pacific Blue-labelled staining anti-CD3 antibody). Our results clearly demonstrate that neonatal iNKT cells expressed variable, but generally higher, levels of CD62L compared with adult iNKT cells (supplementary Fig. S1 and see Fig. 3 for more representative datasets out of 10 subjects tested). We also confirmed that neonatal iNKT cells express the IL-2 receptor common γ chain CD132 (Fig. 1c), normally expressed on resting T cells and necessary for IL-2 receptor signalling.22 On the other hand, expression of the IL-2 receptor β chain CD122 was undetectable in flow cytometry (Fig. 1c), even though the CD122 mRNA was detected by real-time PCR (not shown).
Figure 3.

Neonatal and adult invariant natural killer T (iNKT) cell CD127 and FOXP3 expression, and lack of suppressing effect of neonatal iNKT cells on polyclonal T-cell responses. (a) Expression of Foxp3, CD127 (IL-7 receptor α chain) in CD1d-tetramer+ CD3+ iNKT or T cells (middle four panels are gated according to upper left panel). Expression of CD25 in FOXP3/CD127-gated populations (right histograms). (b) Suppression of proliferation (measured by [3H]thymidine incorporation) in T cells stimulated by anti-CD3 in the presence of irradiated allogeneic antigen-presenting cells plus either a 1 : 1 ratio of neonatal iNKT cells or different ratios of regulatory T (Treg) : T cells. (c) Proliferation (CFSE dilution measured at 120 hr) in unstimulated T cells (shaded area; results were identical to T cells stimulated in presence of a 1 : 1 ratio of Treg cells) or in T cells stimulated by anti-CD3 in the presence of irradiated allogeneic antigen-presenting cells plus an equal ratio of neonatal iNKT cells (clear area).
As the pattern of expression of surface markers, including CD69 and HLA-DR,10,13 on neonatal iNKT cells is indicative of a resting rather than activated T-cell memory phenotype, we tested whether they expressed KLF-2, a transcription factor highly expressed in quiescent, non-proliferating T cells and down-regulated following activation.23 Pure (> 98%) populations of T or iNKT cells were obtained by flow cytometry cell sorting and mRNA expression was measured by real-time PCR. Neonatal iNKT cells clearly expressed ex vivo KLF-2 mRNA levels comparable to resting T (CD3+ CD25–) cells or resting memory (CD45+ CD25–) T cells. In contrast, T cells activated following exposure to phytohaemagglutinin or staphylococcal enterotoxin B and sorted by positive expression of CD25 expressed substantially less KLF-2 mRNA (Fig. 1d), consistent with previous reports.23 Altogether, our results demonstrate that iNKT cells are abundant earlier in gestation and display a quiescent, rather than activated memory T-cell phenotype.
Expression of CD25 on neonatal iNKT cells is observed earlier in gestation and is not the result of activation from labour
Invariant NKT cells are highly abundant at the materno–fetal placental interface and are potentially involved in the induction of labour.24,25 To exclude the possibility that expression of a memory T-cell phenotype could be the consequence of recent activation by labour, we analysed cord blood mononuclear cells collected from women undergoing elective ‘cold’ Caesarean section deliveries in the absence of clinically detectable labour. As shown in Fig. 2, iNKT cells from three representative donors in each group reproducibly expressed CD45RO, CD62L and CD25. Importantly, expression of CD45RO and CD25 on neonatal iNKT cells was comparable between neonates born by vaginal delivery following normal labour and neonates who were delivered by Caesarean section without labour and in the absence of documented placental infection (i.e. chorioamnionitis; Fig. 2). These results confirm that expression of CD25 is not the result of a recent activation by labour.
Figure 2.
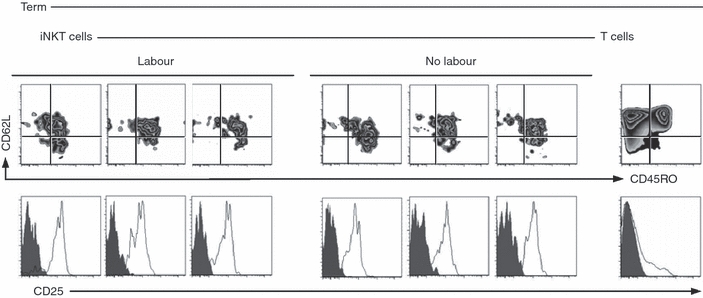
Phenotype of neonatal invariant natural killer T (iNKT) cells in the absence or presence of labour. Surface marker (clear area) and fluorescence-minus-one negative control staining (shaded area) for CD45RO, CD62L and CD25 expression on iNKT (expression in T cells shown as control) shown for three representative, out of five tested term neonates delivered naturally (labour) or by Caesarean section in absence of labour (no labour).
CD25+ neonatal iNKT cells are not functionally suppressive cells
Constitutive expression of CD25 on CD4+ T cells is correlated with immunoregulatory functions and expression of the transcriptional regulator FOXP3.26 We therefore investigated whether neonatal iNKT cells may share other phenotypic markers in common with Treg cells and suppress immune responses. CD25-expressing neonatal iNKT cells did not express FOXP3, normally highly expressed in Treg cells (Fig. 3a), but did express high levels of CD127 (the IL-7 receptor α chain), which is absent on Treg cells and also characteristic of naive T cells.27,28 To formally exclude the possibility that CD25-expressing neonatal iNKT cells are suppressive, CD3+ CD1d-tetramer+ neonatal iNKT cells were sorted and compared with different proportions of CD4+ CD25high Treg cells for their ability to suppress polyclonal T-cell responses. As shown in Fig. 3(b,c), even at a 1 : 1 ratio, neonatal iNKT cells did not suppress T-cell proliferation, but rather resulted in a 15-fold increase in proliferation. As expected, addition of Treg cells suppressed T-cell proliferation (Fig. 3b).
Enhanced proliferation threshold in neonatal iNKT cells
Immunological activation can be defined as a state of heightened capacity to proliferate and mediate effector functions. We tested whether CD25-expressing neonatal iNKT cells display a heightened proliferative capacity. To this end, we activated mononuclear cells with two prototypic CD1d glycolipid antigens: α-GC and OCH, which is less potent structural derivative of a-GC [29], and measured expression of CD69, used here as an early indicator of activation (Fig. 4a and supplementary Fig. S2a for a representative dataset), as well as late proliferation events detected using CFSE dilution (Fig. 4b and supplementary Fig. S2b for a representative dataset). When compared among donors, activation thresholds were similar between neonatal and adult iNKT cells (Fig. 4c). However, substantially lower amounts of agonists were required to trigger proliferation in neonatal iNKT cells compared with adult iNKT cells (Fig. 4d). As shown in Fig. 4(d), this difference was even more remarkable with OCH. Importantly, proliferation thresholds were comparable in both CD4+ and CD4– adult iNKT cells (Fig. 4e).
Figure 4.
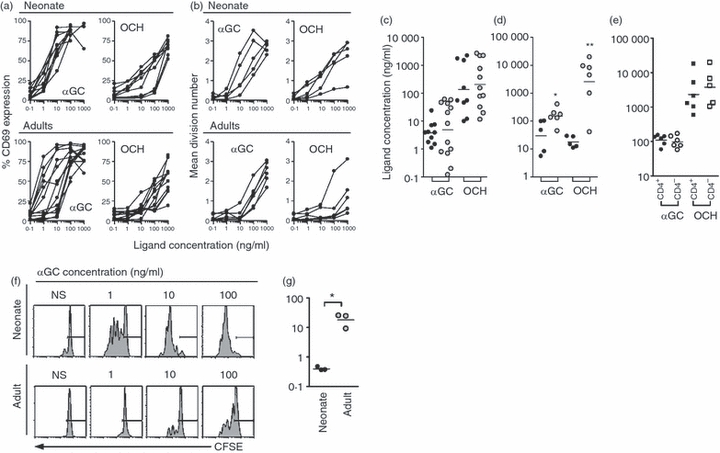
Activation and proliferation threshold in neonatal and adult invariant natural killer T (iNKT) cells. (a) Activation (measured by CD69 expression at 48 hr and presented as percentage CD69-expressing cells) and (b) proliferation (measured by CFSE dilution at 72 hr and presented as an average number of cell divisions) compared between neonatal and adult iNKT cells stimulated with increasing concentrations of α-galactosylceramide (α-GC) or OCH (each line represents a different donor). Concentration of ligand required to achieve half-maximal CD69 expression (c) or proliferation (d) in neonatal (dark circles) or adult (shaded circles) iNKT cells (bar represent median). (e) Proliferation threshold to α-GC or OCH in adult CD4+ and CD4– iNKT cells. (f) Representative CFSE-dilution profiles in CD3+ CD1d-tetramer+ iNKT cells stimulated with α-GC presented by the CD1d-expressing myeloid leukaemia cell line K562, in the presence of anti-CD28. Background stimulation by the equivalent non-CD1d-transfected cell line was < 10% (not shown). NS = unstimulated. (g) Concentration of α-GC required to achieve half-maximal proliferation in iNKT cells from neonatal (dark circles) or adult (shaded circles) donors. *P < 0.05 and **P < 0.01 by Mann–Whitney U-test.
Differences in iNKT-cell proliferation threshold were not the result of enhanced CD1d-restricted presentation by neonatal antigen-presenting cells or of a differential requirement for CD28 co-stimulation, as demonstrated using purified T cells from either neonatal or adult donors exposed to α-GC presented by a common MHC-negative, CD1d-transfected K562 myelogenous leukaemia cell line. Indeed, a substantially lower proliferation threshold was again seen in neonatal iNKT cells stimulated by α-GC in the presence of a soluble anti-CD28 antibody, when compared with their adult counterparts (Fig. 4f,g). A similar difference in proliferation threshold was also obtained in the absence of exogenous anti-CD28 co-stimulation, although proliferation was generally lower in both age groups (not shown).
Neonatal iNKT cells require de novo TCR/CD28 co-stimulation to proliferate
Substantial inter-donor variability was observed in iNKT-cell activation and proliferation thresholds (Fig. 4a–d), presumably because of intrinsic differences in avidity for CD1d-restricted antigens. To obviate some of this potential variability, we used an ‘antigen-presenting cell-independent’ assay. We reasoned that this system would allow better evaluation of whether this heightened proliferation threshold is ‘intrinsic’ to neonatal iNKT cells. Sorted T cells (including iNKT cells) were stimulated with plate-bound stimulating anti-CD3 (i.e. OKT3) and soluble anti-CD28, in the presence of an excess of recombinant IL-2 (50 U/ml) to avoid the effects of limiting IL-2 concentrations in the cell culture (Fig. 5a–d). In this system, we confirmed the strict requirement for TCR and CD28 co-stimulation in both neonatal and adult iNKT cells (Fig. 5a). Activated T cells express CD25 and readily proliferate in the presence of IL-2.30 We therefore investigated whether this may also hold true for neonatal iNKT cells. Addition of IL-2, however, did not stimulate neonatal iNKT cell proliferation (Fig. 5a). Notably, iNKT cell proliferation was strictly dependent on CD25, as shown by a complete inhibition of proliferation in cells stimulated in the presence of a blocking anti-human CD25 antibody (Fig. 5a). Using CFSE-time series models,19,31 we detected no statistically significant difference in the time of initiation of the first cell division or rate of subsequent divisions (P > 0·05 between trend lines, by linear regression) between neonatal iNKT, adult iNKT and T cells (Fig. 5b). However, a significantly greater proportion of neonatal iNKT cells reproducibly progressed into cell cycle in comparison with adult T, iNKT or neonatal T cells (Fig. 5c; results are representative of five donors tested in each age group). Notably, we also confirmed the substantially greater proliferation in neonatal iNKT compared to adult iNKT, neonatal T or adult T cells by measuring thymidine incorporation in purified cell sub-populations (Fig. 5d).
Figure 5.

T-cell receptor/CD28 co-stimulation requirements and comparison of proliferation in neonatal invariant natural killer T (iNKT), adult iNKT and T cells. (a) T cells obtained from either a neonate or an adult were cultured (120 hr) in the absence of stimulation (unstim) or exposed to anti-CD3, interleukin-2 (IL-2) only, anti-CD3 and anti-CD28 stimulation ± an excess recombinant IL-2 (50 U/ml) or anti-CD3 and anti-CD28 stimulation in the presence of a blocking anti-CD25 antibody (gated on iNKT cells). (b) Time of initiation (intercept of line of best fit at mean division one) and rate of cell division (slope of line of best fit) in neonatal iNKT (dark grey circles), adult iNKT (light grey circles), neonatal T (dark grey squares) or adult T (light gray squares) cells. (c) Proportion of proliferating (CFSElow) iNKT cells or T cells (at 72 hr) following stimulation with anti-CD3 and anti-CD28. Results of panel (a), (b) and (c) are representative of five donors in each age group. (d) [3H]Thymidine incorporation (72 hr) in sorted neonatal (dark bars) and adult (grey bars) iNKT or T cells stimulated by anti-CD3 and anti-CD28 in the presence or absence of IL-2. Cells (5000/condition) were tested in duplicates (bars represent SEM). Results of (d) are representative of two independent experiments.
‘Priming’ of neonatal iNKT cells due to de novo CD25 expression
Antigen-driven activation and proliferation of T cells generally occurs in two IL-2-dependent steps. First, continuous engagement of the TCR and CD28 molecules is required to drive the production of both IL-2 and CD25 expression (referred to here as the early induction phase of proliferation). Second, continuous exposure to IL-2 interacting with the IL-2 receptor complex (inclusive of CD25 molecule), drives T cells through the cell cycle – referred to herein as the maintenance phase of proliferation; see ref.14 In the early induction phase of proliferation, IL-2 is also critical in driving CD25 expression, therefore acting as a positive-regulator of proliferation.32 To test whether de novo CD25 expression on neonatal iNKT cells could obviate the early requirement for IL-2 in driving cells into cell cycle, we stimulated T cells with plate-bound anti-CD3 and soluble anti-CD28 and measured the effect of early versus late IL-2 inhibition on the induction phase of proliferation. Expression of CD25 increased in both neonatal and adult iNKT in response to CD3/CD28 co-stimulation and was partially blocked by an anti-IL-2 blocking antibody added at the time of stimulation (Fig. 6a). As expected, we only detected proliferation (i.e. CFSE dilution) in CD25-expressing cells (not shown).
Figure 6.
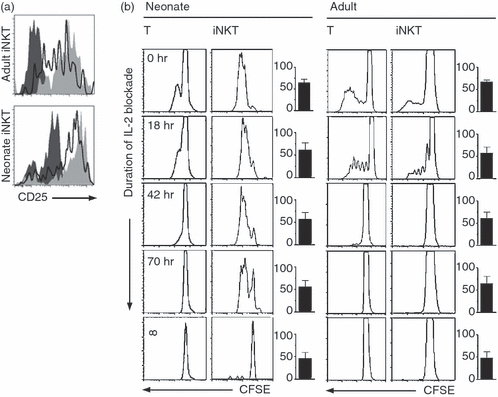
Importance of CD25 expression and effect of early interleukin-2 (IL-2) blocking during the induction phase of invariant natural killer T (iNKT) cell proliferation. (a) CD25 expression (at 48 hr) in unstimulated (dark grey area) or stimulated (light grey area) neonatal and adult iNKT cells is partially blocked by an anti-IL-2 antibody (clear area). (b) Stimulation in presence of anti-IL-2 washed out after the specified number of hours (∞ = control condition where the antibody was not washed out; bottom four panels) abrogated proliferation in neonatal or adult T cells, but not in neonatal iNKT cells. CFSE histograms are gated on live CD3+ CD1d– (T) or CD3+ CD1d+ (iNKT) cells at 120 hr of stimulation. Bar graphs represent overall percentage of viable cells in each condition. Proliferation was identical in cells stimulated in presence of recombinant IL-2, or in presence of anti-IL-2 washed immediately after its addition (not shown). Results are representative of four independent experiments in each age group.
More importantly, proliferation could be completely inhibited by IL-2 blockade in neonatal iNKT cells as well as in T and adult iNKT-cell cultures (Fig. 6c, bottom panels), confirming the importance of IL-2 in driving T cells into cell cycle during the induction phase of proliferation. Remarkably, blocking of IL-2 only during the induction phase of proliferation (i.e. by washing out the IL-2 blocking antibody after a specific time period, followed by exposure to recombinant IL-2) completely inhibited proliferation in T and adult iNKT cells, but not in neonatal iNKT cells (Fig. 6c). These data, which are representative of four donors tested in each age group, confirmed that IL-2 is required early in the induction phase of proliferation in both T and adult iNKT cells, but not in neonatal iNKT cells.
Discussion
Natural killer T cells play an important immune regulatory role in autoimmune diseases and malignancies, although their ontogeny in humans is insufficiently understood. In this study, we expand on previous findings that neonatal NKT cells constitutively express the high-affinity IL-2 receptor α chain CD25. However, in these earlier studies authors have not elucidated the functional impact of this CD25-expressing phenotype and in the absence of this important data, may have precipitously concluded that this phenotype simply reflected activation from a previous encounter with an undefined endogenous ligand in utero.10,11 CD1d is expressed on human fetal trophoblastic placental cells and iNKT cells are abundant at the decidual materno–fetal interface, comprising about 0·5% of T cells.24 Stimulation of iNKT cells by α-GC during pregnancy triggers cytokine-mediated CD1d-dependent fetal abortion in C57BL/6J mice.25 Although the role of iNKT cells in the physiology of normal labour is not entirely elucidated, the possibility that CD25 expression on neonatal iNKT could be related to activation in this context needed to be addressed. Alternatively, iNKT-cell activation might have also been the result of activation by ascending intrauterine micro-organisms, which are well-reported to be associated with labour.25,33 CD25 expression in neonatal iNKT cells, however, is clearly not the result of labour or placental infection, as evidenced by a consistent expression in cord blood samples obtained from either preterm or full-term placenta without evidence of labour or detectable inflammation in the mother.
We demonstrate that neonatal NKT cells, in fact, are not activated in spite of their constitutive CD25 expression. It is not clear what drives the constitutive expression of CD25 on neonatal iNKT cells. Nonetheless, neonatal iNKT cells present several features that make their phenotype clearly distinct from other CD25-expressing activated T cells and Treg cells. These features include the absence of detectable proliferation upon exposure to IL-2 alone, a strict requirement for co-stimulation of the TCR and CD28 for activation and proliferation de novo, the expression of other quiescent T-cell markers CD127, CD62L and the transcription factor KLF-2, the absence of other markers of recent activation, such as CD69 and HLA-DR10,13 and finally, the lack of suppression of polyclonal T-cell responses. Altogether, these findings are more consistent with the theory that expression of CD25 by iNKT cells reflects a distinct early life developmental stage.
Despite this non-activated state, CD25-expressing neonatal iNKT cells proliferate with substantially lower antigenic stimulation following activation, thereby attenuating avidity differences observed among adult iNKT cells. Cord blood T cells are largely naive and generally require greater antigenic stimulation to proliferate compared with adult peripheral blood T cells.34,35 Therefore, the heightened proliferation threshold in neonatal iNKT clearly distinguishes them from other cord blood T cells. Both antigenic receptor affinity maturation and structural reorganization of the antigenic receptor mechanism contribute to lowering the activation threshold in (secondary) memory T-cell responses.36 However, the heightened proliferation threshold in neonatal iNKT is also clearly independent of their CD45RO-expressing memory T-cell phenotype.
The biological impact of the heightened proliferation threshold in neonatal iNKT cells is not clear. Neonatal iNKT cells display a more plastic cytokine programme at birth and generally show a diverse antigenic receptor repertoire.10,12,37 In the presence of limiting antigen stimulation, this mechanism may be particularly important in a rapidly growing fetus or neonate in humans to facilitate the expansion of lower affinity iNKT-cell clones and ensure stability in the iNKT antigenic repertoire upon repeated antigenic challenge. Also, it is not known whether a certain degree of diversity in the iNKT-cell repertoire is required for maintaining immunological function, although there is evidence for increased relapses of multiple sclerosis in subjects with a more limited iNKT-cell repertoire.38
Our findings that both neonatal and adult iNKT cells display similar activation thresholds, but that a greater proportion of neonatal iNKT cells proliferate following activation is in keeping with the existing role for early, sustained IL-2/CD25 interactions in the induction of T-cell proliferation. Although we cannot completely exclude that other unidentified factors might contribute to lowering the proliferation threshold in neonatal iNKT cells, our findings are highly indicative that their increased antigenic sensitivity is primarily the result of a constitutive CD25 expression. The importance of sustained exposure to IL-2 in the early phase of proliferation is further demonstrated in vitro in models showing a lower proportion of precursor cells undergoing cell cycle and a slower rate of subsequent divisions in the presence of limiting IL-2 concentrations.17 In humans, CD25 expression is also critical to T-cell proliferation in vivo, as evidenced by markedly reduced polyclonal T-cell responses in CD25-deficient patients.39,40 Following induction of proliferation, high levels of CD25 are detectable in both neonatal and adult iNKT cells, likely explaining why we did not detect significant differences in division rates after initiation of the cell cycle.
High levels of CD25 expression were comparably detectable in both CD4+ and CD4– neonatal iNKT cells (data not shown), implying a similarly low threshold for proliferation between the two iNKT subsets. Because of the very low abundance of CD4– iNKT cells in cord blood, we were not able to accurately determine proliferation thresholds in neonatal CD4– iNKT cells, although we clearly demonstrate that both adult CD4+ and CD4– iNKT-cell subsets displayed comparable proliferation thresholds, as also reported by others.8 Therefore the heightened proliferation threshold we report is independent of a CD4-expressing phenotype and is not the result of an age-related decline in CD4+ : CD4– iNKT-cell ratios. Given a structurally restricted combinatorial iNKT-cell receptor rearrangement, CD25 expression may be important for repertoire stability, particularly in the CD4– iNKT-cell subset and which appears to primarily expand through peripheral homeostatic proliferation.
Remarkably, CD25 expression has also been detected in a high proportion of circulating fetal T cells, suggesting that this phenotype may predominate in other early life T-cell subsets.41,42 Parallels can be drawn with a recently identified subset of polyclonal CD25+ CD45RO+ CD8+ memory T cells presumably constituting a peripheral reservoir of antigenic receptor diversity in aging individuals with reduced thymic output.43,44 However, a major phenotypic difference with neonatal iNKT cells is the fact that the latter do not spontaneously divide in the presence of IL-2 alone.44 This lack of spontaneous response to IL-2 in the absence of TCR stimulation may reflect a low IL-2R β chain expression which is essential for IL-2 receptor signalling.14 Alternatively, IL-2 receptor signalling may be functionally silenced in resting neonatal iNKT cells.
In conclusion, we demonstrate that CD25-expressing neonatal iNKT cells are able to proliferate with a remarkably reduced antigenic threshold following activation. Our experiments further indicate a role for the constitutive CD25 expression in priming neonatal iNKT cells to sidestep the initial IL-2 requirement and proliferate with remarkably greater sensitivity following TCR activation. Further studies are required to clarify the role of this unique phenotype in iNKT-cell ontogeny and its significance in human health and diseases.
Acknowledgments
We thank Mrs Chandra Pham and Kristi Finlay for help with cord blood collection and neonatal subject recruitment, Laura Sly for scientific reviewing, Amanda Bonnell for editing of the manuscript, Dong Jun Zhang and Dr Peter van den Elzen for preparation of α-galactosylceramide, and the NIH Tetramer Facility for providing the CD1d-restricted MHC tetramers. This study was funded (in part) by grants from the Division of Neonatology, Child & Family Research Institute, the British Columbia Lung Association (to P.M.L.) and CIHR (HOP 57834 to M.K.L.). P.M.L. acknowledges support from the Child & Family Research Institute and Canadian Institutes of Health Research's – Canadian Child Health Clinician Scientist Program. M.K.L. is a Canada Research Chair in Transplantation and an MSFHR Scholar. A.Y.W. holds a Canada Graduate Award and a CIHR Training Program in Transplantation fellowship.
Glossary
Abbreviations:
- APC
allophycocyanin
- FBS
fetal bovine serum
- α-GC
α-galactosylceramide
- IL-2
interleukin-2
- iNKT
inducible natural killer T
- KLF-2
Krüppel-like factor 2
- PCR
polymerase chain reaction
- TCR
T-cell receptor
- Tregs
regulatory T cells
Disclosures
The authors disclose no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Oligonucleotide sequences used for gene expression quantification by real-time polymerase chain reaction.
Figure S1. CD45RO and CD62L expression in adult inducible natural killer T (iNKT), neonatal T or iNKT cells. Expression of both markers were compared to staining controls using the same combination of fluorescently labelled antibodies except for inclusion of isotype controls (fluorescent-minus one plus isotype control).
Figure S2. Representative data set used to calculate thresholds for CD69 induction or proliferation (using CFSE dilution) in inducible natural killer T (iNKT) cells. Peripheral or cord blood mononuclear cells were stimulated with graded concentration of a-galactosylceramide or OCH. The proportion of (a) CD69-expressing or (b) proliferating cells in each division from a normal fit of data were determined using FLOWJO (Tree Star, Inc., Ashland, OR).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4–8– T cells. J Exp Med. 1994;180:1171–6. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Cava A, Van Kaer L, Fu Dong S. CD4+ CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–7. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.de Lalla C, Festuccia N, Albrecht I, et al. Innate-like effector differentiation of human invariant NKT cells driven by IL-7. J Immunol. 2008;180:4415–24. doi: 10.4049/jimmunol.180.7.4415. [DOI] [PubMed] [Google Scholar]
- 4.Fowlkes BJ, Kruisbeek AM, Ton-That H, Weston MA, Coligan JE, Schwartz RH, Pardoll DM. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly expresses a single Vβ gene family. Nature. 1987;329:251–4. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- 5.Sandberg JK, Stoddart CA, Brilot F, Jordan KA, Nixon DF. Development of innate CD4+α-chain variable gene segment 24 (Vα24) natural killer T cells in the early human fetal thymus is regulated by IL-7. Proc Natl Acad Sci U S A. 2004;101:7058–63. doi: 10.1073/pnas.0305986101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J Exp Med. 2002;195:637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, Metelitsa LS. Distinct homeostatic requirements of CD4+ and CD4– subsets of Vα24-invariant natural killer T cells in humans. Blood. 2004;104:4150–6. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- 9.Hager E, Hawwari A, Matsuda JL, Krangel MS, Gapin L. Multiple constraints at the level of TCRα rearrangement impact Vα14i NKT cell development. J Immunol. 2007;179:2228–34. doi: 10.4049/jimmunol.179.4.2228. [DOI] [PubMed] [Google Scholar]
- 10.D’Andrea A, Goux D, De Lalla C, et al. Neonatal invariant Vα24+ NKT lymphocytes are activated memory cells. Eur J Immunol. 2000;30:1544–50. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.van Der Vliet HJ, Nishi N, de Gruijl TD, von Blomberg BM, van den Eertwegh AJ, Pinedo HM, Giaccone G, Scheper RJ. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood. 2000;95:2440–2. [PubMed] [Google Scholar]
- 12.Eger KA, Sundrud MS, Motsinger AA, Tseng M, Kaer LV, Unutmaz D. Human natural killer T cells are heterogeneous in their capacity to reprogram their effector functions. PLoS ONE. 2006;1:e50. doi: 10.1371/journal.pone.0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prussin C, Foster B. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159:5862–70. [PubMed] [Google Scholar]
- 14.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 15.Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–6. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 16.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–42. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 17.Deenick EK, Gett AV, Hodgkin PD. Stochastic model of T cell proliferation: a calculus revealing IL-2 regulation of precursor frequencies, cell cycle time, and survival. J Immunol. 2003;170:4963–72. doi: 10.4049/jimmunol.170.10.4963. [DOI] [PubMed] [Google Scholar]
- 18.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–56. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF, Hodgkin PD. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat Protoc. 2007;2:2057–67. doi: 10.1038/nprot.2007.297. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Loza MJ, Metelitsa LS, Perussia B. NKT and T cells: coordinate regulation of NK-like phenotype and cytokine production. Eur J Immunol. 2002;32:3453–62. doi: 10.1002/1521-4141(200212)32:12<3453::AID-IMMU3453>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Perola O, Ripatti A, Pelkonen J. T-lymphocyte subpopulations do not express identical combinations of interleukin-2 receptor chains in the early phase of their activation and proliferation. Scand J Immunol. 2000;52:123–30. doi: 10.1046/j.1365-3083.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuo CT, Veselits ML, Leiden JM. LKLF: a transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–90. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 24.Boyson JE, Rybalov B, Koopman LA, et al. CD1d and invariant NKT cells at the human maternal–fetal interface. Proc Natl Acad Sci U S A. 2002;99:13741–6. doi: 10.1073/pnas.162491699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito K, Karasawa M, Kawano T, et al. Involvement of decidual Vα14 NKT cells in abortion. Proc Natl Acad Sci U S A. 2000;97:740–4. doi: 10.1073/pnas.97.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levings MK, Sangregorio R, Roncarolo MG. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee A, Farrand KJ, Dickgreber N, Hayman CM, Jurs S, Hermans IF, Painter GF. Novel synthesis of α-galactosyl-ceramides and confirmation of their powerful NKT cell agonist activity. Carbohydr Res. 2006;341:2785–98. doi: 10.1016/j.carres.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–4. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 31.Lavoie PM, Dumont AR, McGrath H, Kernaleguen AE, Sekaly RP. Delayed expansion of a restricted T cell repertoire by low-density TCR ligands. Int Immunol. 2005;17:931–41. doi: 10.1093/intimm/dxh273. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 33.Newton ER. Preterm labor, preterm premature rupture of membranes, and chorioamnionitis. Clin Perinatol. 2005;32:571–600. doi: 10.1016/j.clp.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Bussel JB, Cunningham-Rundles S, LaGamma EF, Shellabarger M. Analysis of lymphocyte proliferative response subpopulations in very low birth weight infants and during the first 8 weeks of life. Pediatr Res. 1988;23:457–62. doi: 10.1203/00006450-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Hassan J, Reen DJ. Reduced primary antigen-specific T-cell precursor frequencies in neonates is associated with deficient interleukin-2 production. Immunology. 1996;87:604–8. doi: 10.1046/j.1365-2567.1996.476587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farber DL. T cell memory: heterogeneity and mechanisms. Clin Immunol. 2000;95:173–81. doi: 10.1006/clim.2000.4858. [DOI] [PubMed] [Google Scholar]
- 37.Kadowaki N, Antonenko S, Ho S, Rissoan MC, Soumelis V, Porcelli SA, Lanier LL, Liu YJ. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193:1221–6. doi: 10.1084/jem.193.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demoulins T, Gachelin G, Bequet D, Dormont D. A biased Vα24+ T-cell repertoire leads to circulating NKT-cell defects in a multiple sclerosis patient at the onset of his disease. Immunol Lett. 2003;3:223–8. doi: 10.1016/j.imlet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Roifman CM. Human IL-2 receptor α chain deficiency. Pediatr Res. 2000;48:6–11. doi: 10.1203/00006450-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–7. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Jenkinson EJ, Kingston R, Owen JJ. Importance of IL-2 receptors in intra-thymic generation of cells expressing T-cell receptors. Nature. 1987;329:160–2. doi: 10.1038/329160a0. [DOI] [PubMed] [Google Scholar]
- 42.Moretta A, Valtorta A, Chirico G, Chiara A, Bozzola M, De Amici M, Maccario R. Lymphocyte subpopulations in preterm infants: high percentage of cells expressing P55 chain of interleukin-2 receptor. Biol Neonate. 1991;59:213–8. doi: 10.1159/000243346. [DOI] [PubMed] [Google Scholar]
- 43.Herndler-Brandstetter D, Veel E, Laschober GT, et al. Non-regulatory CD8+ CD45RO+ CD25+ T-lymphocytes may compensate for the loss of antigen-inexperienced CD8+ CD45RA+ T-cells in old age. Biol Chem. 2008;389:561–8. doi: 10.1515/bc.2008.052. [DOI] [PubMed] [Google Scholar]
- 44.Herndler-Brandstetter D, Schwaiger S, Veel E, et al. CD25-expressing CD8+ T cells are potent memory cells in old age. J Immunol. 2005;175:1566–74. doi: 10.4049/jimmunol.175.3.1566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


