Abstract
The degree of behavioral control that an organism has over an aversive event is well known to modulate the behavioral and neurochemical consequences of exposure to the event. Here we review recent research that suggests that the experience of control over a potent stressor alters how the organism responds to future aversive events as well as to the stressor being controlled. More specifically, subjects that have experienced control show blunted behavioral and neurochemical responses to subsequent stressors occurring days to months later. Indeed, these subjects respond as if a later uncontrollable stressor is actually controllable. Further, we review research indicating that the stress-resistance induced by control depends on control-induced activation of ventral medial prefrontal cortical (vmPFC) inhibitory control over brainstem and limbic structures. Furthermore, there appears to be plasticity in these circuits such that the experience of control alters the vmPFC in such a way that later uncontrollable stressors now activate the vmPFC circuitry, leading to inhibition of stress-responsive limbic and brainstem structures, i.e., stressor resistance. This controllability-induced proactive stressor resistance generalizes across very different stressors and may be involved in determining individual difference in reactions to traumatic events.
Keywords: coping, behavioral control, stress, learned helplessness, medial prefrontal cortex, serotonin
The focus of the meeting on which the present volume is based was “Stress, coping, and disease”. Coping processes have often been viewed as major factors in determining how organisms respond to stressors to which they are exposed (Southwick et al., 2005). By coping is generally meant behavioral and psychological efforts to master, reduce, minimize, or tolerate aversive events. A number of investigators have attempted to distinguish between different coping styles or strategies that individuals may adopt. At the human level it has often been argued that coping efforts are either problem solving or emotion-focused in nature (Parrish et al., 2008). In the former an individual strives to do something active to mitigate the negative circumstance that she is in, whereas in the latter he attempts to reduce the emotional consequences of the negative circumstance. At the animal level investigators have generally distinguished between active and passive coping styles (Koolhaas et al., 1999). Active coping is often similar to what is meant by fight/flight, whereas passive coping is closer to conservation/withdrawal.
The concept of stressor controllability is closely related to that of coping. By behavioral control is meant the ability to alter the onset, termination, duration, intensity, or pattern of a stressor (Maier & Seligman, 1976). Clearly, the typical situation in which an organism has control would be one that could be described as active coping, although it should be noted that the inhibition of behavior can be a controlling response (Maier, 1970), and it is unclear whether this would be described as active or passive coping. In the typical stressor controllability study, animals that do and do not have control over the stressor are compared. Within this context, the subjects without control are simply viewed as not having behavioral control, but within a coping context perhaps they could be viewed as passively coping.
Typically, stressor controllability is manipulated by comparing subjects, usually rats, that receive either escapable (ES) or yoked inescapable (IS) tailshocks. In our laboratory this occurs in small Plexiglas boxes with a wheel mounted on the front wall. The rat’s tail extends from the rear of the box and electrodes are attached directly to the tail. Tailshock is used because the logic requires that the animal without control (IS) be unable to alter any aspect of the stressor. Both animals are exposed to a series of tailshocks (80-100 depending on the experiment) occurring aperiodically. Each tailshock persists until the ES subject turns the wheel a programmed number of times, at which time the tailshock terminates for both the ES subject and its yoked IS partner (e.g., Amat et al., 2006). Thus, the ES rat has behavioral control over the duration of each tailshock, while the IS subject is exposed to the exact same tailshocks, but has no control over any aspect of the procedure. Clearly, the ES animals are provided with an active coping response. It is an interesting question whether the IS subject should be viewed as passively coping, since if it is to “cope” that is its only option, or simply as having no control and not coping.
Exposure to a stressor alters not only behavior and physiology at the time of the stressor episode, but also changes how the organism responds to subsequent aversive stimuli. There has been considerable study of how genetic predisposition, the nature of the stressor, individual differences in coping strategy, etc., determine how an organism reacts to an aversive situation, but relatively less attention has been given to exactly how exposure to a stressor at time A influences how the individual reacts to a stressor at a later time B. Even less effort has been directed at exploring whether coping factors at time A influence reactions at time B. The purpose of the present paper is to review recent work examining whether and how the controllability of a potent stressor at time A alters behavioral and neurochemical reactions to an uncontrollable stressor at time B. The data will suggest that although quite often exposure to a potent stressor at time A sensitizes reactions to subsequent aversive events, organisms become resistant to later stressors if the initial stressor is one over which the organism was given behavioral control. However, in order to describe this recent research it will be necessary to summarize some of our prior work on stressor controllability, serotonin (5-HT) and the dorsal raphe nucleus (DRN), and the ventral medial prefrontal cortex (vmPFC).
Stressor Controllability
It has been known for many years that potent uncontrollable stressors such as IS produce a constellation of behavioral changes that persist for a number of days following IS. Thus, rats exposed to IS later fail to learn to escape in a different apparatus such as a shuttlebox, are less active in the presence of aversive stimuli, are less aggressive and less dominant, are less interactive socially with both adult and juvenile conspecifics, eat and drink less, are neophobic, etc (Maier & Watkins, 1998). It has been argued that this pattern reflects a persistent state of anxiety induced by IS (Maier & Watkins, 1998), but this is not an issue here. What is important here is that none of these consequences follow the stressor episode if the tailshocks are controllable (ES). That is, the ability to terminate each of the shocks with a behavioral response completely blocks the behavioral impact of the stressor, even though the stressor is physically identical to the ISs. It is important to understand that this does not mean that the ES situation is not “stressful”. For example, ES and IS induce the same magnitude and duration HPA axis response, and this is true at the level of corticosterone, ACTH, and CRF mRNA in the paraventricular nucleus (Helmreich et al., 1999; Maier et al., 1986). This is not to argue that conditions could not be found under which stressors differing in controllability would produce different HPA responses. However, under precisely the same conditions that produce the behavioral differences, HPA differences are not apparent.
Stressor Controllability and the DRN
The initial exploration of the role of the DRN in stressor controllability phenomena derived from a search for a region of the brain that projected to all, or most, of the regions that are the proximate mediators of the behaviors that are altered by IS. Fear behavior, for example, is mediated in the amygdala, and so the question was whether this region receives projections that facilitate its output. The available literature suggested that the DRN sends 5-HT projections to the regions in question (Graeff et al., 1997), and that 5-HT release in these areas produced changes in the appropriate direction (e.g., inhibition of escape, increases in fear, etc.). The reasoning, then, was that the behavioral pattern that follows IS would be reproduced if IS activated DRN 5-HT neurons and ES did not. Since the behavioral sequelae of IS persist for a number of days, and it would be most unlikely for DRN 5-HT neurons to remain active for days following IS, a more likely scenario was that IS would perhaps sensitize DRN 5-HT neurons for a number of days so that the behavioral testing conditions (e.g., the fear test) would now produce exaggerated release of 5-HT in the appropriate projection regions.
A variety of research has supported a critical role for IS-induced activation of the DRN. First, IS does lead to much greater activation of DRN 5-HT neurons than does ES as assessed by Fos expression in 5-HT labeled neurons (Grahn et al., 1999) and 5-HT release within the DRN itself (Maswood et al., 1998). Extracellular levels of 5-HT within the DRN are a measure of DRN 5-HT activity because 5-HT is released by firing DRN neurons from axon collaterals within the DRN, as well as in projection regions (Tao et al., 2000). Furthermore, this intense excitation of DRN 5-HT neurons does sensitize them so that they later release exaggerated amounts of 5-HT in projection regions. For example, Amat et al. (1998) reported that although 2 brief footshocks had no effect on extracellular levels of 5-HT in the basolateral amygdala (a projection region of the DRN) in control animals or subjects that had previously received ES, a large increase occurred in response to the 2 footshocks in rats that had received IS 24 hr earlier. The fact that IS but not equal ES activates and sensitizes DRN 5-HT neurons does not mean that these processes mediate the behavioral changes produced by IS, but not ES. However, lesion of the DRN (Maier et al., 1993) and pharmacological blockade of DRN 5-HT activity (Maier et al., 1995b), both at the time of IS or at the time of later behavioral testing, completely prevent the behavioral consequences of IS. In addition, pharmacological activation of DRN 5-HT neurons, in the absence of any stressor at all, produces the same behavioral changes that are produced by IS (Maier et al., 1995a). Thus, DRN 5-HT activation is both necessary and sufficient for the production of the behavioral sequelae of IS.
Stressor Controllability and the vmPFC
The data briefly reviewed above support a role for the DRN and its 5-HT projections in stressor controllability effects, but do not indicate the nature of its involvement. Since IS has a much different effect on activity in this structure than does ES it is natural to suppose that the DRN “detects” or determines the degree of control that the organism has over the stressor. However, for the DRN to make this “computation” it would be required to have somatomotor input concerning whether motor responses such as wheel turning have occurred or not, and somatosensory input concerning whether stimuli such as shock are present or absent. Control is defined by the conditional probability of shock termination being greater after some particular response (e.g., turning the wheel) than in the absence of this response, and detection of this circumstance requires the appropriate inputs. However, the DRN does not receive these sorts of projections.
The foregoing suggests that it is likely that controllability is detected by other structures, and that projections from these structures then regulate the DRN accordingly. The DRN receives excitatory input from a number of structures when the organism is exposed to aversive stimuli (Amat et al., 2001), and it is possible that the presence of uncontrollability leads the putative controllability/uncontrollability detector to facilitate DRN 5-HT activity, or it is possible that the presence of controllability leads to DRN 5-HT inhibition—either process would lead the DRN to be more active when the stressor is uncontrollable than when it is controllable. The computation of degree of control would seem to be a cortical function, and the DRN receives virtually all of its cortical input from prelimbic (PL) and infralimbic (IL) regions of the vmPFC (Peyron et al., 1998; Vertes, 2004). Interestingly, glutamatergic pyramidal neuronal projections from the vmPFC to the DRN synapse preferentially onto GABAergic interneurons that then inhibit 5-HT cells (Jankowski & Sesack, 2004). Thus, stimulation of vmPFC output neurons to the DRN would be expected to inhibit 5-HT cells within the DRN, and this is indeed the case (Hajos et al., 1998).
Thus, if the vmPFC detects controllability information, or alternatively, if the vmPFC regulates DRN 5-HT neurons after this information is detected elsewhere, then the anatomy suggests that the presence of control should be the “active ingredient”, leading to vmPFC inhibition of DRN 5-HT activity during a stressor. To test this possibility, Amat et al. (2005) inactivated the vmPFC via microinjection of the GABA agonist muscimol during exposure to ES and yoked IS and examined DRN 5-HT activation and later behavior. If vmPFC output to the DRN inhibits the DRN during ES as opposed to facilitating the DRN during IS, then vmPFC inactivation during the stressors should lead ES to a) activate DRN 5-HT neurons to the level that normally occurs with IS and b) produce the same later behavioral changes as are produced by IS. The results were quite clear. Muscimol microinjection in the vmPFC led ES to now activate the DRN to the levels observed during IS, as measured by both extracellular 5-HT within the DRN and Fos expression in 5-HT labeled cells. Consistent with these findings, vmPFC during ES led ES to produce the same constellation of behavioral changes as does IS including shuttlebox escape failure (Amat et al., 2005), reduced social investigation (Christianson et al., 2009), and potentiated drug reward (Rozeske et al., 2008). Conversely, intra-vmPFC muscimol had no effect at all on IS subjects—the DRN was activated and behavioral changes produced as are typical.
If vmPFC output is responsible for mediating the effects of control, then an even stronger test would be to activate the vmPFC during ES and IS. Activation should have the effect of making IS to appear as if it were ES. Indeed, pharmacological activation of vmPFC neurons with the GABA antagonist picrotoxin (pyramidal output neurons are under tonic GABAergic inhibition) during IS and ES eliminated the DRN 5-HT activation otherwise produced by IS as well as the typical behavioral sequelae of IS (Amat et al., 2008).
These data suggest that having control activates vmPFC output to the DRN, thereby inhibiting the DRN activation produced by neural input deriving from aversive stimulation. To directly assess this possibility, Baratta et al. (2009) retrogradely labeled vmPFC cells that project to the DRN by iontophoresis of the retrograde tracer Fluorogold onto DRN neurons. ES, relative to IS, induced expression of the IEG/activation marker Fos in the retrogradely labeled cells, directly demonstrating that the presence of control activates neurons that project from the vmPFC to the DRN.
Although the experiments described above implicate the vmPFC in the mediation of stressor controllability phenomena and suggest that control inhibits, rather than a lack of control facilitates, stress-induced DRN activity, the precise role of the vmPFC remains unclear. It is possible that the detection of control occurs in the vmPFC as it receives a rich array of necessary inputs. In addition, the vmPFC has been implicated in contingency learning, as opposed to simple Stimulus-Response learning (Balleine & Dickinson, 1998). By contingency is roughly meant the statistical correlation between behavior and outcomes, rather than just their temporal pairing. This is noted because the concepts of contingency and control are quite similar. In this regard, it can be noted that inactivation of the vmPFC in the study by Amat et al. (2005) did not reduce the ES subjects’ ability to learn to turn the wheel to escape, consistent with the conclusion that the vmPFC is not involved in simple S-R learning. It is also possible that the control detection process occurs elsewhere, and that the information is relayed to the vmPFC that then regulates other brain regions, such as the DRN, accordingly. The latter possibility would be in keeping with the “executive functioning” that has been regarded as the hallmark of the mPFC (Robbins, 2000).
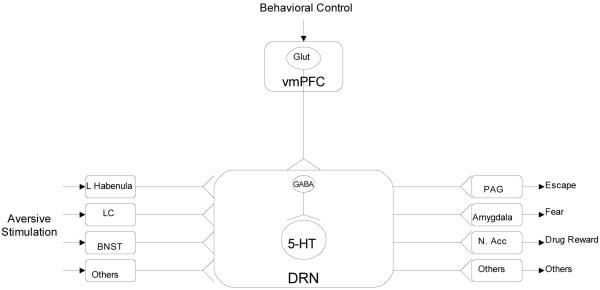
The general conception that this work leads to is schematized in Fig. 1. Aversive stimuli lead to the activation of a number of brain regions, which all project to and activate DRN 5-HT neurons. The DRN is thus an integrative site, combining inputs from various aversive stimulus properties. The DRN sends 5-HT projections to a number of brain areas that are proximate mediators of behavior, and modulates activity in these regions. Thus, the DRN functions as a kind of “gain control”, increasing and decreasing activity in regions that regulate behavior. Thus, for example, the DRN does not mediate fear, but rather can amplify fear. Indeed, DRN lesions do not alter basal fear conditioning, they only eliminate the potentiation of fear produced by IS (Maier et al., 1993). The DRN here is viewed to play a role similar to that of the central nucleus of the amygdala in mediating fear. Importantly, the DRN is under the inhibitory control of the vmPFC, and behavioral control appears to activate this inhibitory control.
Fig. 1.
Scheme of how stressor controllability modulates the behavioral impact of aversive stimuli. Glut=glutamate, vmPFC=ventral medial prefrontal cortex, L. habenula=lateral habenula, LC=locus coeruleus, BNST=bed nucleus of the stria terminalis, GABA=gamma aminobutyric acid, 5-HT=serotonin, DRN=dorsal raphe
The Proactive Influence of Control over a Stressor
As noted above, the experience of potent stressors alters how the organism reacts to subsequent stressors. The issues to be explored here are whether, and by what mechanism(s), the behavioral controllability of the initial stressor alters reactions to subsequent stressors. The recent work from our laboratory directed at these questions derives from older reports indicating that a session of ES prevented a later exposure to IS from producing shuttlebox escape deficits (Seligman & Maier, 1967), even if the two experiences occurred in different environments (Williams & Maier, 1977). This was demonstrated to be a stressor controllability effect as an initial experience with yoked IS did not have this blunting effect. This immunizing effect of prior ES has proved to be quite general, with demonstrated blockade of the effects of later IS on reducing social interaction (Christianson et al., 2009) as well as drug reward (Rozeske et al., 2008). Furthermore, the proactive blunting of the behavioral consequences of IS has been shown to be quite enduring, persisting for at least 2 months between ES and later IS (unpublished results).
The most obvious possibility would be that the prior experience with ES somehow prevents later IS from activating DRN 5-HT neurons. To examine this possibility we (Amat et al., 2006) measured extracellular levels of 5-HT and Fos expression in the DRN during IS after the subjects had received ES, IS, or control treatment 7 days earlier. Indeed, prior exposure to ES completely blocked the DRN activation normally produced by IS. That is, the DRN responded as if the stressor was controllable, even though it was not. Thus, exposure to ES, but not to IS, blocks both neurochemical and behavioral responses to IS.
Role of the vmPFC in the Production of Stressor Resistance
As reviewed above, activation of the vmPFC during ES has proven necessary for the presence of control to blunt the impact of the ongoing stressor. Thus, it is reasonable to suppose that engagement of the vmPFC during ES might also be necessary for ES to produce immunization with regard to the effects of future uncontrollable stressors. To examine this possibility, the vmPFC was inactivated with muscimol during the initial ES experience, and now ES no longer blocked either the DRN 5-HT activation or the behavioral consequences of IS administered 7 days later (Amat et al., 2006).
The experiment described above indicates that immunization requires the vmPFC at the time of the initial controllable stressor. Perhaps immunization occurs because the experience of ES alters the vmPFC in such a way that even uncontrollable aversive events then activate it, thereby inhibiting the DRN and perhaps other stress-responsive structures. If this is so, then the vmPFC would be needed not only to acquire information about control but also to use this information to regulate the DRN at the time of later uncontrollable aversive stimulation. Thus, the vmPFC was inactivated at the time of later IS rather than earlier ES, and this manipulation also prevented immunization (Amat et al., 2006). Now, IS both activated the DRN and produced behavioral consequences such as shuttlebox escape failure, even though the subjects had experienced prior ES. These data suggest that the vmPFC is a site of neural plasticity within the stressor controllability paradigm. It is often argued that lasting neural plasticity requires the synthesis of new proteins, and so Amat et al. (2006) microinjected the protein synthesis inhibitor anisomycin in the vmPFC during ES, and this prevented ES from blocking the neurochemical and behavioral changes produced by later IS.
There are various ways to conceptualize the implications of the data above, but one idea is that perhaps the activation of vmPFC output neurons during ES “ties” or associates vmPFC neuronal activation to some aspect of the ES experience, to tailshock for example, so that after exposure to ES even IS now activates vmPFC output, thereby leading to the inhibition of the DRN and the blockade of behavioral changes that depend on DRN activation/sensitization. If the joint occurrence of aversive stimulation and ES-induced vmPFC activation is sufficient to tie the two together, then perhaps simply activating the vmPFC during stressor exposure would be sufficient to lead to stressor immunization. That is, perhaps control is not needed at all, just vmPFC activity during a stressor. To test this idea, the vmPFC was activated by picrotoxin during ES, IS, or control treatment (Amat et al., 2008). IS administered 7 days after IS+vmPFC activation failed to activate the DRN or produce typical IS-induced behavioral changes. Thus, even IS produces immunization if the vmPFC is activated during its administration. Interestingly, vmPFC activation with picrotoxin by itself, in the absence of tailshock, failed to lead to immunization. Immunization required the conjoint presence of the stressor and the activation of the vmPFC, consistent with the argument that they become associated.
Social Defeat and Fear Conditioning
The research reviewed above suggests that the experience of control over tailshock blunts the DRN response to later uncontrollable tailshock and the behavioral changes that result from DRN 5-HT activation. It also suggests that this resistance to the effects of IS is mediated by ES-induced alterations in the vmPFC. Several questions suggest themselves. Perhaps the most obvious concerns whether the resistance to later stress effects produced by exposure to control over tailshock is restricted to later tailshock and very similar stimuli, or whether the resistance is more general.
To begin to explore this issue we chose to study the effects of ES on neurochemical and behavioral responses to a stressor that has as few external stimuli in common with tailshock and the wheel-turn apparatus as possible. Social defeat (SD) seemed to fit this requirement. In SD paradigms a target subject is exposed to a larger aggressive resident animal, and the outcome is almost always that the resident attacks the target, with the target subject rapidly adopting species-specific defeat postures. In the procedure that we adopted, the target rat was introduced into the cage of a resident male for 45 min. Thus, the SD procedure involves no shock or other discrete aversive stimuli, no apparatus similar to a wheel turn box, the presence of a conspecific, and in our procedure even occurs on another floor of the building so as to minimize the presence of common odor cues.
We first had to determine behavioral changes that might result from SD so that it could be determined whether prior ES would block them. As SD might be uncontrollable from the subjects’ perspective as defeat of the target is essentially inevitable, we determined whether SD would produce behavioral sequelae similar to those of IS. Indeed, SD produced shuttlebox escape failure and reduced juvenile social investigation 24 hr later (Paul et al., 2008). Gardner et al. (2005) had found a somewhat similar SD procedure to induce Fos in the DRN, so we determined whether SD would increase 5-HT in the DRN, and it did so. The important result was that ES occurring 7 days before SD blocked both the DRN activation and the behavioral consequences (poor escape and reduced social investigation) of SD. Yoked IS did not reduce the effects of later SD, and indeed, exacerbated them. Thus, it was control over tailshock that was responsible for the blunting of the impact of later SD (Maier, unpublished data).
These data clearly show that the experience of behavioral control over a potent aversive event alters the organism in a way that is much more general than how it responds to the same stimulus over which it had had control. It has yet to be determined whether alterations in the vmPFC are responsible for this generalized resistance produced by control over tailshock, but one interpretation would be that during ES vmPFC activation becomes associated with something much more general than tailshock, something common to a broad range of stressors, something such as fear/anxiety (see below). Determining how this generalized resilience operates will be a challenge.
A second question concerns whether control activates vmPFC projections to stress-responsive structures other than the DRN, thereby regulating behaviors controlled by these other structures. The amygdala is an obvious candidate. The role of the amygdala in fear and fear conditioning is well known (LeDoux, 2007). The view has emerged that the association between neutral stimuli such as tones and aversive stimuli such as footshocks form in the basal nucleus, and from there input is provided to the central nucleus (CE), either directly or via the lateral nucleus. The CE, in turn, projects to the proximate mediators of fear responses, to the periaqueductal gray for example, resulting in freezing behavior. Thus, a tone or a context that has been paired with footshock produces fear responses because the CE is activated.
Interestingly, the vmPFC sends glutamatergic projections to a number of regions within the amygdaloid complex (Vertes, 2006). The prelimbic region (PL) sends somewhat complex projections to the basolateral region. The infralimbic region (IL) sends a clear projection to a region of the amygdala called the intercalated cell region (ITC). These cells are almost entirely GABAergic, and in turn project to the CE (CE). As would be expected, activation of the IL with picrotoxin activates these ITC GABAergic cells, and stimulation of the IL results in the inhibition of CE output neurons and fear behaviors (Berretta et al., 2005).
Given this anatomy, Baratta et al. (2007) gave rats either ES, yoked IS, or control treatment. 7 days later all subjects received fear conditioning in which a tone was paired with footshock, followed 1 day later by fear testing to determine how much conditioning had occurred. Freezing was measured both to the conditioning context and to the tone (presented in a novel context) that had been paired with shock. The wheel turn apparatus and the conditioning boxes were sufficiently different that fear behavior did not generalize from the ES/IS treatment to the conditioning situation. That is, there was no freezing in the boxes before footshock, an outcome that would confound differences in conditioning. In keeping with prior reports (Rau et al., 2005), IS potentiated fear conditioning. More importantly for the present paper, ES retarded fear conditioning—less fear was conditioned to both the context and the tone.
Because there has been a great deal of recent interest in the extinction of conditioned fear, Baratta et al. (2007) determined whether experience with ES might not facilitate fear extinction. The logic of this question required a design in which ES and IS were administered after fear conditioning. This is because it is necessary that the amount of fear conditioning be equal in the various groups, otherwise, any apparent extinction differences could be attributed to acquisition differences. Subjects thus first received fear conditioning. For simplicity, there was no tone and only fear conditioning to the experimental context was assessed. Then, 1 day later, the rats were exposed to either ES, yoked, IS, or control treatment. 7 days later, all subjects began fear extinction consisting of daily 5 min exposures to the conditioning context, without the occurrence of footshock, that continued until a criterion of extinction had been reached. Remarkably, ES that occurred after fear conditioning hastened extinction of conditioned fear. This is remarkable because ES is not negatively fearful or stressful, it is quite aversive, yet it actually reduced fear responding relative to no exposure to a stressor at all.
Although the natural interpretation was that ES facilitated extinction learning, a more detailed observation questioned this possibility. The fear response in the conditioning context did disappear more rapidly in the ES subjects, but fear was diminished by the second minute of the very first extinction session. Clearly, this is too rapid for the subjects to have learned that footshock would no longer occur. Indeed, the first footshock did not occur on the conditioning day until the subjects had been in the context for 3 min.
If ES did not facilitate extinction learning, then how could it have produced the more rapid disappearance of fear? Another possibility would be that the experience of ES reduces the expression of fear behavior. That is, the stimulus context might still have high associative strength for the footshock UCS in ES subjects, but the behavior that such an association would normally produce is inhibited.
Perhaps a consideration of the amygdala circuitry briefly reviewed above would make this distinction more intelligible. The association between CS and UCS occurs in basolateral regions of the amygdala, but the IL does not project to this portion of the amygdala. Instead, the IL inhibits the CE projections to regions that produce fear responses; that is, the expression of fear.
Thus, it is possible that ES alters the IL in such a way that it is later activated by fear, thereby reducing fear expression. This could occur because the IL is sensitized by ES or because IL activity becomes associated with fear, or something that is part of fear. However, by either process, the argument is that the experience of ES does not alter the subsequent fear conditioning or extinction process, but rather reduces the expression of the fear behavior that is elicited by stimuli that signal footshock. The possibility that ES exposure reduces fear expression can be tested by selectively inactivating the IL during each of the phases of the experimental procedure in which an initial experience with ES was shown to interfere with fear conditioning. Recall that in this experiment schematized in Fig. 3, bottom row, animals first received either ES or IS (Phase 1), then 7 days later fear conditioning (Phase 2), and then 1 day later testing for the amount of fear that was conditioned (Phase 3).
Consider the predictions to be made by the hypotheses that ES interferes with fear expression:
Phase 1
If ES activates the IL, thereby altering it in such a way that it is later activated by fear, then inactivation of the IL during ES should prevent ES-induced reduction of fear measured in Phase 3. This is because the IL would now not be activated during fear, and therefore not either sensitized or “tied” to fear.
Phase 2
In contrast to inactivation during Phase 1, inactivation of the IL during the fear conditioning should have no effect, that is, prior ES should still retard fear conditioning. This is because the association between CS and UCS occurs in basolateral regions of the amygdala not in the ITC-CE pathway, the target of IL projections. Thus, the association should form normally even if the IL-ITC-CE pathway is active during the conditioning session.
Phase 3
The most interesting question concerns the prediction to be made if the IL is inactivated only during the last stage of the experiment, the test for fear. Since the IL was not inactivated during Phase 1 it should either become sensitized or tied to fear. The critical aspect of the fear expression hypothesis is that the experience of ES does not later fear learning, and so this should proceed normally in Phase 2. The hypothesis supposes that in the test phase the elicitation of fear activates the IL in subjects that had experienced ES, and so fear behavior is reduced because the ITC is activated, the ITC inhibiting CE output. Thus, inactivation of the IL during the test phase should lead to normal expression of fear behavior. That is, IL inactivation should reveal the fear conditioning that was there all along in ES subjects, an unmasking of the learning that was present, but whose output in behavior was inhibited. Clearly, the idea that exposure to ES interferes with the actual fear conditioning process would predict that IL inactivation of Phase 3 would be without impact as from this view Es would have undermined the conditioning in Phase 2. If conditioning was reduced, then fear should still be less in Phase 3 even if the IL was inhibited.
Baratta et al. (2008) conducted precisely the experiment just outlined. Not surprisingly, muscimol microinjected into the IL during ES blocked the reduction in later fear conditioning produced by ES. Also perhaps not surprisingly, intra-IL muscimol administered before the fear conditioning did influence the ES-induced reduction in fear conditioning, nor did it have any effect on fear conditioning in controls. The critical question was what intra-IL muscimol before fear testing would do. In vehicle-injected controls ES in Phase 1 reduced the fear measured in Phase 3 testing. However, IL inactivation during testing eliminated the reduction in fear behavior in ES subjects and revealed the fear that had indeed been conditioned. Thus, it would appear that experiencing control over an aversive stimulus alters the IL in such a way that it is later activated under fear conditions, leading to the inhibition of fear behavior. Clearly, ES reduced the expression of fear, not the development of fear conditioning.
In sum, the experience of control over tailshock does not alter only how the organism reacts to later shock and regulates not only how the DRN responds to later events. The experience of ES had a substantial effect on how the subjects reacted to SD, an event quite different than tailshock. Indeed, SD is sufficiently different from tailshock that it is more than possible that experiencing control over a potent aversive stimulus alters the organism in a profound manner. In coping terms, perhaps it shifts how the organism attempts to deal with future threat. This is clearly mediated, at least in part, by changes in the vmPFC and its regulation of other structures such as the DRN. The experiments involving fear conditioning suggest that this regulation is not limited to the DRN.
Safety Signal and the Sensory Insular Cortex
To this point the focus has been on behavioral control as an environmental variable that modulates how the occurrence of an aversive event alters the contemporaneous and future impacts of the event. However, control is not the only experiential variable that modulates the consequences of stressors, and it is important to inquire whether the mPFC mediates their effects as well. There has been considerable recent interest in “safety signals” (SSs) as stress-modulators. SSs refer to stimuli that signal the absence of an aversive event. In Pavlovian terms, these are stimuli paired with the absence of an unconditioned stimulus (UCS); that is, Pavlovian conditioned inhibitors. Stimuli that occur at the termination of each of a series of aversive events such as shocks become potent SSs (Maier et al., 1976) and inhibit fear responses. This is because they signal a period of time free from shock, and indeed, are as far from the next shock as is possible. Interestingly. The provision of a SS (e.g., a 5 sec diminution of the houselights at the end of each tailshock) has been shown to eliminate the shuttlebox escape deficits (Minor et al., 1990) and reduced social investigation (Christianson et al., 2008) produced by IS. It has been suggested that SSs blunt the behavioral effects of the IS because it reduces the total time that the subjects are afraid during the session (Jackson & Minor, 1988).
Since SSs blunt the immediate effects of tailshock as does behavioral control, it might be expected that SSs would require the vmPFC. However, Christianson et al. (2008) reported that inactivation of the vmPFC during the IS session did not reduce the elimination of the behavioral consequences of IS produced by SSs. In exploring whether there is a cortical region required for SSs to operate, Christianson et al. (2008) focused on the posterior insula because it has a number of properties that would make it sensitive to SSs (Benison et al., 2007). Indeed, inactivation of the posterior insula with muscimol prevented SSs from blunting IS effects, but did not reduce the impact if behavioral control. That is, there was a double-dissociation between the vmPFC and the posterior insula in mediating the stress buffering effects of control and SSs. These data may help to explain an important difference between control and SSs, namely that control blunts the effects of later uncontrollable stressors, while SSs do not (Maier & Warren, 1988). It is known that there is plasticity in the vmPFC, but this issue has not been examined for the insula.
Thus, not all stress-buffering factors use the same neural circuitry, and it is not known how different circuitries that might be used interact or alter downstream systems. The vmPFC, for example, inhibits stressor-induced DRN activation, and interestingly, the posterior insula does not (Christianson et al., 2008). There remains much to be discovered concerning how these, and perhaps other, cortical structures regulate limbic and brainstem stress-responsive circuitries.
Conclusions
Numerous studies have documented that how individuals are affected by aversive events is determined not just by the physical characteristics of the stimuli, but also by complex cognitive factors having to do with how the events are appraised and what type of coping is possible. Stressor controllability studies fit within this general framework. The research reviewed here has highlighted the role of the vmPFC in mediating the effects of control. Aversive stimulation activates a number of brainstem and limbic structures such as the DRN that, in turn, mediate the behavioral sequelae of stressors. The available evidence suggests that these structures are not themselves sensitive to stressor controllability. Perhaps it is reasonable that more “primitive” regions of the brain are insensitive to control as more primitive organisms have a more limited behavioral repertoire that can be used to behaviorally cope with challenges. For these organisms, adjustments to challenges tend to be physiological rather than behavioral, and behavioral control is not a factor. As organisms evolved and developed a more elaborate behavioral repertoire, control should become a more important factor, and it would make sense for “higher” centers to be sensitive to this dimension. Since the physiological adjustments that are used to adjust to stressors can be catabolic and costly, it would also make sense that these would be inhibited if behavioral coping is possible. It is as if when behavioral control is possible the vmPFC says “cool it” to the DRN and other stress-responsive structures. Another way to view this is that organisms have preprogrammed species-typical ways to deal with challenges. However, when the environment affords a novel or individualistic way to cope or deal with the challenge (turning a wheel while restrained in a box), higher organisms have the flexibility to use these means, and this requires inhibition of the preprogrammed responses.
The top-down inhibitory control over stress-responsive structures that occurs when behavioral control is present is in keeping with more general views of PFC function. The PFC has been ascribed the role of mediating executive functions or cognitive control--the ability to orchestrate thought and action in accordance with internal goals (Miller & Cohen, 2001). These functions often involve a top-down coordination of “lower” motor and sensory processes, and furthermore often specifically inhibitory control is critical to these operations in order to suppress inappropriate tendencies. The Stroop task is the classic example in humans. Subjects either read words or name the color in which the words are written. To do this, subjects have to selectively attend to one attribute. This is especially true when naming the color of a conflict stimulus (e.g. the word RED displayed in green), because there is a strong preprogrammed tendency to read the word (“red”), which competes with the response to the color (“green”). The PFC is necessary to be able to inhibit the inappropriate tendency. One way to view the present argument is that we are extending the idea of executive function from the domain of the interaction between cognitive processes to the regulation of emotion.
That behavioral control modulates the immediate effects of the stressor that are being controlled has been known for many years. However, even more striking, control has a long-lasting proactive effect and alters how the organism responds to subsequent stressors, even if they are quite different from the original stressor that was controlled. Both behaviorally and neurochemically, the subjects respond to a subsequent stressor as if it is controllable, even if it is not controllable. The vmPFC is critical to this process, both at the time of the original and the later stressor—inactivation of the vmPFC at either stage eliminates the proactive immunizing effects of control. There appears to be plasticity within the vmPFC so that after it is activated while a stressor is present it then later becomes activated in the presence of another stressor, thereby leading to inhibition of lower stress-responsive structures. This striking effect of experiencing control likely depends on the stressor being a highly salient event in the life of the organism. A session of tailshock delivered while in a small wheel-turn box is outside of the rat’s previous life experience. The limits of the immunizing effects of control are not yet known.
The work reviewed here articulates well with recent studies in human subjects. There is a growing literature concerning brain mechanisms that are involved in “emotion regulation”. In a particularly relevant set of experiments (Urry et al., 2006), subjects were asked to attempt to make fearful stimuli more or less fearful by imagining them to be more or less threatening. As might be expected, amygdala activity was increased in subjects making stimuli more fearful, and decreased in fear minimizing subjects. Of note here, mPFC activity moved in the inverse direction—increased in the minimizing subjects and decreased in the fear maximizers. An obvious interpretation is that subjects were able to alter fearfulness of the stimuli via regulation of mPFC inhibitory control over the amygdala. This sort of mPFC-amygdala interaction may also be involved in a number of clinical phenomena. For example, PTSD is precipitated by the experience of a stressor, and PTSD patients have been observed to show a pattern of enhanced amygdala and diminished PFC activity when confronted with fear provoking stimuli (Bremner et al., 1999; Phan et al., 2006; Shin et la, 2005). Not all individuals that experience a traumatic event develop PTSD, and a loss of PFC inhibitory control may be critical. The present research suggests that prior experiences with control/coping with regard to potent stressors may be important in determining the outcome of trauma.
Acknowledgements
The preparation of this manuscript was supported by NIH Grant MH50479
Footnotes
A publisher’s error resulted in this article not appearing in the Special Issue: Stress, Coping, and Disease. The article is presented here for the reader’s convenience and for the continuity of the special issue: Stress, Coping, and Disease, Brain Research 1293, October 1, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Prior experience with behavioral control over stress blocks the behavioral effects of later uncontrollable stress: Role of the ventral medial prefrontal cortex. Journal of Neuroscience. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Lucero TR, Amat J, Watkins LR, Maier SF. Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learn Mem. 2008;15:84–87. doi: 10.1101/lm.800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective Activation of Dorsal Raphe Nucleus-Projecting Neurons in the Ventral Medial Prefrontal Cortex by Controllable Stress”. Journal of Neuroscience submitted. 2009 doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benison AM, Rector DM, Barth DS. Hemispheric mapping of secondary somatosensory cortex in the rat. J Neurophysiol. 2007;97:200–207. doi: 10.1152/jn.00673.2006. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, Baratta MV, Paul ED, Campeau S, Watkins LR, Barth DS, Maier SF. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. J Neurosci. 2008;28:13703–13711. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009 doi: 10.1080/10253890802510302. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev. 1997;21:791–799. doi: 10.1016/s0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Watkins LR, Deak T, Maier SF, Akil H, Watson SJ. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 1999;11:121–128. doi: 10.1046/j.1365-2826.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Jackson RL, Minor TR. Effects of signaling inescapable shock on subsequent escape learning: implications for theories of coping and “learned helplessness”. J Exp Psychol Anim Behav Process. 1988;14:390–400. [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Maier SF, Busch CR, Maswood S, Grahn RE, Watkins LR. The dorsal raphe nucleus is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behav Neurosci. 1995;109:759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behav Neurosci. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness:theory and evidence. Journal of Experimental Psychology:General. 1976;105:3–46. [Google Scholar]
- Maier SF, Warren DA. Controllability and safety signals exert dissimilar proactive effects upon nociception and escape performance. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:18–25. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability, anxiety, and serotonin. Cognitive Therapy and Research. 1998;6:595–613. [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minor TR, Trauner MA, Lee CY, Dess NK. Modeling signal features of escape response: effects of cessation conditioning in “learned helplessness” paradigm. J Exp Psychol Anim Behav Process. 1990;16:123–136. [PubMed] [Google Scholar]
- Parrish CL, Radomsky AS, Dugas MJ. Anxiety-control strategies:is there room for successful exposure treatment. Clin Psychol Rev. 2008;28:14001412. doi: 10.1016/j.cpr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Paul ED, Amat J, Aleksejev LR, Watkins LR, Maier SF. Escapable stress blocks the behavioral and neurochemical response to later social defeat stress in the rat. Society for Neuroscience Meetings; Washington DC: 2008. [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Robbins TW. From arousal to cognition: the integrative position of the prefrontal cortex. Prog Brain Res. 2000;126:469–483. doi: 10.1016/S0079-6123(00)26030-5. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. The Medial Prefrontal Cortex Regulates the Differential Expression of Morphine-Conditioned Place Preference Following a Single Exposure to Controllable or Uncontrollable Stress. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman MEP, Maier SF. Failure to escape traumatic shocks. Journal of Experimental Psychology. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential effect of local infusion of serotonin reuptake inhibitors in the raphe versus forebrain and the role of depolarization-induced release in increased extracellular serotonin. J Pharmacol Exp Ther. 2000;294:571–579. [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. Journal of Experimental Psychology:Animal behavior processes. 1977;3:240–253. [Google Scholar]