Abstract
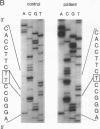
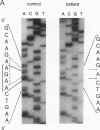
Argininemia results from a deficiency of arginase (EC 3.5.3.1), the last enzyme of the urea cycle in the liver. We examined the molecular basis for argininemia by constructing a genomic library followed by cloning and DNA sequencing. Discrete mutations were found on two alleles from the patient, a product of a nonconsanguineous marriage. There was a four-base deletion at protein-coding region 262-265 or 263-266 in exon 3 that would lead to a reading-frame shift after amino acid residue 87 and make a new stop codon at residue 132. The other was a one-base deletion at 77 or 78 in exon 2 that would lead to a reading-frame shift after residue 26 and make a stop codon at residue 31. For confirmation, genomic DNAs from the patient and from her parents were amplified by the polymerase chain reaction method. The patient was shown to be a compound heterozygote, inheriting an allele with the four-base deletion from the father and the other allele with the one-base deletion from the mother. These data seem to be the first evidence of a case of argininemia caused by two different deletion mutations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernar J., Hanson R. A., Kern R., Phoenix B., Shaw K. N., Cederbaum S. D. Arginase deficiency in a 12-year-old boy with mild impairment of intellectual function. J Pediatr. 1986 Mar;108(3):432–435. doi: 10.1016/s0022-3476(86)80891-5. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. D., Knox W. E. Arginase isozymes of rat mammary gland, liver, and other tissues. J Biol Chem. 1973 Aug 25;248(16):5785–5789. [PubMed] [Google Scholar]
- Grody W. W., Argyle C., Kern R. M., Dizikes G. J., Spector E. B., Strickland A. D., Klein D., Cederbaum S. D. Differential expression of the two human arginase genes in hyperargininemia. Enzymatic, pathologic, and molecular analysis. J Clin Invest. 1989 Feb;83(2):602–609. doi: 10.1172/JCI113923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi Y., Takiguchi M., Amaya Y., Kawamoto S., Matsuda I., Mori M. Molecular cloning and nucleotide sequence of cDNA for human liver arginase. Proc Natl Acad Sci U S A. 1987 Jan;84(2):412–415. doi: 10.1073/pnas.84.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi Y., Takiguchi M., Matsuda I., Mori M. Sequence heterogeneity of human liver arginase cDNAs and restriction fragment length polymorphism of the gene locus. Jinrui Idengaku Zasshi. 1988 Sep;33(3):305–313. doi: 10.1007/BF02032860. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Raper S. M. The heterogeneity of arginases in rat tissues. Biochem J. 1976 Feb 1;153(2):469–478. doi: 10.1042/bj1530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Amaya Y., Murakami K., Tokunaga F., Iwanaga S., Kobayashi K., Saheki T., Kimura S., Mori M. Complete nucleotide sequence of cDNA and deduced amino acid sequence of rat liver arginase. J Biol Chem. 1987 May 5;262(13):6280–6283. [PubMed] [Google Scholar]
- Matsuda I., Yamamoto J., Nagata N., Ninomiya N., Akaboshi I. Lysosomal enzyme activities in cultured lymphoid cell lines. Clin Chim Acta. 1977 Nov 1;80(3):483–486. doi: 10.1016/0009-8981(77)90141-3. [DOI] [PubMed] [Google Scholar]
- Ohtake A., Takiguchi M., Shigeto Y., Amaya Y., Kawamoto S., Mori M. Structural organization of the gene for rat liver-type arginase. J Biol Chem. 1988 Feb 15;263(5):2245–2249. [PubMed] [Google Scholar]
- RATNER S., MORELL H., CARVALHO E. Enzymes of arginine metabolism in brain. Arch Biochem Biophys. 1960 Dec;91:280–289. doi: 10.1016/0003-9861(60)90502-6. [DOI] [PubMed] [Google Scholar]
- Reddi P. K., Knox W. E., Herzfeld A. Types of arginase in rat tissues. Enzyme. 1975;20(5):305–314. doi: 10.1159/000458952. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes R. S., Dizikes G. J., Klisak I., Grody W. W., Mohandas T., Heinzmann C., Zollman S., Lusis A. J., Cederbaum S. D. The gene for human liver arginase (ARG1) is assigned to chromosome band 6q23. Am J Hum Genet. 1986 Aug;39(2):186–193. [PMC free article] [PubMed] [Google Scholar]
- Spector E. B., Rice S. C., Cederbaum S. D. Immunologic studies of arginase in tissues of normal human adult and arginase-deficient patients. Pediatr Res. 1983 Dec;17(12):941–944. doi: 10.1203/00006450-198312000-00003. [DOI] [PubMed] [Google Scholar]
- TOMLINSON S., WESTALL R. G. ARGININOSUCCINIC ACIDURIA. ARGININOSUCCINASE AND ARGINASE IN HUMAN BLOOD CELLS. Clin Sci. 1964 Apr;26:261–269. [PubMed] [Google Scholar]
- Takiguchi M., Haraguchi Y., Mori M. Human liver-type arginase gene: structure of the gene and analysis of the promoter region. Nucleic Acids Res. 1988 Sep 26;16(18):8789–8802. doi: 10.1093/nar/16.18.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi M., Murakami T., Miura S., Mori M. Structure of the rat ornithine carbamoyltransferase gene, a large, X chromosome-linked gene with an atypical promoter. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6136–6140. doi: 10.1073/pnas.84.17.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]