Abstract
An 8-month-old child with an immunodeficiency disorder characterized by abnormal lymphocyte function and by low IgG and IgA levels had combined liver and small bowel transplantation under tacrolimus and steroid immunosuppression for the treatment of short gut syndrome and hepatic cirrhosis. The patient developed an early postoperative episode of Pnemocystis carinii pneumonia, and a subsequent surgical complication, prompting discontinuance of tacrolimus. A skin rash eventually shown to be graft-versus-host disease (GVHD) developed in the flank on the 12th post-transplant day and gradually became generalized. Peritonitis, sepsis, multisystem organ failure including the liver allograft led to death on the 23rd post-operative day. The mechanisms leading to post-transplant GVHD under the specific circumstances in this case are discussed.
Keywords: short gut syndrome, immnodeficiency, small bowel transplantation, graft versus host disease, tacrolimus
Graft-versus-host disease (GVHD) is a complex disorder that occurs after alloactivation of transplanted immunocompetent donor lymphoid cells to tissues of the recipient. The major targets are epithelial cells of the skin, intestine and liver. Under conditions of recipient cytoablation, this complication develops after allogeneic bone marrow transplantation with variable severity in 40 to 80% of recipients even if the donor has a good MHC match (1). GVHD under the treatment circumstances of whole-organ transplantation has been uncommon, although not rare (2).
Fear that GVHD would preclude transplantation of lymphoid-rich MHC disparate intestinal allograft was instilled by experiments with the parent-to-offspring F1 hybrid rat model in which the recipient could not reject the graft, but was vulnerable to invariably lethal GVHD (3). The unchallenged impression left by these results for many subsequent years was that GVHD was going to be a critical and perhaps non-resolvable management problem in clinical cases. Additional anxieties concerned the transplantation of gastrointestinal contents, enclosed in a viscus which if damaged would be the inevitable nidus for uncontrolled sepsis.
The consequent widespread pessimism about clinical application of intestinal transplantation was abruptly changed between 1989 and 1992 by 2 factors. The first was the emergence of FK506 (now tacrolimus) as a superior immunosuppressant with which rat bowel alone, or as part of a multi-visceral complex, could be transplanted with routine success (4). The second was the discovery of donor leukocytes in the skin, lymph nodes, blood, and other locations in human recipients of various organ allografts as long as 30 yr post-transplantation (2). It was postulated (the 2-way paradigm) that the 2 cell populations reciprocally modulated immune responsiveness, including the induction of mutual non-reactivity as the basis of organ allograft acceptance (5).
Appreciation of the duality of the immune reaction following organ transplantation (HVG and GVH) allowed the expectation that intestinal transplantation would be feasible clinically without a major risk of GVHD providing neither of the “cancelling” arms was cytoablated [including the allograft (6)] and if tacrolimus-based immunosuppression was given. This prediction has been realized, and has allowed the rapid growth of intestinal and multi visceral transplantation (7). However, we report here the first example of full-blown GVHD in a liver/intestinal recipient. The patient, whose immune system was weakened by a pre-existing immune deficiency state, was further imperilled by the inability to give adequate immunosuppression following a surgical complication.
Case report
A white female offspring of unrelated parents with no family history of anomalies or immune deficiency presented with a large duodenal cyst and complete intestinal atresia from the ligament of Treitz to the sigmoid colon. She was supported with total parenteral nutrition (TPN) and subsequently developed multiple episodes of catheter sepsis (enterococcus, Staphylococcus aureus, and Serratia marcescens), pseudomonas pneumonia and eventually cirrhosis. Persistent lymphopenia prompted an immunologic work-up at 4 months of age (Table 1). Antibody responses to protein antigens were not assessed, but tests for HIV and adenosine deaminase deficiency were negative.
Table 1.
Immunolgic work-up at 4 months of age*
| Values Test | Patient’s Value | Normal Age Adjusted |
|---|---|---|
| Quantitative Immunoglobulin | ||
| IgG | 81 mg/dl | 192–668 |
| IgA | 5 mg/dl | 11–92 |
| IgM | 20 mg/dl | 9–73 |
| Adenosine deaminase | 81 nM/mgHb/H | 20–70 |
| WBC | 20.6 × 1 000/mm3 | 6 000–17 500 |
| Total lymphocytes | 15% | 33–63% |
| = 3090/mm3 | 1 980–11 025 | |
| Total T-cells | 62% | 62–86% |
| = 1916/mm3 | 612–2572 | |
| Suppressor cells | 30% | 14–38% |
| = 927/mm3 | 133–969 | |
| Helper cells | 70% | 35–59% |
| = 2163/mm3 | 288–1 736 | |
| Helper/Suppressor ratio | 2.33 | 0.71–3.23 |
| Total B-cells | 12% | 3–27% |
| = 371/mm3 | 0–648 | |
| Mitogen studies | Patient | Control |
| PHA: | ||
| AB plasma | 270512 CPM’s | 8767 CPM’s |
| Autologous plasma | 48829 CPM’s | 6225 CPM’s |
| PWM: | ||
| AB plasma | 612 CPM’s | 605 CPM’s |
Note the low serum IgG and IgA concentrations. The phytohemagglutinin (PHA) results were consistent with presence of an inhibitory substance of PHA mitogenic response in plasma
On August 8, 1991, at the age of 8 months, an enblock liver and intestinal transplant was performed (6–8) following 7 d of antibiotic preparation. The donor was an 8-d-old male of the same blood type (0), weighting 3.4 kg. The recipient’s HLA antigens were A1, B8, BW6, DR1, DR3, DQW1, DQW2, DRW53. The donor’s antigens were A24, A31, B35, B39, BW6, DR4, DR12, DQW3, DRW52, DRW53. The lymphocytotoxic crossmatch was negative. Intraoperative blood loss was 500 ml which was replaced with packed red blood cells. Immunosuppression included intravenous tacrolimus 0.15 mg/kg/d and methylprednisolone at 1.25 mg/kg/d.
Figure 1 outlines significant postoperative events and immunosuppressive management. The postoperative course was uneventful until the 4th postoperative day (POD) when she was diagnosed with Pneumocystis carinii pneumonia (PCP) and treated with intravenous trimethoprim/sulfamethoxazole. Intravenous immunoglobulin also was administered because of the IgG deficiency. A liver biopsy on day 5 showed ischemic damage and extra-medullary hematopoiesis but no evidence of rejection. Methylprednisolone was stopped, and the tacrolimus dose was decreased. On the 8th postoperative day, the duodenojejunal anastomosis leaked and was revised. When an intraoperative biopsy of the intestinal allograft showed only ischemic injury, immunosuppression was stopped.
Fig. 1.
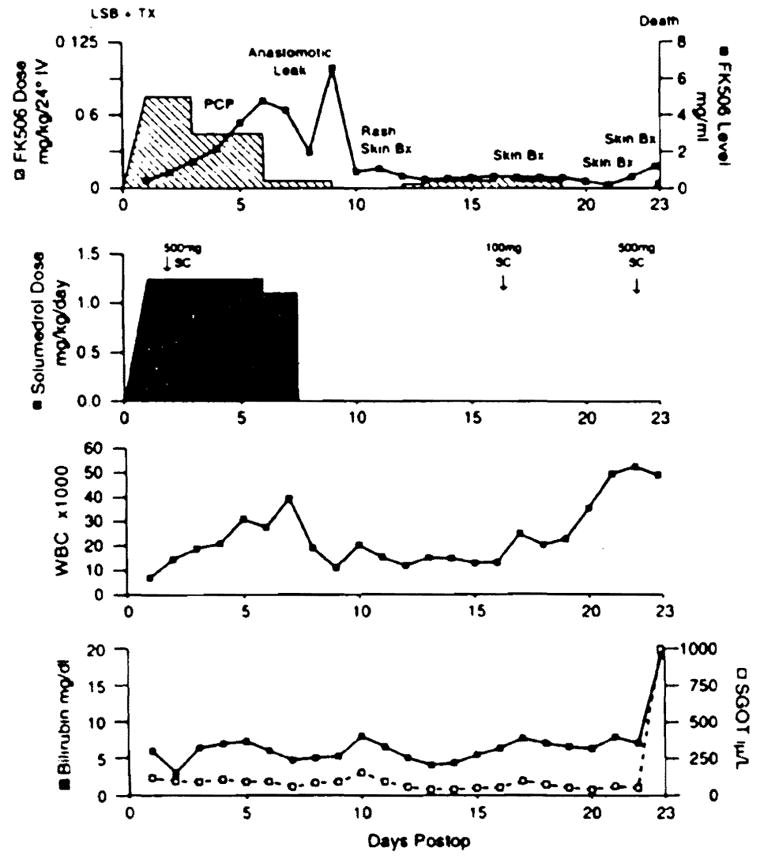
The complex clinical course in this patient was further compounded by an early surgical complication with peritonitis and sepsis. The subsequent deviation from immunosuppressive protocol as seen in this figure precipitated the subsequent immunologic events. LSB TX: Liver/Small Bowel Transplant; PCP: Pneumocystis carinii pneumonia.
On POD 12, edema and erythema were noted in the left flank and left lower abdominal quadrant, which rapidly extended to both legs and the chest. Respiratory, renal, and hepatic function declined and hemodynamic instability required vasopressor support. Three geographically separated skin biopsies on successive days showed dermal lipidosis with sheets of weakly PASD+ and KPI+ cells which were initially ascribed to parenteral alimentation. Other findings were dermal edema, endothelial swelling, hemorrhage, as well as necrosis of hair follicles (Fig. 2), epidermal thickening with basal cell hyperplasia, and occasional pyknotic cells at the follicular openings with satellitosis. Tacrolimus and prednisone treatment was resumed (Fig. 1).
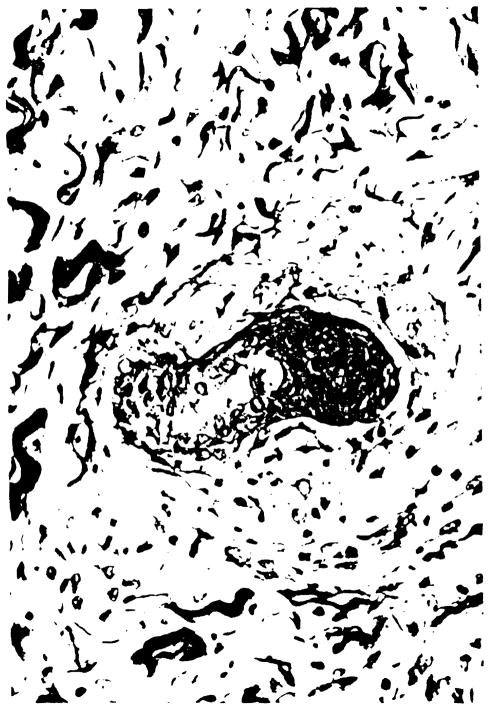
Fig. 2.
Skin biopsy performed on post-operative day 18 showing necrosis of a hair follicle and a sparse mononuclear cell infiltration in the dermis.
The clinical impression of GVHD was confirmed with another skin biopsy on post-operative day 22. This showed occasional single cell necrosis, with exocytosis of lymphocytes, an activated basal layer with focal vacuolation, follicular plugging and a light perivascular infiltrate of activated lymphoid cells (Fig. 3), consistent with GVHD. Formalin-fixed, paraffin-embedded tissue was used for Y chromosome analysis. A commercially available probe for the DY21 and DY23 loci of the Y (donor) chromosome was utilized with a fluorescence-based chromosome in situ detection system (Oncor, Inc., Gatthersburg, MD), according to the manufacturer’s instructions. Less than 1% of the total leukocyte population was positive for the Y chromosome. The positive cells were found both within the dermis and epidermis in small numbers (Fig. 4). Staining was not observed in the patient’s tissue in which probe was deleted. Both positive and negative control tissues yielded the expected results.
Fig. 3.

Skin biopsy performed on post-operative day 22 showing an inflammatory infiltrate in the epidermis and adjacent dermis with damaged keratinocytes (arrow).
Fig. 4.
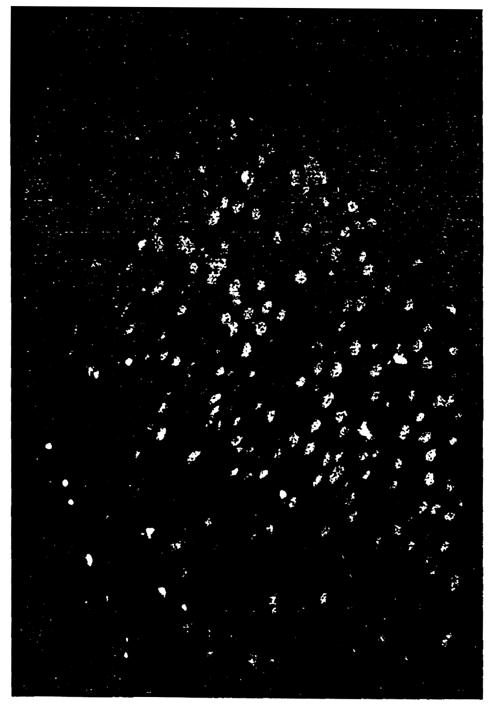
Y chromosome analysis of skin biopsy from post-operative day 22. Donor (male) cells appear as while specks among the red background of recipient tissue.
A liver biopsy, also on POD 22, revealed centrilobular necrosis with a neutrophilic infiltrate, canalicular cholestasis and patchy acute triaditis with damaged bile ducts, focally associated with a few lymphocytes. Tacrolimus and prednisone doses were increased without benefit, and she died the following day. The family refused autopsy.
Discussion
The discovery of microchimerism in different kinds of long-surviving organ allograft recipients (9) suggested that allograft acceptance involved a mutual “nullification” of the coexisting donor and recipient cell populations, which also explained why lymphoid-rich organs like the intestine did not commonly cause GVHD (5, 9, 10). In our patient, this protective mechanism was lost, first because of the pre-existing immune deficiency of the recipient, and second because of the reduction and then discontinuance of immunosuppression following an intestinal anastomotic leak and a lung infection. The donor leukocyte population then overwhelmed the host in spite of being vastly outnumbered.
This is the first recorded example of cellular GVHD associated with intestinal transplantation. However, humoral GVHD caused by a small-bowel allograft has been recorded by Grant et al. (11). In this case, antihost isoagglutinins secreted by the passenger leukocytes of an ABO compatible but not identical intestinal allograft caused fatal hemolysis of the recipient’s red blood cells, similar to the complication that can be caused under the same circumstances by liver allografts (12) and other organs (13).
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland. Supported in part by FIS (95/5071)
Abbreviations
- GVH
Graft-versus-host
- GVHD
Graft-versus-host disease
- HVG
Host-versus-graft
- MHC
Major histocompatibility complex
- PCP
Pneumocystis carinii pneumonia
- POD
Postoperative day
- TPN
Total parenteral nutrition
- VNTR
Variable number tandem repeal
References
- 1.Deeg HJ, Henslee-Downey PJ. Management of acute graft-versus-host disease. Bone Marrow Transpl. 1990;6:1. [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: The basis of graft acceptance. Hepatology. 1993;17(6):1127. [PMC free article] [PubMed] [Google Scholar]
- 3.Monchik GJ, Russell PS. Transplantation of the small bowel in the rat: technical and immunologic considerations. Surg. 1971;70:693. [PubMed] [Google Scholar]
- 4.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small-bowel allotransplantation under FK 506. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetris AJ, Murase N, Trucco M, Thomas AW, Rao AS. The lost chord: Microchimerism and allograft survival. Immunol Today. 1996;17:577. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Todo S, Tzakis A, et al. The many faces of multi visceral transplantation. Surg Gynecol Obstet. 1991;172:335. [PMC free article] [PubMed] [Google Scholar]
- 7.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantation. Ann Surg. 1995;22(3):270. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft-versus-host disease, and tolerance after different kind of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation. 1995;60:158. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant D, Garcia B, Wall W, et al. Graft-versus-host disease after clinical, small bowel/liver transplantation. Transplant Proc. 1990;22(6):2464. [PubMed] [Google Scholar]
- 12.Ramsey G, Nusbacher J, Starzl TE, Lindsay GD. Isohemagglutinins of graft origin after ABO-unmatched liver transplantation. N Engl J Med. 1984;311:1167. doi: 10.1056/NEJM198411013111807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ascher NL, Najarian JS. Liver transplantation: editorial. N Eng J Med. 1984;311:1179. doi: 10.1056/NEJM198411013111810. [DOI] [PubMed] [Google Scholar]