Abstract
Toll-like receptors (TLRs) are critical components of the innate immune system, acting as pattern recognition molecules and triggering an inflammatory response. TLR associated gene products are of interest in modulating inflammatory related pulmonary diseases of the neonate. The ontogeny of TLR related genes in human fetal lung has not been previously described and could elucidate additional functions and identify strategies for attenuating the effects of fetal inflammation. We examined the expression of 84 TLR related genes on 23 human fetal lung samples from three groups with estimated ages of 60 (57-59d), 90 (89-91d), and 130 (117-154d) days. Using a false detection rate algorithm, we identified 32 genes displaying developmental regulation with TLR2 having the greatest up-regulation of TLR genes (9.2 fold increase) and TLR4 unchanged. We confirmed the TLR2 up-regulation by examining an additional 133 fetal lung tissue samples with a fluorogenic polymerase chain reaction assay (TaqMan®) and found an exponential best-fit curve over the time studied. The best-fit curve predicts a 6.1 fold increase from 60d to 130d. We conclude that TLR2 is developmentally expressed from the early pseudoglandular stage of lung development to the canalicular stage.
Introduction
Toll-like receptors (TLRs) are a critical component of the mammalian innate immune system and act as a vital trigger of inflammation in the body. Most, but not all, TLR proteins signal through a complex intracellular cascade utilizing the MyD88 pathway. TLRs function as an inflammation switch and consequently are of interest in the study of neonatal inflammatory conditions. TLRs have been implicated in various conditions affecting newborns, including necrotizing enterocolitis (1), bronchopulmonary dysplasia (2-4), preterm birth (5), inflammatory bowel disease (6), differential response to bacterial sepsis (7-8), and as mediators of the sequelae of maternal smoking (9). While TLRs are expressed in abundance on inflammatory cells (spleen, dendritic cells, etc.) and on cells in direct contact with the external environment (pulmonary type 2 cells and intestinal enterocytes), they are also found on other organ cells (ovary and kidney) (10) where their function is unknown.
TLR2 and TLR4 recognize LPS and peptidoglycans respectively providing an innate immune response against the most common pathogens involved in chorioamnionitis and neonatal sepsis/pneumonia. In utero activation of TLR4 with LPS has been shown to mature lungs and alter the septaion of alveoli in fetal sheep (11). Both TLR2 and TLR4 show a several fold increase in mRNA expression in ovine and murine fetal lung from late gestation to term and continuing into adulthood (12, 13). In contrast, expression in murine liver tissue is similar in fetal, newborn, and adult stages without developmental expression (12). TLR4 on non-immune cells of murine lung preserves and maintains the lung architecture. TLR4 -/- mice given bone marrow transfusions from wild type mice had absence of TLR4 on the parenchymal cells, but not on inflammatory cells and developed a gradual loss of lung elasticity and irreversible airspace enlargement (14). Thus TLR2 and TLR4 are present on both inflammatory and non-inflammatory cell lines, display developmental expression distinct from other organ systems and have the ability to alter the structure of pulmonary tissue in murine and ovine models. However, there are no studies describing the ontogeny of TLRs on human fetal lung tissue. Elucidating the patterns of developmental expression of TLRs in human fetal lung could provide support for the interaction of TLRs in both normal and dysregulated neonatal pulmonary conditions associated with inflammation. The goals of the study are to identify developmentally regulated TLR signaling pathway genes with a focus on TLR2 and TLR4 specifically. The primary objective was to identify the developmental changes of mRNA expression in 84 genes involved in the TLR signaling pathway in human fetal lung tissue in three groups of varying estimated gestational age. The secondary objective was to confirm the results by quantitative RT-PCR and provided more detailed characterization of the relationship between TLR2 and TLR4 expression with fetal age.
Methods
Samples
Human fetal lung tissue samples were obtained from the Laboratory of Developmental Biology, University of Washington (Seattle, WA), a National Institute of Child Health and Human Development supported tissue retrieval program. Limited information is available concerning the health status of the mothers, but tissues are from elective terminations and are presumed to be free of abnormalities or inflammation. The post-mortem interval prior to collection and freezing was <2 h for all the samples. The use of these tissues was approved by the University of Missouri-Kansas City Pediatric Health Sciences Review Board.
RNA extraction and purification
Tissues were stored at −70°C prior to preparation. Frozen lung tissues (20 to 30 mg) were homogenized and total RNA extracted according to the QIAGEN RNeasy protocol (QIAGEN, Valencia, CA) with an on-column DNase I treatment. Briefly, frozen tissue samples were placed in the provided lysis buffer and homogenized with a rotor-stator homogenizer for at least 30 seconds, simultaneously disrupting and homogenizing the samples. Samples were then centrifuged for 3 minutes and the supernatant transferred to a new tube and mixed with 70% ethanol by pipetting. This mixture was applied to the provided spin column and centrifuged for 15 s with the flow-through discarded. As per protocol, RNase free DNase I was added onto the column and incubated at room temperature for 30 min to remove all DNA contaminants efficiently. Afterwards, the column was washed three times with provided buffers and the purified total RNA eluted off with two applications of RNase-free water placed directly on the column and spun through. Extracted RNA quality was assessed using the microfluidics-based Experion RNA StdSens analysis chip/kit with the Experion automated electrophoresis station (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Samples were chosen with an RNA Quality Index (RQI) > 8.5.
RNA processing and plate-array analysis
Total RNA from each sample was converted to cDNA via a reverse transcriptase reaction using the SABiosciences First Strand kit and analyzed with a commercially available TLR RT2 Profiler PCR Array (SABiosciences, Frederick, MD). The protocol for the First Strand kit involves an additional genomic DNA elimination step followed by preparation of a reverse transcriptase cocktail and incubation of 10 μl at 42°C for 15 minutes. The cDNA synthesis reaction was stopped by heating to 95°C for 5 minutes. 91 μl of water was added to the cDNA product and the solution stored at -20°C until further analysis. cDNA was analyzed on the commercially available TLR Profiler Array using the supplied protocol. The protocol involved adding 102 μl of cDNA reaction product to 1350 μl of supplied SABiosciences RT2 qPCR Master Mix and bringing the volume up to 2700 μl with water. 25μl of this experimental cocktail was added to each well on the 96-well plate which was then loaded in an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems Inc, Foster City, CA) and run in a two-step cycling program with one initial cycle of 95°C for 10 minutes and 40 cycles of 15 sec at 95°C and one minute at 60°C. Each sample was tested once with these arrays.
Sample normalization
The cycle threshold (Ct) for the housekeeping gene beta actin (ACTB) was determined on each array. The relative expression of each TLR related gene (CtExp) was normalized with ACTB by subtracting the latter from the former. Because the level of gene expression is inversely proportional to the Ct and the amount of product doubles with each cycle of the real-time PCR, the value ΔCt is converted to the gene expression level by raising 2 to the negative ΔCt (2-ΔCt). This protocol provided a Ct value for each well corresponding to the relative expression levels of the mRNA of 84 TLR mediated signal transduction pathway genes for each sample. Data from one sample was not used as the expression of the housekeeping genes was significantly anomalous indicating a processing error on the entire array.
Array data analysis
The data for the 84 TLR pathway related genes were analyzed by linear regression with estimated gestational age related to ACTB normalized expression levels using JMP 8 (SAS Cary, NC). The p-values for slope of each gene’s regression line were organized and processed with a false discovery rate algorithm with a detection rate of 10%. The use of the false detection rate identifies genes likely to be developmentally expressed and, therefore, meriting further investigation.
Expression data for both TLR2 and TLR4 specifically were analyzed by pooling the 23 samples into three groups based on estimated gestational age corresponding approximately to different stages of organogenesis. Samples were organized around 60 days (57-59d), 90 days (89-91d), and 130 days (117-154d), representing the early and late pseudoglandular stages and the canalicular stage of lung development, respectively. Median expression levels for both TLR2 and TLR4 for each group were compared with Kruskal Wallis analysis of variance using SPSS 17.0 (SPSS Inc. Chicago, IL).
RNA TaqMan® analysis
In order to confirm and expand on the TLR2 findings in the first stage of this research, we analyzed extracted total RNA from 133 human fetal lung tissue samples with a TLR2 RT-PCR TaqMan® assay and normalized to 18S ribosomal RNA (TaqMan ribosomal RNA Control Reagents, Applied Biosystems, Foster City, CA). This experimental protocol included a one step reverse transcriptase reaction prior to the real-time PCR. We used the same tissue population described above. After determining the RNA concentration on a Nanodrop instrument (Thermo Scientific, Waltham, MA), each sample was diluted to 5μg/μl and mixed with 5μl of Master Mix without UNG, 0.25μl of Multiscribe and RNase Inhibitor mix, 0.5μl of TLR2 Gene Assay 20x mix, and 1.25μl of water. Three μl of RNA solution was added to this 7μl of TaqMan® reaction mix for a total reaction volume of 10 μl. On each 96 well plate, 18 samples were loaded in triplicate reactions with a cDNA standard (107-102 molecules) and non template controls. The TLR2 RNA content of each lung tissue sample was analyzed using the Applied Biosystems (Foster City, CA) TaqMan® RNA-to-CT™ 1-Step Kit and a TLR2 TaqMan® Gene Expression Assays. The cycler protocol involved an initial reverse transcriptase step (30 minutes at 50°C then 10 minutes at 95°C) followed by 40 cycles of real-time PCR (15 seconds at 95°C and one minute at 60°C). 18S rRNA was measured in each sample in triplicate reactions and compared to an 18S rRNA cDNA standard (108-103 molecules) using the TaqMan® Ribosomal RNA Control Reagents in an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA) according to the manufacturer’s recommendations. We obtained a value for the relative amount of each sample by using the slope of the logarithmic curve for the standard run on each plate and the mean Ct for each experimental RNA sample. These values were then normalized using the mean Ct for each corresponding 18S rRNA. A regression analysis was then performed relating estimated gestational age with TLR2 gene expression using both a linear and logarithmic trendline and with corresponding ANOVA tables using Microsoft Excel 2003 (Microsoft, Redmond, WA).
Results
Plate array analysis
Analysis of all 84 TLR related genes using a false detection rate of 10% was performed on the p-values for the slope of the regression line and revealed 32 genes that were differentially expressed (Table 1). Five of the identified genes were down-regulated from the 60 day group to the 130 day group including interleukin-1 alpha and its associated kinase 1 while 27 genes were found to be up-regulated over the period studied including TLR2, 3, 5, 6, 7, 8, and 10 as well as interleukin-8 (IL8) and interleukin-1 alpha associated kinase 2.
Table 1. TLR Signaling Pathway Genes with Identified Developmental Expression.
Genes identified with a 10% False Detection Rate as displaying developmental regulation with their fold change from the 60d group (expression set to 1) to the 130d.
| Gene | Fold Increase in 130d Group |
|---|---|
| Interleukin 8 | 10.34 |
| Colony stimulating factor 2 (granulocyte-macrophage) | 10.19 |
| Toll-like receptor 2 | 9.20 |
| Chemokine (C-X-C motif) ligand 10 | 7.28 |
| Interleukin 12A (natural killer cell stimulatory factor 1, cytotoxic lymphocyte maturation factor 1, p35) | 4.59 |
| Toll-like receptor 1 | 4.13 |
| Toll-like receptor 10 | 4.06 |
| Interleukin-1 receptor-associated kinase 2 | 3.96 |
| Toll-like receptor 8 | 3.93 |
| Chemokine (C-C motif) ligand 2 | 3.26 |
| Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha | 3.11 |
| ECSIT homolog (Drosophila) | 3.07 |
| TANK-binding kinase 1 | 2.94 |
| Toll-like receptor adaptor molecule 1 | 2.91 |
| Heat shock 70kDa protein 1A | 2.48 |
| Toll-like receptor 7 | 2.37 |
| Interferon regulatory factor 1 | 2.28 |
| Lymphocyte antigen 86 | 2.19 |
| Toll-like receptor 6 | 2.07 |
| CD86 molecule | 1.86 |
| Toll-like receptor 5 | 1.85 |
| Toll-like receptor adaptor molecule 1 | 1.77 |
| Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (p105) | 1.75 |
| Caspase 8, apoptosis-related cysteine peptidase | 1.47 |
| Receptor-interacting serine-threonine kinase 2 | 1.47 |
| Toll-like receptor 3 | 1.43 |
| Lymphocyte antigen 96 | 1.42 |
| *Ubiquitin-conjugating enzyme E2N (UBC13 homolog, yeast) | 0.85 |
| *Conserved helix-loop-helix ubiquitous kinase | 0.72 |
| *Interleukin-1 receptor-associated kinase 1 | 0.53 |
| *Interleukin 1, alpha | 0.51 |
| *High-mobility group box 1 | 0.49 |
genes with decreased expression over three stages of development.
TLR2 and TLR4 pooled median analysis
Analyzing the pooled sample means of TLR2 and TLR4 from the three pooled age groups revealed similar normalized relative gene expression (TLR2=0.000777 and TLR4=0.00112, respectively) in the 60 day group (57–59d). Setting the expression level in this youngest group arbitrarily to 1, we then calculated the fold change for the subsequent groups. When compared to the 60 day pooled samples, median TLR2 expression showed a 3.9 fold increase in the 90 day group (89–91 d) and 9.2 fold increase in the 130 day group (117–154 d) (Figure 1) (p<0.001). Data reported reflect normalization with the beta actin housekeeping gene. We normalized the data with housekeeping genes hypoxanthine phosphoribosyltransferase 1 and beta-2-microglobulin and the findings were preserved independent of the normalizing gene used (data not shown). TLR4 expression remained low and was not different among the three groups (p=0.949) (Figure 1).
Figure 1.

TLR fold increase in Human Fetal Lung. Expression levels of TLR2 (□) and TLR4 (■) in human fetal lung tissue. TLR mRNA expressed as relative values with value of the mean of the 60d group set to 1. 95% confidence interval bars shown. TLR2 expression is significantly different in the 90 and120 day groups compared to the 60 day group (p<0.001) while TLR4 is unchanged (p=0.906).
TLR2 TaqMan® analysis
The expanded results (n=133 samples) for TLR2 using the TaqMan® assay with expression normalized with 18S ribosomal RNA for each sample were plotted against estimated gestational age (Figure 2). Analysis with linear regression confirmed the developmental expression. The natural logarithm of the TLR2 expression plotted against age of the samples provides a best fit line with an R2 = 0.359 and p-value < 0.001 indicating an exponential increase over the time studied of the TLR2 gene.
Figure 2.
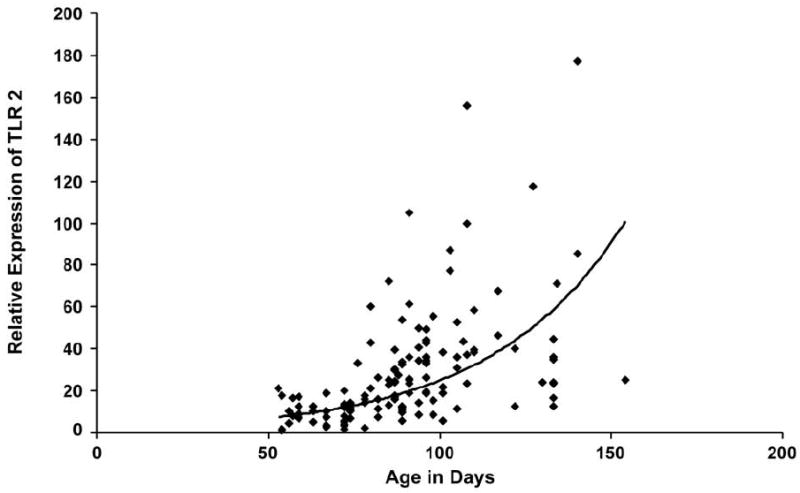
TLR2 Expression with increasing gestational age. Plot of TLR2 expression in 133 fetal lung samples versus estimated gestational age. Best fit exponential curve shown (y=1.9021e0.0258x). R2 = 0.359 and p<0.001.
Discussion
Using a false detection rate algorithm, we identified 32 TLR signaling pathway genes that appear to be developmentally regulated in developing prefunctional lung. Five of these 32 genes appeared to be down-regulated over the period of investigation, while there was coordinated up-regulation of 27 genes (Table 1). The lung tissues studied were at stages of development at which gas exchange and exposure to external environment are not occurring rendering it unlikely that a direct interface for infection would be present as a stimulus for up-regulation. Among the up-regulated genes, TLR2, colony stimulating factor 2 and IL-8 had among the highest fold increases. TLR4 was found in the 60 day lung samples at a concentration comparable to TLR2, however, there was no change in the TLR4 expression level with increasing gestational age. TLR2 activation results in expression of IL8 in B-cells (15). However, the tissue samples studied are presumed to be derived from elective terminations of pregnancy and, as such, are assumed to have been evacuated from a non-inflamed milieu. TLR2 can be triggered by endogenous ligands and has been shown to lead to IL-8 stimulation in both gingival and renal epithelial cells (16,17). Thus, the coordinated and proportional up-regulation could imply translation of the detected TLR2 mRNA to functional protein or indicate a developmental function of IL-8 in addition to TLR2. The identified genes displaying developmental expression provide candidates for further investigation of the interplay of the TLR cascade during pulmonary development.
We confirmed a developmental up-regulation of TLR2 gene expression in human fetal lung tissue during the period of development from the early pseudoglandular stage through the late pseudoglandular and the canalicular stages. While the curve of best-fit for TLR2 expression was exponential for the period studied, the levels of TLR2 mRNA in the canalicular stage were approximately equal to two adult samples tested (data not shown). This is the first description of human fetal lung expression of TLR signaling pathway genes.
The full role of TLRs in fetal development is still evolving. There are suggestions of non-immune developmental functions of TLRs from the preservation of tissue architecture by TLR4 in mice (11) to the emerging role of TLR8 in normal neurite development (18). TLRs have been found to be expressed on non-immune pulmonary cells in both sheep and rabbits (13,19) and TLR2 has been shown to lead to the production of IL-8 from epithelial cells in renal and gingival cells. The differing patterns of our TLR2 and TLR4 expression from published ovine and murine studies (12-13) caution against over-generalization of animal TLR studies to humans. Thus, while the role of TLRs in human fetal lung development is unknown, the increased expression of TLR2 occurring in the late pseudoglandular and canalicular stages of development suggests a possible novel developmental function.
There are several limitation to our study including a lack of staining of the samples to indicate the cell types expressing TLRs within the lung homogenate and in the inability to test samples closer to viability and on to term gestation. A further limitation of this study is the assumption that lung samples were otherwise normally developing fetuses though we have no means for independent confirmation.
In conclusion TLR2, but not TLR4, is developmentally expressed during lung development prior to the ability of gas exchange. This pattern of expression stands in contrast to similar animal studies and suggests a role of TLR2 in human lung organogenesis.
Acknowledgments
The authors wish to thank Drs. Howard Kilbride and Jason Newland for critical reading of this manuscript.
Statement of Financial Support: Supported in part by the Center for Infant Pulmonary Disorders, Children’s Mercy Hospital and Clinics, NHLBI R01 HL 70560 (WET), and Children’s Mercy Hospital Research Vision. The project entitled ’Laboratory of Developmenal Biology’ was supported by NIH Award Number 5R24HD000836 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
Abbreviations
- ACTB
beta actin
- Ct
cycle threshold
- TLR
Toll-like Receptor
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joshua E Petrikin, Department of Pediatrics, University of Missouri-Kansas City School of Medicine, Kansas City, MO 64108.
Roger Gaedigk, Division of Pediatric Pharmacology and Medical Toxicology, University of Missouri-Kansas City School of Medicine, Kansas City, MO 64108.
J Steven Leeder, Division of Pediatric Pharmacology and Medical Toxicology, University of Missouri-Kansas City School of Medicine, Kansas City, MO 64108.
William E Truog, Department of Pediatrics, University of Missouri-Kansas City School of Medicine, Kansas City, MO 64108.
References
- 1.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: Toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49:589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887–L892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri N, Whyte MK, Sabroe I. Reducing the toll of Inflammatory Lung Disease. Chest. 2007;131:1550–1556. doi: 10.1378/chest.06-2869. [DOI] [PubMed] [Google Scholar]
- 4.Raymond T, Schaller M, Hogaboam CM, Lukacs NW, Rochford R, Kunkel SL. Toll-like receptors, Notch ligands and cytokines drive the chronicity of lung inflammation. Proc Am Thorac Soc. 2007;4:635–641. doi: 10.1513/pats.200706-067TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krediet TG, Wiertsema SP, Vossers MJ, Hoeks S, Fleer A, Ruven H, Rijkers GT. Toll-like Receptor 2 Polymorphism is associated with preterm birth. Pediatr Res. 2007;62:474–476. doi: 10.1203/PDR.0b013e31813c9401. [DOI] [PubMed] [Google Scholar]
- 6.Abreu MT, Arditi M. Innate immunity and Toll-like receptors: clinical implications of basic science research. J Pediatr. 2004;144:421–429. doi: 10.1016/j.jpeds.2004.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Sadeghi K, Berger A, Langgartner M, Prusa A, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E. Immaturity of infection control in preterm and term newborns is associated with impaired Toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 8.Viemann D, Dubbel G, Schleifenbaum S, Harms E, Sorg C, Roth J. Expression of Toll-like receptors in Neonatal Sepsis. Pediatr Res. 2005;58:654–659. doi: 10.1203/01.PDR.0000180544.02537.FD. [DOI] [PubMed] [Google Scholar]
- 9.Noakes PS, Hale J, Thomas R, Lane C, Devadason SG, Prescott SL. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J. 2006;28:721–729. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- 10.Fleer A, Krediet TG. Innate immunity: Toll-like receptors and some more. Neonatology. 2007;92:145–157. doi: 10.1159/000102054. [DOI] [PubMed] [Google Scholar]
- 11.Willet KE, Jobe AH, Ikegami M, Newnham J, Brennan S, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res. 2000;48:782–788. doi: 10.1203/00006450-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Harju K, Glumoff V, Hallman M. Ontogeny of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr Res. 2001;49:81–83. doi: 10.1203/00006450-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Hillman NH, Moss TJ, Nitsos I, Kramer BW, Bachurski CJ, Ikegami M, Jobe AH, Kallapur S. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res. 2008;63:388–393. doi: 10.1203/PDR.0b013e3181647b3a. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonell ME, VanDyke TE, Ganley-Leal LM, Nikolajczyk BS. TLR Cross-Talk Specifically Regulates Cytokine Production by B Cells from Chronic Inflammatory Disease Patients. J Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskan MA, Hajishengallis G, Kinane DF. Differential activation of human gingival epithelial cells and monocytes by Porphyromona gingivalis fimbriae. Infect Immun. 2007;75:892–898. doi: 10.1128/IAI.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury P, Sacks SH, Sheerin NS. Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clin Exp Immunol. 2006;145:346–356. doi: 10.1111/j.1365-2249.2006.03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403–411. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]


