Rapidly fatal insulin-dependent diabetes of an autoimmune etiology appears between the ages of 40 and 140 days in 40% to 75% of BB Wistar rats.1–7 A typical insulitis lesion precedes the diabetic state followed by a selective destruction of the beta cells, leaving alpha and delta cells intact;1–4 without insulin treatment, the animals die within a few days. The process is genetically programmed and islet directed,7 but there may be other simultaneous autoimmune disorders such as lymphocytic thyroiditis.8 Cyclosporine (CyA) can mitigate or prevent BB rat diabetes.3–6
FK 506, a potent new immunosuppressive agent, can prevent or reverse allograft rejection after clinical organ transplantatlon,9–11 by suppressing T-cell activation in the same general way as CyA.12, 13 Both drugs inhibit the transcription of messenger RNAs (mRNAs) for early phase lymphokines, such as IL-2, IL-3, and interferon-gamma, but FK 506 is more potent.14 FK 506 can prevent an autoimmune disease in MRL-lpr/lpr mice, which resembles human systemic lupus erythematosus.15 Patients with hemolytic uremic syndrome,16 glomerulonephritis with the nephrotic syndrome,17 psoriasis, (unpublished observations), and pyoderma gangrenosum (unpublished observations) have also been put into remission with FK 506.
In this study, the ability of FK 506 to prevent the spontaneous diabetic syndrome in BB rats was demonstrated with two different doses. Many of the animals continued to be free of diabetes after discontinuance of the drug.
MATERIAL AND METHODS
Animals
The BB rat colony of the University of Pittsburgh (Pittsburgh, PA) was started from initial breeding stocks obtained from the University of Massachusetts (Boston, MA) in August 1986. The rats have been inbred over 10 generations and develop spontaneous diabetes at a 60% to 90% incidence in both sexes between 60 and 190 days of age, with the peak incidence between 80 and 100 days. All animals received tap water and regular rat chow (Purina Rat Chow 5001) ad libitum under controlled lighting conditions.
FK 506
FK 506 was a gift by Fujisawa Pharmaceutical Co., Ltd (Osaka, Japan). The oral form of FK 506, dispersed with hydroxypropyl methylcellulose, was diluted in tap water by sonication, and was administered daily by gastric gavage.
Experimental Design
A total of 59 BB rats was obtained from seven consecutive litters. Animals from each litter were randomly divided into three sex-matched groups. Animals in an untreated group received tap water (group 1, n = 20). In the treatment groups, intragastric FK 506 doses were given at 1.0 mg/kg/d (group 2, n = 19) and 2.0 mg/kd/d (group 3, n = 20). Treatment was started at 30 days of age and continued for 90 days. Body weight and glycosuria (Testape, Eli Lilly, Indianapolis, IN) were measured three times a week. Blood glucose levels were measured by the glucose oxidase method as soon as glycosuria was seen and in all nonglycosuric animals every 14 days. Diabetes was diagnosed if the nonfasted glucose level exceeded 200 mg/dL for 3 consecutive days. At the age of 120 days, following 90 days of treatment, six BB rats from each of the FK 506 treatment groups (groups 2 and 3), were randomly selected and killed for special studies. All other animals were followed until at least 150 days of age, 30 days or more after the discontinuance of FK 506.
Assays
Glucose Tolerance Test
At the age of 120 days on the last day of the treatment period, blood samples were collected from the tail vein before and 5, 10, 15, 30, 60, and 90 minutes after the intraperitoneal injection of 2 g/kg glucose (50% dextrose). Tests were performed in the morning without anesthesia, 12 hours after food withdrawal.
Insulin Extraction
After completing the glucose tolerance tests, the animals were killed and pancreatic tail tissue was diced in small pieces into the cold acid ethanol (absolute ethanol 75.2%, hydrochloric acid 1.4%, distilled water 23.4%) and sonicated vigorously for 10 minutes at 4°C. After centrifugation at 500 rpm for 10 minutes, the supernatant fractions were frozen for subsequent assays of insulin by radioimmunoassay.
Histopathology
Tissues were routinely fixed in formalin, processed, and paraffin-embedded. Hematoxylin and eosin sections from the pancreatic head, body, and tail were examined by a pathologist without access to the experimental group code. Semi-quantitative evaluation of islet-cell inflammation was graded on a scale of 0 to 4 as follows: 0, no inflammation; 1, single hyperchromatic nuclei within or adjacent to islets (in cases of doubt as to whether these did or did not represent inflammatory cells, a grade of 1 was assigned); 2, mononuclear cell clusters occupying an area less than 25% of the islet; 3, inflammatory infiltrates occupying 25% to 50% of total islet area; 4, infiltrates involving greater than 50% of total islet area.
In addition to these lesions, foci of periductal mononuclear inflammation were also quantified, but not graded.
Guinea pig anti-human insulin (Biogenex Labs, San Ramon, CA) and rabbit anti-human glucagon (Amersham International, Amersham, UK) have sufficient cross-reactivity with the respective rat hormones to allow immunohistochemical detection. Localization of these antibodies onto fixed, paraffin-embedded tissues was visualized using the avidin-biotin procedure (Vector Labs Inc, Burlingame, Calif). Color was developed with 3-amino-9-ethyl carbazole (Biomeda Corporation, Foster City, CA). The entire procedure was performed on a computer-driven robot (Code-On®, Fisher Scientific Inc, Pittsburgh, PA) which allowed the placement of parallel control slides.
RESULTS
Incidence of Diabetes
In untreated group 1, 15 of 20 (75%) of the animals developed diabetes between the age of 70 and 103 days (Fig 1). None of the animals treated with daily doses of 2.0 mg/kg FK 506 (group 3) developed glycosuria during treatment or for the 30 days following its discontinuance. With a daily dose of 1.0 mg/kg/d (group 2), three animals became diabetic (15.7%) during therapy when they were 115, 117, and 120 days old, and two others developed diabetes after discontinuance of the drug when they were 132 and 147 days old.
Fig 1.
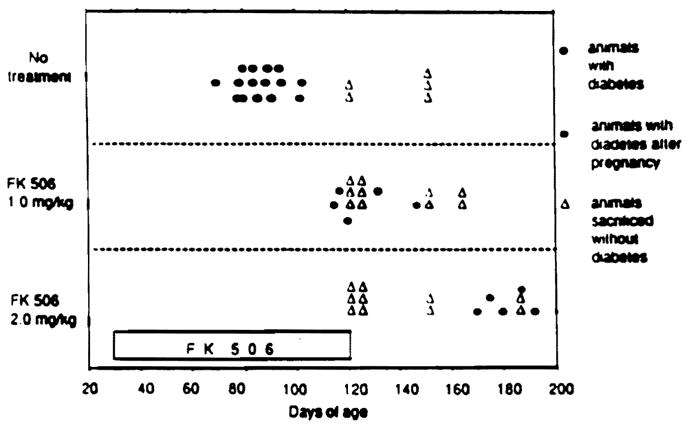
Onset and Incidence of diabetes under treatment with 1.0 mg/kg/d oral FK 506, (n = 19); 2.0 mg/kg/d FK 506, (n = 20); or water, (n = 20) from 30 to 120 days of age.
Several animals in each group were killed for tissue at 120 and between 150 and 185 days. The 10 remaining rats in the high FK 506-dose series (group 3) remained nondiabetic until 5 pregnant females became diabetic after 170 to 190 days. The other five were still nondiabetic 90 to 120 days after stopping FK 506. A long-term nondiabetic state was also frequently achieved in the low-dose group 2 FK 506 series.
Body Weight
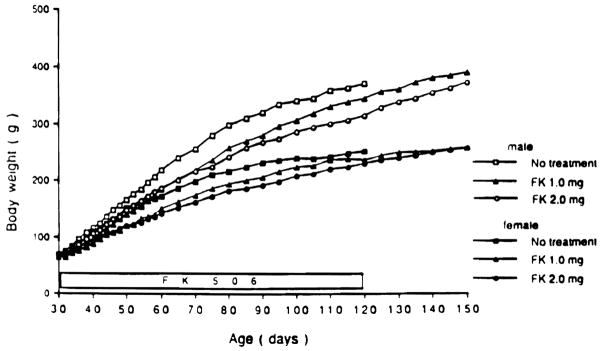
At the beginning of FK 506 treatment, mean body weights were nearly identical in the three groups. Body weight gain was slightly inhibited by FK 506 treatment both in the more rapidly growing males and in the females, but the differences were not statistically significant at most time points (Fig 2).
Fig 2.
Effect of FK 506 treatment on body weight in males and females compared with their male and female water-treated siblings. There were only five water-treated animals left at 120 days.
Blood Glucose and Intraperitoneal Glucose Tolerance Test
Nonfasting blood glucose levels during the 90 days of treatment in those animals that remained nondiabetic are summarized in Fig 3. The values were not different in the three groups, and were not significantly different than in normal Wistar Furth (non-BB) rats (data not shown).
Fig 3.
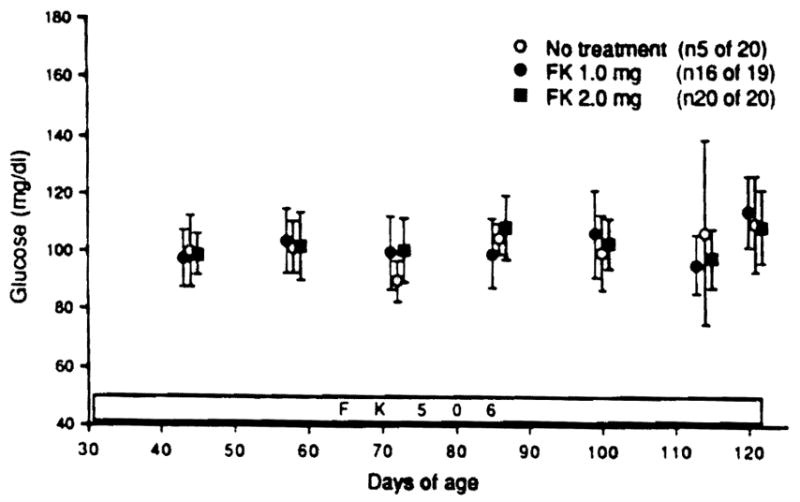
Blood glucose levels at 30 to 120 days of age in nondiabetic survivors during FK 506 treatment (1.0 mg/kg, n = 16; 2.0 mg/kg, n = 20) compared with water treatment (n = 5).
Intraperitoneal GTTs were performed in three nondiabetic animals of group 2 and 6 animals of group 3 at 120 days. The curves were similar to those of normal Wistar Furth rats (Fig 4).
Fig 4.
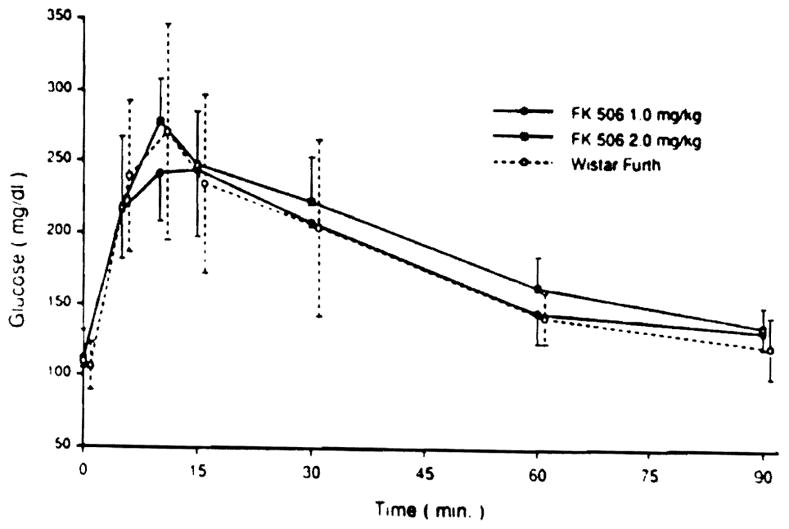
Blood glucose levels during intraperitoneal glucose tolerance test in BB rats treated with oral FK 506 (1.0 mg/kg and 2.0 mg/kg) compared with normal Wistar Furth (non-BB) rats. Glucose (2 g/kg) was administered at 0 minutes.
Blood Chemistry and Pancreatic Insulin Content
These were determined at the end of the FK 506 treatment in six nondiabetic survivors killed in group 2 and five killed in group 3 (the blood specimen from the sixth animal in this group was lost). There was no increase of blood urea nitrogen, creatinine, glutamine-oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), or total bilirubin compared with six normal Wistar Furth (non-BB) rats (Table 1).
Table 1.
Blood Chemistry and Pancreatic Insulin Content at 120 Day of Age
| Group | n | BUN (mg/dL) | Creatinine (mg/dL) | GOT (U/L) | GPT (U/L) | Total Billrubin (mg/dL) | Insulin Content (U/g pancreas) |
|---|---|---|---|---|---|---|---|
| Group 2 | 6 | 24.0 ± 6.9 | 0.8 ± 0.2 | 130.3 ± 93.0 | 33.8 ± 9.3 | 0.7 ± 0.2 | 5.4 ± 3.5 |
| Group 3 | 5 | 29.8 ± 5.4 | 1.0 ± 0.4 | 134.6 ± 69.1 | 35.0 ± 9.4 | 0.6 ± 0.4 | 2.7 ± 2.4 |
| Wistar Furth | 6 | 23.2 ± 4.2 | 0.8 ± 0.1 | 123.2 ± 19.2 | 36.3 ± 2.9 | 0.4 ± 0.2 | 3.2 ± 0.6 |
Values are mean ± SD.
The pancreatic insulin content in these groups 2 and 3 animals was not significantly different than in the normal Wistar Furth rats (Table 2).
Table 2.
Frequency of Pancreatic Lesions in Treated and Untreated BB Rats and in Normal Wistar Furth Rats
| n | Total Islets | Grade |
PDI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (%) | 1 (%) | 2 (%) | 3 (%) | 4 (%) | |||||
| Group 1 | Diabetic | 4 | 144 | 28 | 33 | 27 | 6 | 6 | 56 |
| Nondiabetic | 1 | 81 | 52 | 38 | 10 | 0 | 0 | 14 | |
| Total | 5 | 225 | 37 | 35 | 21 | 4 | 4 | 40 | |
| Group 2 | Diabetic | 1 | 66 | 3 | 15 | 21 | 36 | 24 | 76 |
| Nondiabetic | 5 | 418 | 74 | 25 | 1 | 1 | 0 | 4 | |
| Total | 6 | 484 | 64 | 23 | 4 | 6 | 3 | 14 | |
| Group 3 | Diabetic | 0 | – | – | – | – | – | – | – |
| Nondiabetic | 5 | 334 | 74 | 25 | 0 | 0 | 0 | 2 | |
| Total | 5 | 334 | 74 | 25 | 0 | 0 | 0 | 2 | |
| Wistar Furth | Diabetic | 0 | – | – | – | – | – | – | – |
| Nondiabetic | 4 | 430 | 85 | 15 | 0 | 0 | 0 | 0 | |
| Total | 4 | 430 | 85 | 15 | 0 | 0 | 0 | 0 | |
PDI: penductal inflammation (expressed as percentage of total islets).
Histology
Pancreatic sections were examined from a total of 20 animals (group 1, (n = 5); group 2, (n = 6); group 3, (n = 5); and normal Wistar-Furth controls, (n = 4). A total of 1,473 islets were identified and graded as to degree of insulitis (Table 2). Each group was subdivided into animals that showed evidence of diabetes at death (group 1A, 2A, etc) and those that did not (group 1B, 2B, etc). Lesions of grade 2, 3, and 4, as well as foci of periductal inflammation, were found almost exclusively in animals that were diabetic. These lesions were markedly reduced in BB rats treated with low-dose FK 506 (group 2) and almost completely prevented with high-dose FK 506 treatment (group 3).
In a separate analysis, immunocytochemical studies were performed on tissue sections from 20 animals (group 1, (n = 6); group 2, (n = 7); group 3, (n = 3); and the normal Wistar-Furth controls, (n = 4). The results of these studies are listed in Table 3. In normal Wistar-Furth animals, insulin-containing cells were clearly demarcated and generally inhabited the centers of the islets. Glucagon-containing cells formed a distinct peripheral rim around the islet. With the development of diabetes in control animals, and in two animals of group 2 who developed diabetes in spite of low-dose FK 506 therapy, insulin-containing cells disappeared completely or became faintly stained, the islet size was reduced, and the cells stained only with antiglucagon antibodies (Table 3). In contrast, normal-appearing distributions of insulin and glucagon-containing cells were observed in the five nondiabetic animals treated with low-dose FK 506 (group 2) and three of three high-dose FK 506 (group 3) animals (Table 3).
Table 3.
Immunocytochemical Analysis of Insulin and Glucagon-Containing Cells in Treated and Untreated BB Rats and in Wistar Furth Rats Pancreas
| Group | Animal No. | Insulin | Glucagon | Outcome |
|---|---|---|---|---|
| Group 1 (No treatment) | 1 | − | + | Diabetic |
| 2 | − | + | Diabetic | |
| 3 | − | + | Diabetic | |
| 4 | + | + | Nondiabetic | |
| 5 | + | ND | Subdiabetic | |
| 6 | − | + | Diabetic | |
| Group 2 (FK 506 1.0 mg) | 1 | + | + | Nondiabetic |
| 2 | + | ND | Nondiabetic | |
| 3 | + | ND | Nondiabetic | |
| 4 | + | + | Nondiabetic | |
| 5 | +c | +c | Diabetic | |
| 6 | −d | + | Diabetic | |
| 7 | + | + | Nondiabetic | |
| Group 3 (FK 506 2.0 mg) | 1 | + | + | Nondiabetic |
| 2 | + | + | Nondiabetic | |
| 3 | + | + | Nondiabetic | |
| Wistar Furth | 1 | + | + | Nondiabetic |
| 2 | + | + | Nondiabetic | |
| 3 | + | + | Nondiabetic | |
| 4 | + | + | Nondiabetic |
Subdiabetic: normal blood glucose, abnormal glucose tolerance test.
ND: not done.
Reduced intensity of staining.
Two islets positive, remainder negative.
DISCUSSION
There is much reason to believe that these studies in an autoimmune animal model have direct clinical relevance. Type I human diabetes is associated with specific HLA haplotypes DR3 and DR4 and an environmental triggering event is postulated to precipitate active autoimmunity and selective beta-cell destruction in genetically susceptible persons.18 The same immunosuppressive treatment that can control the autoimmune process in BB rats should be effective in humans. In BB rats, prevention of diabetes was possible when treatment with CyA was started before the onset of beta-cell destruction.3–6,19,20 Subsequent clinical trials with CyA induced clinical remissions of type I diabetes, providing therapy was started soon after the diagnosis was made.21–23 These trials could not reach their full potential because of nephrotoxicity caused by the drug. Recurrence of diabetes usually was observed promptly when CyA was stopped or more slowly if the doses were too low.
The therapeutic index of FK 506 in humans may be better9–11 and allow the promise of these earlier trials to be fulfilled. Administration of 2.0 mg/kg/d of FK 506 completely prevented the development of spontaneous diabetes in BB rats, and greatly reduced the characteristic histopathologic changes in the pancreas. With immunoassay, insulin was found in normal quantities in the protected pancreases (Table 1), and these findings were in conformity with immunocytochemical studies (Table 3). Blood glucose levels of FK 506-treated animals remained in the normal range. Glucose intolerance, which has been described in BB rats under CyA treatment,24 was not observed with the doses of FK 506 used in this study. The therapeutic effect of a higher FK 506 dose far outlasted the time of active treatment. With a lower dose of FK 506 (1.0 mg/kg), the prevention of pancreatic injury was less complete but the posttherapy relapse rate of animals spared from diabetes was low. There was no mortality in this study except for that caused by diabetes, and toxic effects of FK 506 were not noted except for a slightly decreased rate of weight gain. Liver and renal function were unaltered.
SUMMARY
The BB rat is the experimental analogue of human juvenile diabetes mellitus. From 30 through 120 days after birth, 59 BB rats were treated with water (n = 20), or FK 506 in daily intragastric doses of 1 mg/kg (n = 19) or 2 mg/kg (n = 20). Diabetes developed in 75%, 15%, and 0% of the three groups. Animals protected from diabetes by FK 506, and killed in the nondiabetic state at 120 days had normal intraperitoneal glucose tolerance tests, virtual absence histopathologically of autoimmune insulitis, normal pancreatic insulin content, and immunocytochemical confirmation of islet insulin and glucagon content. Forty five to 75 days after stopping FK 506, about ¾ of the animals who were diabetes free at 120 days have remained so. These results provide support for a clinical trial of FK 506 for recent onset diabetes.
Acknowledgments
Supported by research grants from the Veterans Administration; Project grant no. DK 29961 from the National Institutes of Health; and the Renziehausen Grant of the Children’s Hospital of Pittsburgh.
References
- 1.Nakhooda AF, Like AA, Chappel CI, et al. Diabetes. 1977;26:100. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- 2.Nakwooda AF, Like AA, Chappel CI, et al. Diabetologia. 1978;14:199. doi: 10.1007/BF00429781. [DOI] [PubMed] [Google Scholar]
- 3.Stiller CR, Laupacis A, Keowyn PA, et al. Metabolism. 1983;32(suppl 1):69. doi: 10.1016/s0026-0495(83)80014-6. [DOI] [PubMed] [Google Scholar]
- 4.Like AA, Dirodi V, Thomas S, et al. Am J Pathol. 1984;117:92. [PMC free article] [PubMed] [Google Scholar]
- 5.Brayman KL, Annstrong BA, Shaw LM, et al. Surgery. 1987;102:235. [PubMed] [Google Scholar]
- 6.Bone AJ, Walker R, Varey AM, et al. Diabetes. 1990;39:508. doi: 10.2337/diab.39.4.508. [DOI] [PubMed] [Google Scholar]
- 7.Colle E, Guttmann RD, Fuks A. Diabetes. 1986;35:454. doi: 10.2337/diab.35.4.454. [DOI] [PubMed] [Google Scholar]
- 8.Sternthal E, Like AA, Sarantis K, et al. Diabetes. 1981;30:1058. doi: 10.2337/diab.30.12.1058. [DOI] [PubMed] [Google Scholar]
- 9.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S, Fung JJ, Starzl TE, et al. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung JJ, Todo S, Jain A, et al. Transplant Proc. 1990;22:6. [PMC free article] [PubMed] [Google Scholar]
- 12.Kino T, Hatanaka H, Miyata S, et al. J Antibiot. 1987;40:1256. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 13.Sawada S, Suzuki G, Kawase Y, et al. J Immunol. 1987;139:1797. [PubMed] [Google Scholar]
- 14.Tocci MJ, Matkovich DA, Collier KA, et al. J Immunol. 1989;143:718. [PubMed] [Google Scholar]
- 15.Yamamoto K, Mori A, Nakahama T, et al. Immunology. 1990;69:222. [PMC free article] [PubMed] [Google Scholar]
- 16.McCauley J, Bronsther O, Fung J, et al. Lancet. 1989;2:1516. doi: 10.1016/s0140-6736(89)92951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCauley J, Tzakis AG, Fung JJ, et al. Lancet. 1990;335:674. doi: 10.1016/0140-6736(90)90471-g. [DOI] [PubMed] [Google Scholar]
- 18.Eisenbarth GS. New Engl J Med. 1986;314:1360. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 19.Laupacis A, Stiller CR, Gardell C, et al. Lancet. 1983;1:10. doi: 10.1016/s0140-6736(83)91558-1. [DOI] [PubMed] [Google Scholar]
- 20.Like AA, Anthony M, Guberski DL, et al. Diabetes. 1983;32:326. doi: 10.2337/diab.32.4.326. [DOI] [PubMed] [Google Scholar]
- 21.Stiller CR, Dupre J, Gent M, et al. Science. 1984;223:1362. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- 22.Assan R, Feutren G, Debray-Sachs M, et al. Lancet. 1985;1:67. doi: 10.1016/s0140-6736(85)91964-6. [DOI] [PubMed] [Google Scholar]
- 23.Feutren G, Boitard C, Bougneres P, et al. Cyclosporine for Type I diabetes: Lessons from first clinical trials and new perspectives. In: Eisenbarth GS, editor. Immunotherapy of Diabetes and Selected Autoimmune Diseases. Boca Raton, FL: CRC Press; 1989. pp. 61–72. [Google Scholar]
- 24.Stiller CR, Dupre J. Immune interventional studies in Type I diabetes: Summary of the London (Canada) and Canadian-European experience. In: Eisenbarth GS, editor. Immunotherapy of Diabetes and Selected Autoimmune Diseases. Boca Raton, FL: CRC Press; 1989. pp. 73–84. [Google Scholar]
- 25.Yale JF, Grose M, Seemayer TA, et al. Diabetes. 1987;36:749. doi: 10.2337/diab.36.6.749. [DOI] [PubMed] [Google Scholar]