Abstract
Transcription factors belonging to the CCAAT-enhancer binding protein (C/EBP) family have been implicated in the regulation of gene expression during differentiation, development and disease. Autoregulation is relatively common in the modulation of C/EBP gene expression and the murine and human C/EBPα genes have been shown to be auto-activated by different mechanisms. In the light of this finding, it is essential that autoregulation of C/EBPα genes from a wider range of different species be investigated in order to gauge the degree of commonality, or otherwise, that may exist. We report here studies that investigate the regulation of the Xenopus laevis C/EBPα gene (xC/EBPα). The –1131/+41 promoter region was capable of directing high levels of expression in both the human hepatoma Hep3B and the Xenopus kidney epithelial A6 cell lines, and was auto-activated by expression vectors specifying for xC/EBPα or xC/EBPβ. Deletion analysis showed that the –321/+41 sequence was sufficient for both the constitutive promoter activity and auto-activation and electrophoretic mobility shift assays identified the interaction of C/EBPs and Sp1 to this region. Although deletion of either the C/EBP or the Sp1 site drastically reduced the xC/EBPα promoter activity, multimers of only the C/EBP site could confer autoregulation to a heterologous SV40 promoter. These results indicate that, in contrast to the human promoter and in common with the murine gene, the xC/EBPα promoter was subject to direct autoregulation. In addition, we demonstrate a novel species-specific action of Sp1 in the regulation of C/EBPα expression, with the factor able to repress the murine promoter but activate the Xenopus gene.
INTRODUCTION
CCAAT-enhancer binding protein-α (C/EBPα) belongs to a family of transcription factors that all contain a highly conserved C-terminal basic region-leucine zipper (bZIP) domain that consists of a basic region, involved in DNA recognition, and an adjacent helical structure, the leucine zipper, which mediates subunit dimerisation (1,2). In contrast, the N-termini of the proteins, which carry the regulatory and the trans-activation domains, are quite divergent. Six distinct C/EBP isoforms have been identified to date (C/EBPα to ζ) with the majority of these able to recognise similar DNA sequences, at least in vitro, activate gene transcription in vivo and form heterodimers in intrafamilial combinations (1,2). Additionally, in the case of C/EBPα and C/EBPβ, polypeptides of different sizes and trans-activating capabilities can be produced from the same mRNA by alternative use of in-frame AUG codons because of a leaky ribosome scanning mechanism (3,4). For example, two polypeptides of 42 kDa (p42) and 30 kDa (p30) can be produced from the C/EBPα mRNA, with the latter having a lower trans-activation potential than the former (4). Thus, the number of C/EBP proteins that may be present in any given tissue/cell may be much higher than the number of C/EBP-encoding genes.
C/EBPα shows a restricted tissue expression pattern and trans-activates the promoters of several hepatocyte- and adipocyte-specific genes that are important for energy metabolism (1,2,5–7). The gene plays a pivotal role in adipocyte differentiation; for example, ectopic expression of C/EBPα in adipoblasts inhibits cell proliferation and activates genes characteristic for differentiated fat cells (5–9) and, conversely, introduction of antisense C/EBPα RNA into pre-adipocytes blocks adipogenesis (10). In support of the importance of C/EBPα, in vivo, the targeted inactivation of the gene in mice results in a failure of the liver and adipose tissue to synthesise glycogen and store lipids, respectively (11). Additionally, these mice have disturbed hepatic architecture with acinar formation, resembling proliferative or pseudoglandular hepatocellular carcinoma (11–13), and also display an early block in the maturation of granulocytes because of a loss of granulocyte colony stimulating factor (G-CSF) signal-directed maturation (13). An important role for C/EBPα, and other family members, in development has also been suggested by limited studies on Drosophila and Xenopus laevis homologues (14–16).
Studies that have examined the cis-acting elements and the trans-acting factors that are involved in the transcriptional regulation of the C/EBPα gene have, however, been rather limited. The proximal promoter region of the mouse C/EBPα gene has been shown to contain consensus recognition sequences for several transcription factors, including nuclear factor-1 (NF-1), upstream stimulatory factor (USF), Sp1, Myc/Max, C/EBP and activator-protein 2α (AP-2α) (7,17–21). AP-2α [C/EBPα undifferentiated protein (CUP)] and Sp1 have been shown to repress C/EBPα expression (7,19–21). Additionally, both C/EBPα and C/EBPβ can directly auto-activate the promoter by interacting with the C/EBP-recognition sequence (7,17,18). Such direct autoregulation has also been identified for the rat promoter (22). In contrast, autoregulation of the human gene occurs indirectly through C/EBPα-mediated stimulation of USF binding to the proximal promoter region (23). It is, however, currently unclear whether such a novel indirect mode of autoregulation is unique to the human C/EBPα gene or may also be applicable to other species. Similarly, it is unclear whether the repressive action of Sp1 is a general phenomenon or specific to the murine promoter.
We have previously studied the regulation of C/EBP gene expression from several species including X.laevis, a model system widely used to study embryonic development (16,24–27). In the present paper, we report the organisation of the 5′ flanking region of the X.laevis C/EBPα gene (xC/EBPα) and investigate the importance of the regulatory elements within this region, particularly in relation to species-specific differences.
MATERIALS AND METHODS
Materials
The human hepatoma Hep3B and the X.laevis kidney epithelial A6 cell lines were obtained from the European Collection of Animal Cell Cultures and the American Collection of Animal Cell Cultures, respectively. All the cell culture reagents were purchased from Gibco BRL. The Sp1 antiserum was obtained from Santa Cruz.
Cloning and sequencing
A X.laevis genomic library (Stratagene) was screened under conditions of high stringency with a radiolabelled 579 bp PstI/PstI fragment from the xC/EBPα cDNA insert that contained sequences corresponding to the N-terminus of the gene (15,16). The cDNA fragment was radiolabelled with [α-32P]dCTP by the random priming technique using the Megaprime DNA labelling kit (Amersham). The filters were prehybridised for 6 h at 42°C in 50% (v/v) formamide, 6× SSPE, 5× Denhardt’s, 0.1% (w/v) SDS and 10 mg/ml yeast tRNA. Hybridisation with the denatured radiolabelled probe was carried out, overnight, at 42°C in the prehybridisation buffer. The filters were then washed three times (30 min each) at 65°C in 0.1× SSC, 0.1% (w/v) SDS and exposed to X-ray film. From 500 000 p.f.u. screened, a single positive clone was isolated that survived the tertiary round of screening. The coding and the putative promoter regions present in the isolated genomic clone were identified by Southern blot analysis of restriction endonuclease digested recombinant phage DNA probed with radiolabelled probes derived from the xC/EBPα cDNA insert that corresponded to the N- and C-termini of the protein, respectively. The analysis indicated that ∼1.1 kb of the putative promoter region was contained in a 1.5 kb fragment that could be obtained from digestion of recombinant phage DNA with PstI. This 1.5 kb fragment, along with two subfragments obtained by its digestion with HindIII and EcoRV (0.6 kb) and XbaI and SalI (1.1 kb), were subcloned into pUC18 (28) and sequenced using the Thermo Sequenase Fluorescent Labelled Primer Cycle Sequencing kit with 7-deaza-dGTP (Amersham). The sequence of both strands of the entire 1.5 kb fragment was determined using a combination of M13 universal and reverse primers and specific primers designed against the derived sequence. Electrophoresis was carried out using the LI-COR automated DNA Sequencer and the data analysed with the Base Image IR Software Package (MWG-Biotech). In some cases, manual sequencing was performed using the dideoxy chain-termination method and the Sequenase Version 2.0 sequencing kit (US Biochemical).
Preparation of manipulated xC/EBPα promoter–reporter constructs
PCR-based approaches were used to prepare the various xC/EBPα promoter–reporter constructs. The PCR reactions were performed using either Vent DNA polymerase or the ExpandTM system (Boehringer Mannheim), in order to minimise PCR-generated mutations, and this was confirmed by sequencing of all the products following subcloning into the pGEM-T vector (28). To produce a promoter–luciferase construct with the entire cloned putative xC/EBPα promoter region, recombinant pUC18 containing the 1.5 kb PstI fragment (see above) was used as a template for PCR with the M13 universal primer and an oligonucleotide designed against the +41 to +22 region of the xC/EBPα gene (designated as xalpha). The product was phosphorylated using polynucleotide kinase and ATP, and subcloned into dephosphorylated pGL2-Basic vector (28) that had been digested previously with XhoI followed by ‘filling in’ of the protruding ends using Klenow Polymerase and dNTPs. This construct was designated as –1131/+41. Three further deletions were produced similarly using a common xalpha primer and oligonucleotides designed against the –634 to -615, –321 to –302 and –121 to –108 regions, respectively. These DNA constructs were designated as –634/+41, –321/+41 and –121/+41, respectively.
Internal deletions of the C/EBP and the Sp1 binding sites were produced by the overlap-extension method (29) using recombinant pUC18 containing the –321/+41 promoter fragment. The oligonucleotide combinations used were 5′-CAGCGGAGTGCAATGTAACG-3′ and 5′-GCGTTACATTGCACTCCGCT-3′ (delC/EBP) and 5′-GCAATAGAGAAGGCCTCCAG-3′ and 5′-CTGGAGGCCTTCTCTATT-3′ (delSP1). The fragments were subcloned into the pGL2-Basic vector (28) and verified by sequence analysis.
For the preparation of the pC/EBP×4 and pSp1×4 DNA constructs, containing four copies of the C/EBP or Sp1 sites from the xC/EBPα promoter linked to a heterologous SV40 promoter in the pGL2-promoter vector (28), the following oligonucleotides were synthesised: 5′-CCGGAGTGTTTCCAGACAAAGTGTTTCCAGACAAAGTGTTTCCAGACAAAGTGTTTCCAGACAA-3′ and 5′-TCGATTGTCTGGAAACACTTTGTCTGGAAACACTTTGTCTGGAAACACTTTGTCTGGAAACACT-3′ for pC/EBP×4, and 5′-CCGGAAGCAGGGGCGTGGCCAAGCAGGGGCGTGGCCAAGCAGGGGCGTGGCCAAGCAGGGGCGTGGCC-3′ and 5′-TCGAGGCCACGCCCCTGCTTGGCCACGCCCCTGCTTGGCCACGCCCCTGCTTGGCCACGCCCCTGCTT-3′ for pSp1×4. These were designed in such a manner so that, following annealing, they had overhangs which allowed their direct cloning into the XmaI and XhoI sites in the pGL2-promoter vector (28).
Cell culture and transient transfection assays
Monolayers of human hepatoma cell line Hep3B were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% (v/v) heat-inactivated (30 min, 56°C) fetal calf serum (HI-FCS), 100 µg/ml streptomycin and 100 U/ml penicillin, at 37°C in a humidified atmosphere of 5% (v/v) CO2 in air. The Xenopus A6 cell line was grown in medium containing 75% (v/v) NCTC-135 media and 15% (v/v) sterile water supplemented with HI-FCS, streptomycin and penicillin, as above, at 26°C in an incubator containing 20 mM HEPES. DNA transfections were carried out by the calcium phosphate precipitation method (30) and utilised 2–6 µg of reporter plasmid, 0–4 µg of expression construct and 0.5 µg of CMV-β-galactosidase (28) to provide an internal control for transfection efficiency. After 16 h, the cells were washed with phosphate buffered saline and left in fresh culture medium for 36 h. The luciferase and the β-galactosidase activity in cell extracts were then determined using commercially available kits (Promega). The luciferase activity was normalised to the β-galactosidase value, and each transfection was repeated at least three times.
Electrophoretic mobility shift assays (EMSA)
The –82/+41, –218/–82 and –321/–218 xC/EBPα promoter fragments were used for EMSA. For the –82/+41 fragment, the –634/+41 promoter construct in the pGL2-Basic vector was digested with the enzymes BglII and StyI (BglII site occurs in the polylinker of the vector), size-fractionated by electrophoresis on a 2% (w/v) agarose gel and the resulting fragment of ∼125 bp was purified. Isolation of the –218/–82 and –321/–218 fragments required two distinct restriction endonuclease digestions and gel purification: (i) digestion of recombinant –634/+41 plasmid with SacI and StyI, purification of the insert of ∼550 bp, its digestion with HphI and isolation of the –218/–82 fragment; (ii) digestion of recombinant –321/+41 DNA construct, containing the promoter fragment in the opposite orientation to that used for transfection experiments, with NheI and HindIII, purification of the fragment of ∼360 bp, further digestion with HphI and isolation of the –321/–218 insert. The sequences of the oligonucleotides used for EMSA were: 5′-TGCAGATTGCGCAAT-3′ and 5′-TGCATTGCGCAATCT-3′ (D-C/EBP) (16,25), 5′-CATAGTGGCGCAAACTCCCTTACTG-3′ and 5′-CAGTAAGGGAGTTTGCGCCACTATG-3′ [C-reactive protein (CRP)] (24,25), 5′-GCAGAATTTCTTGGGAAAGAAAA-3′ and 5′-GCTATTTTCTTTCCCAAGAAATT-3′ [β-casein (β-cas)] (25), 5′-GATCCTTCGTGACTCAGCGGGATCCTTCGTGAG-3′ and 5′-CCGCTGAGTCACGAAGGATCCCGCTGAGTCACG-3′ (AP-1) (25), 5′-TCGACGGTGTAGGCCACGTGACCGGGTGT-3′ and 5′-CGCACACCCGGTCACGTGGCCTACACCGT-3′ (USF) (23), 5′-TAGATTCGATCGGGGCGGGGCGAG-3′ and 5′-GCCCTCGCCCCGCCCCGATCGAAT-3′ (Sp1) (23), 5′-TAGCTTGGCATTAGGACCCAGTCGAAGGGCAA-3′ and 5′-GGCTTGCCCTTCGACTGGGTCCTAATGCCAAG-3′ (NS), 5′-CAGTGTTTCCAGAC-3′ and 5′-TTGGTCTGGAAACA-3′ (wtC/EBP), 5′-AAGCAGGGGCGTGGC-3′ and 5′-GAGGCCACGCCCCT-3′(wtSP1), and 5′-GCCTTGGCATTA-3′ and 5′-GAACCGTAATCG-3′ (NF-1). These were radiolabelled by ‘fill-in’ reactions using [α-32P]dCTP and Klenow DNA polymerase.
Nuclear and whole cell extracts were prepared essentially as described by Ramji et al. (24) and Timchenko et al. (23), respectively. The protease inhibitors (0.5 mM PMSF, 1 µg/ml pepstatin A, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml I-S soybean trypsin inhibitor) and DTT (0.5 mM) were added to all the buffers before use. The protein concentration of the nuclear extract was determined using the BCA protein assay kit as described by the manufacturer (Pierce).
For EMSA analysis, 4 µg of protein was incubated in a 20 µl total reaction volume containing 34 mM potassium chloride, 5 mM magnesium chloride, 0.1 mM DTT and 3 µg poly(dI-dC). After 10 min on ice, 32P-labelled probes (40 000 c.p.m.) were added and the incubation continued for 30 min at room temperature. Following the addition of 5 µl of a 20% (w/v) Ficoll solution to each sample, the free probe and the DNA–protein complexes were resolved on 6% (w/v) polyacrylamide gels in 0.25× TBE (22.5 mM Tris base, 22.5 mM boric acid, 0.5 mM EDTA). The gels were dried under vacuum and exposed to X-ray film. For antibody supershift assays, samples of the nuclear extracts and appropriate antisera were incubated for 30 min on ice prior to the addition of the radiolabelled probe (24,25).
RESULTS
Cloning and sequence analysis of the xC/EBPα promoter
The xC/EBPα genomic clone was isolated as described in Materials and Methods and its identity confirmed by sequence analysis of fragments that contained the entire coding region (data not shown). An overall identity of 93% was found to exist between the predicted amino acid sequence of the coding region present in the cDNA and genomic clones, with almost total identity in the various domains/regions that have been implicated as being important for the function of C/EBPα, including the basic region, the leucine zipper, the three putative trans-activation domains, the two in-frame methionine residues in the coding region and a sequence in the putative 5′UTR with a potential to code for a short upstream open reading frame that has been shown to act in cis to inhibit its translation in several cell lines (1,2,4,31). It is likely that allelic variations, which are relatively common in X.laevis because of a genomic tetraplodization event that occurred over 30 million years ago (32,33), are responsible for some variation seen between the xC/EBPα cDNA and genomic sequences.
In order to investigate the regulation of the xC/EBPα promoter, the nucleotide sequence was determined of both strands of a 1.5 kb PstI genomic fragment that contained ∼1.1 kb of the putative promoter region. The sequence has been deposited in the EMBL, GenBank and DDBJ databases under the accession number AJ250304. Before carrying out detailed analysis of the sequence elements that are present in this region, the transcription start site was determined by primer extension analysis. At least four extension products that differed by a single nucleotide were obtained in three independent experiments (data not shown), and indicated that the transcription of the xC/EBPα gene begins 125–128 bp from the first translation initiation codon in the coding region. This compares well with the corresponding distance of 126 bp identified for the human, mouse and rat C/EBPα genes (17,18,22,23). The A residue at position 125 was therefore arbitrarily assigned as +1.
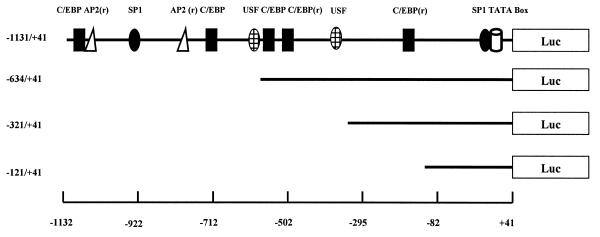
Despite a high level of conservation between the predicted amino acid sequence of xC/EBPα and homologous genes from other species (e.g. 81% identity with the Rana catesbeiana homologue, 69–75% with human, rodent and avian members) (2,7,15,34,35), the degree of sequence identity in the putative promoter regions was relatively low (35–42%) (data not shown). Such a low level of sequence identity has also been seen between the promoter regions of the mouse and the human C/EBPα genes (53%) (23). Despite this low overall sequence similarity, a computer search (36) of the xC/EBPα promoter region for the presence of consensus recognition sequences of known transcription factors in the TRANSFAC database showed the existence of putative sites for several factors that have been demonstrated to be important for the expression of C/EBPα genes (7,17–23,34). Figure 1 shows a schematic representation of the putative sites of four such transcription factors (i.e. C/EBP, USF, Sp1, AP2) along with the xC/EBPα promoter–luciferase constructs used in this study.
Figure 1.
Schematic representation of the xC/EBPα promoter–luciferase DNA constructs. The relative position of the putative TATA box and binding sites for the transcription factors C/EBP, Sp1, Ap2 and USF are shown with r indicating that the consensus sequence occurs in the reverse orientation. See text for details.
Analysis of the xC/EBPα promoter activity in transfected cells
In order to identify the regions in the xC/EBPα promoter that were important for the transcription of this gene, three different promoter–luciferase DNA constructs were prepared initially. Thus, the –1131/+41, –634/+41 and –321/+41 promoter regions were amplified by PCR and subcloned upstream of the luciferase gene in the pGL2-Basic-luciferase vector as described in Materials and Methods. The human hepatoma Hep3B and the Xenopus kidney epithelial A6 cell lines were used for all the transfection experiments. The Hep3B cells were chosen because they are used widely as a model for the analysis of promoter elements that are involved in both the constitutive and inducible expression of genes in hepatocytes, including the C/EBP family members, from different species (see 37,38 and references therein) and, additionally, possess a relatively low level of endogenous C/EBPα expression, thereby allowing auto-activation to be monitored relatively easily. The A6 cells represent the only extensively characterised Xenopus cell line that has been used for promoter-dissection experiments (e.g. 39), and provides an ideal host model system for the analysis of the xC/EBPα promoter.
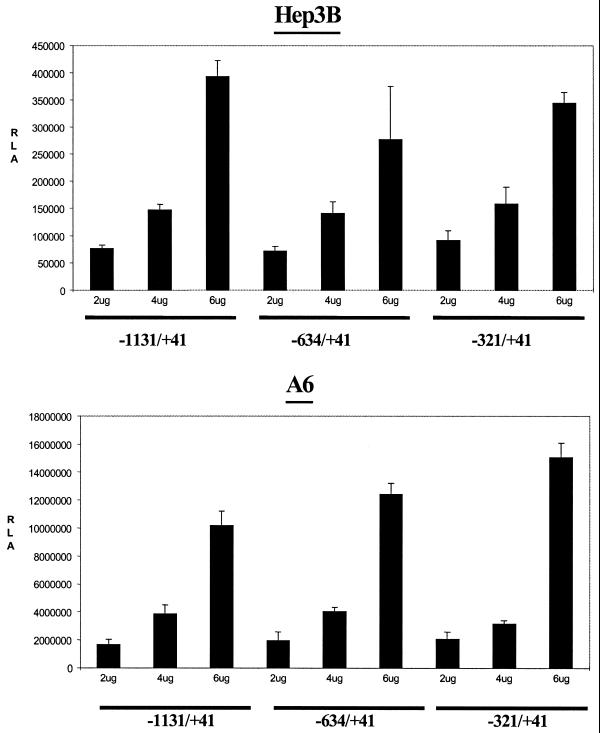
Thus, the three xC/EBPα promoter–luciferase DNA constructs were transfected into the two cell lines along with the CMV-β-galactosidase expression plasmid in order to provide an internal control for transfection efficiency. The RSV luciferase and the pGL2-Basic-luciferase vectors were used as positive and negative controls, respectively. The cells were harvested 36 h after transfection, and the luciferase activity was determined and normalised to the β-galactosidase value. The values obtained using the pGL2-Basic-luciferase vector were subtracted from those obtained using the various xC/EBPα promoter–luciferase DNA constructs in the same vector (typically 3–5%). As shown in Figure 2, a concentration-dependent increase in the relative luciferase activity was obtained in both cell lines. Additionally, in contrast to the activity of the pGL2-Basic-luciferase and the CMV-β-galactosidase vectors, the xC/EBPα promoter activity was substantially higher in the homologous A6 cell line (Fig. 2). The deletions also had no significant effect on the relative luciferase activity, thereby indicating that the –321/+41 region was sufficient to drive the constitutive C/EBPα promoter activity.
Figure 2.
The activity of xC/EBPα promoter deletion constructs in transfected cells. Three different concentrations of the xC/EBPα promoter constructs, –1131/+41, –634/+41 and –321/+41, were transfected into Hep3B or A6 cells as indicated. The luciferase activity at each point was normalised to the β-galactosidase activity, and the values are expressed as relative luciferase activity (RLA). Each value represents the mean ± SD from three independent experiments.
Both C/EBPα and C/EBPβ can activate the xC/EBPα promoter
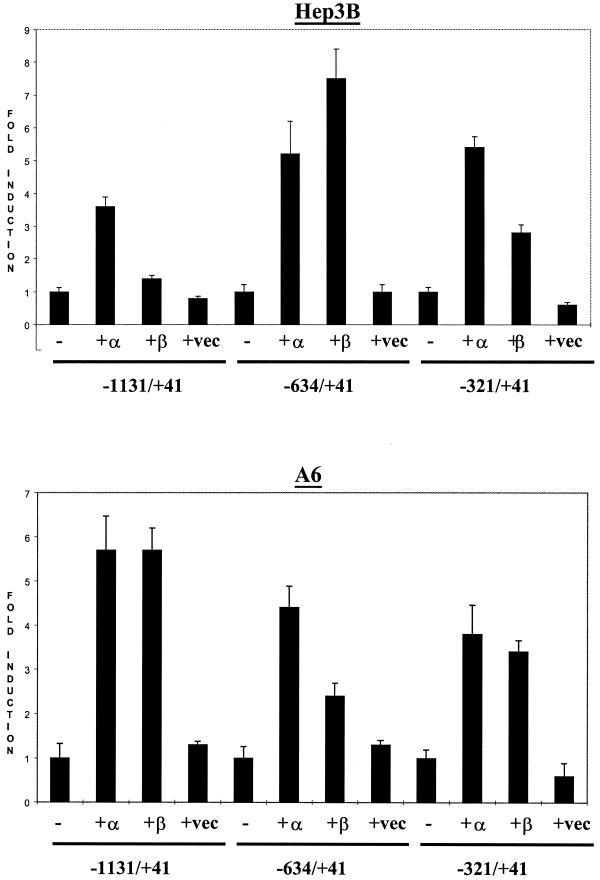
The mouse and the rat C/EBPα promoters have been shown to be activated by both C/EBPα and C/EBPβ (17,18,22). In contrast, the human promoter can only be activated by the C/EBPα expression vector (23). In order to examine the auto-activation properties of the xC/EBPα promoter, the transfection experiments with the three promoter–luciferase DNA constructs, detailed above, were repeated in the presence of co-transfected expression plasmids coding for xC/EBPα or the LAP form of xC/EBPβ, which has been demonstrated to act as a potent transcriptional activator (16). The parent pCS2+ plasmid (16), without any cloned insert, was used as a control. Figure 3 shows that all three xC/EBPα promoter–luciferase DNA constructs could be auto-activated by the xC/EBPα expression plasmid, with an ∼3.5- to 5-fold induction of luciferase activity in Hep3B cells and ∼4- to 5.5-fold increase in the A6 cell line. This level of induction was therefore higher than the ∼3-fold increase seen with the mouse and the human promoters (17,18,23). A slightly different profile was obtained when an expression plasmid coding for the LAP form of xC/EBPβ was used in the transfection experiments. In Hep3B cells, although only a marginal increase in the luciferase activity was seen with the –1131/+41 region, an increase between 3- and 7.5-fold was obtained with the two deletion constructs (Fig. 3). In contrast, all three xC/EBPα promoter–luciferase DNA constructs were activated in A6 cells by the xC/EBPβ expression plasmid (approximate induction of 3.5- to 6-fold). As expected, co-transfection of the parent pCS2+ expression plasmid did not produce any significant changes in the luciferase activity with all the three xC/EBPα promoter constructs (Fig. 3). Overall, therefore, these results indicate that the xC/EBPα promoter can be activated by both the xC/EBPα and the xC/EBPβ expression plasmids, and that the –321/+41 region contains sufficient information for this activation. Additionally, deletions of the putative USF binding sites (i.e. absent in the –321/+41 DNA construct) (see Fig. 1) have little effect on the auto-activation of the xC/EBPα promoter.
Figure 3.
Autoregulation of the xC/EBPα promoter. The three xC/EBPα promoter constructs, –1131/+41, –634/+41 and –321/+41, were co-transfected into Hep3B or A6 cell lines along with recombinant plasmid DNA driving the expression of either xC/EBPα (+α), the LAP form of xC/EBPβ (+β) or the pCS2+ vector (+vec). The relative luciferase activity obtained using the promoter-less pGL2-Basic vector, in the absence or the presence of the various pCS2+ plasmids, was subtracted from the corresponding values obtained using the xC/EBPα promoter constructs. The normalised activity for each of these promoter constructs alone (–) has arbitrarily been assigned as 1, with those obtained in the presence of the co-transfected expression plasmids being shown as fold induction with respect to this value. Each value represents the mean ± SD from three to four independent experiments.
Analysis of DNA–protein interactions with the minimal xC/EBPα promoter region
In order to examine the interaction of DNA binding proteins with the –321/+41 xC/EBPα promoter sequence, EMSA were carried out using three fragments from this region (–82/+41, –218/–82 and –321/–218) that were produced by restriction endonuclease digestions as described in Materials and Methods. Sequence analysis indicated the absence of any transcription factor binding sites between the junctions of these fragments. Extracts with high levels of xC/EBPα binding activity were used for the experiments and were obtained by transfection of Hep3B or A6 cells with the pCS2+xα plasmid that directs the expression of the xC/EBPα protein. Initial analysis showed a similar pattern with both nuclear and whole cell extracts and the former were therefore used for all subsequent experiments. The strategy was based on similar studies that have been carried out on the human C/EBPα promoter (23). In order to examine whether the various DNA–protein complexes represented specific interactions of the proteins with the radiolabelled probe, competition experiments were performed using an excess of the appropriate unlabelled fragment. Additionally, to identify whether any of the DNA–protein complexes represented binding by the C/EBP family members, the competition analysis was extended to include an excess of double stranded oligonucleotides for a high affinity C/EBP binding site (D-C/EBP; see Materials and Methods) (16,25), with an AP-1 binding site oligonucleotide acting as a control.
For all three fragments, at least two DNA–protein complexes were obtained using extracts from Hep3B or A6 cells, each of which could be competed by an excess of the corresponding unlabelled fragment (data not shown). From these, only a single DNA–protein complex, obtained using the –218/–82 promoter fragment, could be competed specifically by an excess of the C/EBP binding site oligonucleotide (data not shown).
Both C/EBPα and C/EBPβ interact with the –218 to –82 promoter region
Because the xC/EBPα promoter could be auto-activated by expression plasmids specifying for both C/EBPα and C/EBPβ and EMSA on the minimal promoter region showed the interaction of the C/EBPs with the –218/–82 sequence (Fig. 3; data not shown), we next decided to investigate further the role of the C/EBPs in autoregulation. Oligonucleotide containing the putative recognition sequence present in this region, designated as wt-C/EBP, was synthesised and used for EMSA with nuclear extracts from Hep3B or A6 cells that had been transfected with either the pCS2+xα expression plasmid or the parent pCS2+ vector. As shown in Figure 4A, the C/EBP DNA binding activity was substantially higher in extracts from Hep3B or A6 cells that had been transfected with the xC/EBPα expression plasmid compared to the parent control vector. Such a dramatic increase in DNA binding activity was specific to the C/EBPs and was not seen when a control NF-1 binding site oligonucleotide was included in the analysis (Fig. 4A). The DNA–protein complex could be competed by an excess of the corresponding unlabelled wt-C/EBP oligonucleotide or those containing the C/EBP binding site from the promoter of the CRP or the β-cas genes, but not by an Sp1 or NF-1 binding site oligonucleotide (Fig. 4B). In order to investigate further the interaction of the C/EBPs with the wt-C/EBP oligonucleotide, antibody interference EMSA experiments were carried out with antisera against the two C/EBP isoforms (α and β) using D-C/EBP oligonucleotide and pre-immune rabbit IgG as controls. The extracts and antisera were incubated together prior to the addition of the wt-C/EBP ologonucleotide. Despite the relatively low signal intensity obtained from the DNA–protein complexes with wt-C/EBP oligonucleotide in this experiment, the production of the antibody–protein–DNA ‘super-shift’ complex was seen with both antisera but not the pre-immune serum (Fig. 4C), thereby indicating that both C/EBPα and C/EBPβ interacted with this site.
Figure 4.
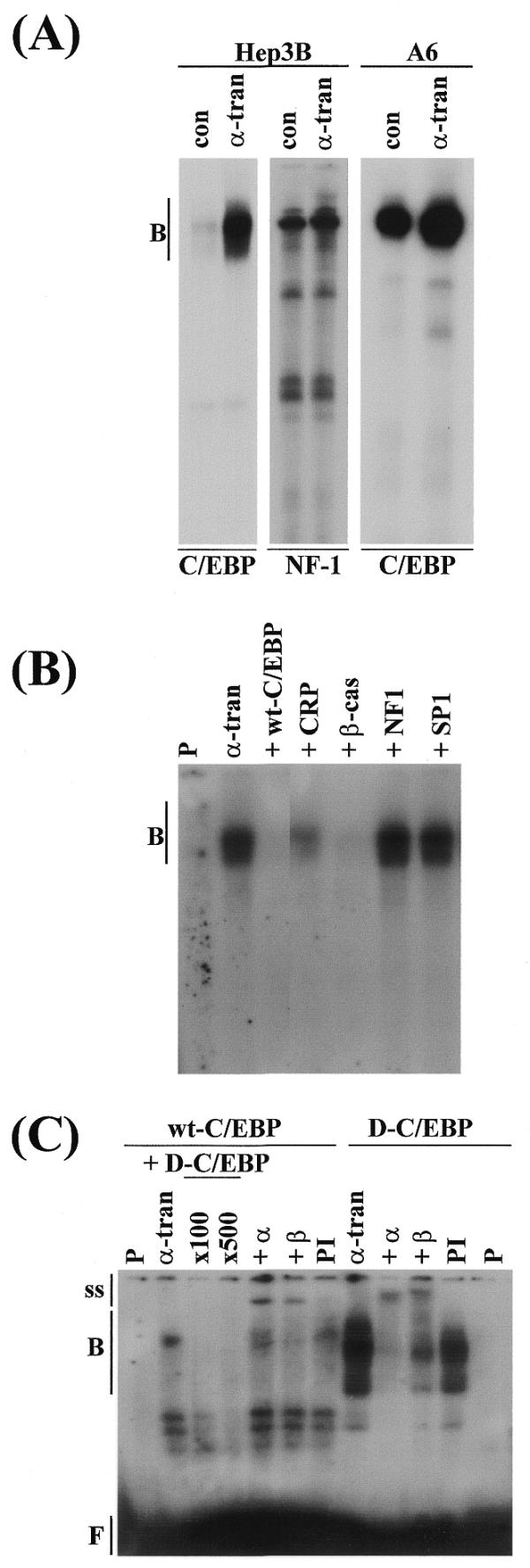
Interaction of the C/EBPs with the –218/–82 sequence. (A) Nuclear extracts from Hep3B or A6 cells, which had been transfected with the control pCS2+ vector (con) or the xC/EBPα expression plasmid (α-tran) were used for EMSA with radiolabelled wt-C/EBP or NF-1 binding site oligonucleotides. (B) Competition experiments were carried out using extracts from Hep3B cells transfected with the xC/EBPα expression plasmid and a 400-fold molar excess of oligonucleotides for: the corresponding unlabelled wt-C/EBP sequence (+wt-C/EBP), a C/EBP binding site from the C-reactive protein gene promoter (+CRP), a C/EBP binding site from the β-cas gene promoter (+β-cas), a NF-1 recognition sequence (+NF1) and a Sp1 binding site (+SP1). (C) Antibody interference EMSA was carried out using extracts from Hep3B cells transfected with the xC/EBPα expression plasmid and radiolabelled wt-C/EBP or D-C/EBP oligonucleotides, in the absence (α-tran) or the presence of antisera against C/EBPα (+α) or C/EBPβ (+β), or pre-immune serum (PI). For EMSA with wt-C/EBP oligonucleotide in (C), competition experiments were also carried out using a 100- and 500-fold molar excess of unlabelled D-C/EBP oligonucleotide (+D-C/EBP). Vertical arrows labelled B, ss and F indicate the position of the major DNA–protein complexes, antibody–DNA–protein supershifted complexes and free probe, respectively. P represents the profile obtained using the radiolabelled oligonucleotide alone; the free probe in (A) and (B) has migrated from the gel.
The C/EBP recognition sequence is required for both constitutive expression and auto-activation
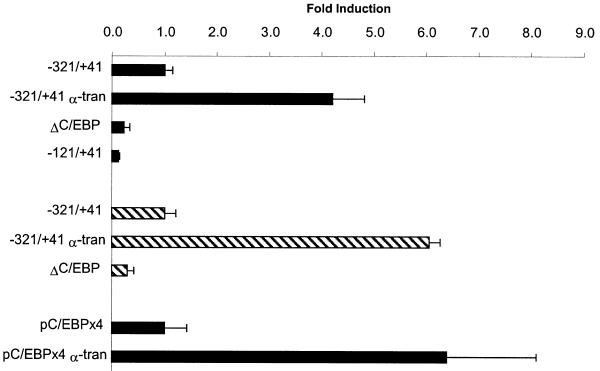
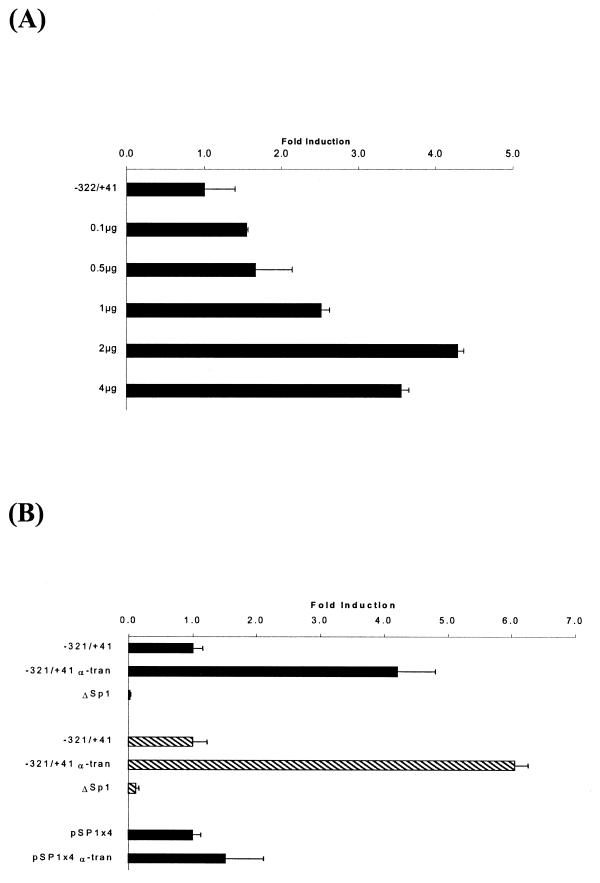
In order to investigate further the importance of the C/EBP recognition sequence in the minimal xC/EBPα promoter, an internal deletion mutant that lacked this site was prepared by PCR using the del-C/EBP oligonucleotide (see Materials and Methods). EMSA experiments confirmed that this deletion abolished the interaction of the C/EBPs, present in nuclear extracts from Hep3B or A6 cells, with the wt-C/EBP sequence (data not shown). The DNA construct, ΔC/EBP, was then used in transient transfection assays in both Hep3B and A6 cell lines along with the –321/+41 promoter construct. As shown in Figure 5, deletion of the C/EBP site resulted in a drastic reduction in the constitutive xC/EBPα promoter activity. Such a dramatic reduction in reporter gene activity was also seen when another 5′ deletion construct that lacked this C/EBP site (i.e. –121/+41) was included in the analysis (Fig. 5). In order to examine whether this C/EBP site is essential for auto-activation, four copies of the sequence were linked to the heterologous SV40 promoter in the pGL2-promoter vector (28) and used for transient transfection assays in Hep3B cells. As shown in Figure 5, a 6-fold increase in luciferase gene activity was obtained in the presence of co-transfected pCS2+xα plasmid.
Figure 5.
Analysis of the importance of the C/EBP binding site in the minimal xC/EBPα promoter region. The –321/+41, –121/+41, ΔC/EBP and pC/EBP×4 promoter–reporter DNA constructs were transfected into Hep3B or A6 cell lines (filled and hatched histograms, respectively) in the presence of the pCS2+xα plasmid that drives the expression of xC/EBPα (α-tran) or the control parent pCS2+ vector. The control activity obtained with the –321/+41 or pC/EBP×4 DNA constructs has arbitrarily been assigned as 1, with the others being shown with respect to this value. Each value represents the mean ± SD from three independent experiments.
Sp1 binds to the –82 to +41 region of the xC/EBPα promoter
Analysis of the sequence of the –82 to +41 region showed the presence of a consensus Sp1 recognition sequence, GGGGCGTGG, at position –42 to –34. Although consensus Sp1 binding sites have been identified in the promoter of the human, mouse, rat and chicken C/EBPα genes (7,17–23,34), their precise function has only been determined in detail in the murine promoter. Here, Sp1 was shown to bind to a GC box at the 5′ end of the C/EBP recognition sequence and, thereby, repress C/EBP-mediated promoter activation (21). In the light of this finding, we decided to investigate the role of the Sp1 site in the xC/EBPα promoter further and in more detail. In order to identify whether Sp1 was involved in the various DNA–protein interactions seen with the –82/+41 promoter region, competition EMSA were performed using extracts from Hep3B or A6 cells that had been transfected with the pCS2+xα expression plasmid, and a 500-fold molar excess of a consensus Sp1 binding site oligonucleotide. As a control, a similar molar excess of oligonucleotides corresponding to a high affinity C/EBP binding site (D-C/EBP), sequences corresponding to the –53 to –81 region of the xC/EBPα promoter (i.e. outside the consensus Sp1 site; NS) and a consensus USF site were included. As shown in Figure 6A, all the DNA–protein complexes obtained with the –82/+41 xC/EBPα promoter region could be competed out using an excess of Sp1 binding site oligonucleotide but not with the other sequences. In order to further confirm the interaction of Sp1 with this region, competition experiments were carried out using oligonucleotide designed against the Sp1 site present in the –34 to –42 region of xC/EBPα promoter (designated as wt-Sp1) and a slightly larger oligonucleotide that contained deletion of this sequence (referred to as del-Sp1). As shown in Figure 6B, the wt-Sp1, but not del-Sp1, competed for the binding of nuclear proteins to this region. Additionally, when both the wt-Sp1 and del-Sp1 oligonucleotides were used for similar EMSA with extracts from Hep3B or A6 cells, which had previously been transfected with the pCS2+xα expression plasmid, specific DNA binding was only seen with wt-Sp1 oligonucleotide (data not shown). In order to confirm the interaction of Sp1 with the wt-Sp1 oligonucleotide, antibody supershift assays were performed using antisera against Sp1. As shown in Figure 6C, antibody–DNA–protein supershifted complex was obtained using extracts from both cell lines.
Figure 6.
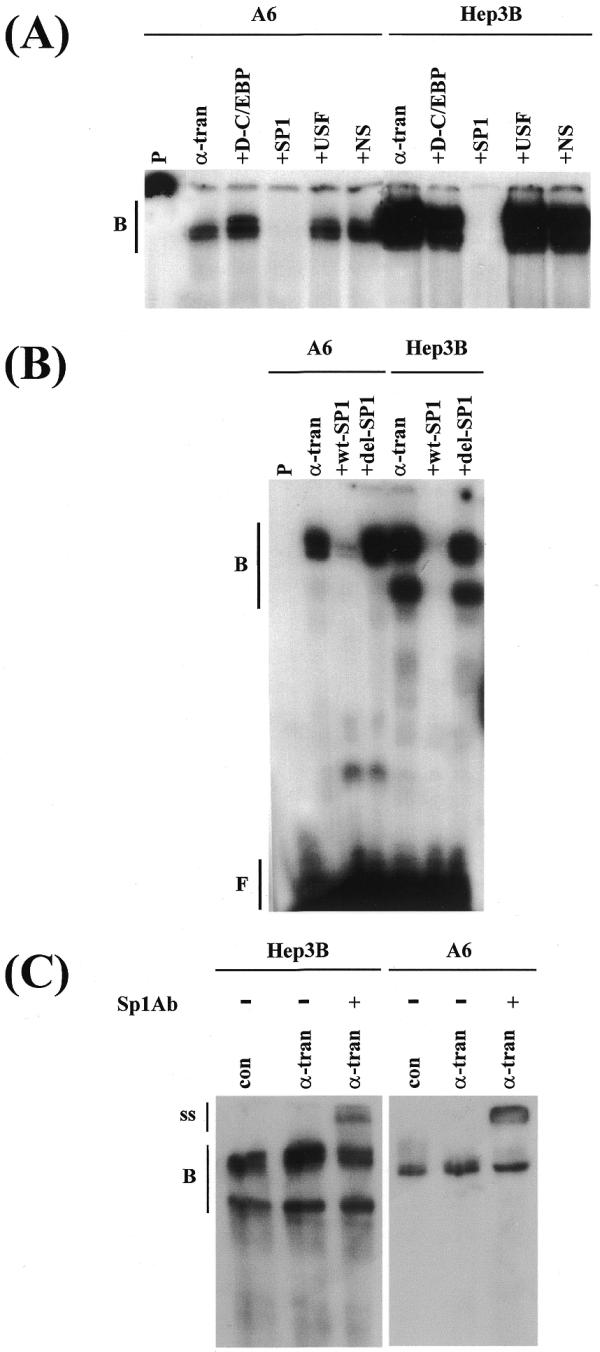
Binding of Sp1 to the –82/+41 region. EMSA were carried out using nuclear extracts from Hep3B or A6 cells that had been transfected with the pCS2+ control vector (con) or the pCS2+xα expression plasmid (α-tran) and either –82/+41 promoter fragment (A and B) or an oligonucleotide containing the putative Sp1 recognition sequence (C). Competition experiments used a 500-fold excess of oligonucleotides for: a high affinity C/EBP binding site (+D-C/EBP), consensus Sp1 recognition sequence (+SP1), consensus USF binding site (+USF), sequences corresponding to the –53 to –81 region of the xC/EBPα promoter (+NS), the Sp1 site from the xC/EBPα promoter (+wt-SP1) or a larger sequence containing deletion of this site (+del-SP1). In (C), + indicates the profile obtained with extracts from pCS2+xα-transfected cells in the presence of antiserum against Sp1. Vertical lines labelled B, ss and F show the position of the DNA–protein complex, the antibody–DNA–protein-supershifted complex and the free probe, respectively.
The Sp1 binding site is necessary for constitutive expression but not auto-activation
In order to investigate the importance of the Sp1 binding site in the xC/EBPα promoter, co-transfection experiments were initially carried out using the –321/+41 promoter–luciferase DNA construct and increasing concentrations of Sp1 expression plasmid. As shown in Figure 7A, the Sp1 expression plasmid was found to increase the xC/EBPα promoter activity. To analyse the importance of the Sp1 site further, an internal deletion mutant that lacked the putative recognition sequence was prepared by PCR using the del-SP1 oligonucleotide that curtails the binding of this factor (data not shown). Transient transfection assays into both Hep3B and A6 cell lines showed that the deletion of this site produces a drastic reduction of the constitutive xC/EBPα promoter activity (Fig. 7B). To examine whether this Sp1 site is also involved in the auto-activation of the xC/EBPα gene, four copies of the recognition sequence were linked to the heterologous SV40 promoter in the pGL2-promoter vector. As shown in Figure 7B, the luciferase activity was similar both in the absence or the presence of co-transfected xC/EBPα expression plasmid, thereby indicating that the site was involved in constitutive promoter activity but not auto-activation.
Figure 7.
Analysis of the importance of the Sp1 binding site in the minimal xC/EBPα promoter region. (A), the –321/+41 construct was transfected in the absence or presence of different concentrations of the Sp1 expression plasmid as indicated and (B), the –321/+41, ΔSp1 and pSP1×4 promoter–reporter DNA constructs were transfected into Hep3B or A6 cell lines (filled and hatched histograms, respectively) in the presence of the pCS2+xα plasmid (α-tran) or the parent pCS2+ vector. The control activity obtained with the –321/+41 or pSP1×4 DNA constructs has arbitrarily been assigned as 1, with those obtained in the presence of the co-transfected pCS2+xα or Sp1 expression plasmid being shown with respect to this value. Each value represents the mean ± SD from two independent experiments.
DISCUSSION
We report in this paper the organisation of the xC/EBPα promoter and an investigation of the nuclear factors that regulate the expression of this gene. Transient transfecion assays showed that the –321/+41 region contained sufficient information for both the constitutive promoter activity and activation by xC/EBP expression plasmids. Both Sp1 and the C/EBPs were identified as important contributors to the regulation of xC/EBPα expression with both sites being involved in constitutive expression and the C/EBP recognition sequence being absolutely essential for autoregulation. In addition, the studies demonstrate that the regulation of the C/EBPα gene involves species-specific mechanisms for both auto-activation and the action of Sp1.
Autoregulation is relatively common in the control of genes encoding transcription factors from several species, including X.laevis, and includes basal transcription factors, cell cycle regulated factors, stimulus-responsive factors and cell-type-specific factors (40,41). The advantages for such type of control are enormous (40). First, it offers the cells a relatively simple regulation mechanism without the involvement of a battery of other factors, which in turn, would need to be subject to another hierarchy of regulation. Thus, given that eukaryotes may contain thousands of independent transcriptional activators, autoregulation may be necessary to limit the complexity required for completely independent regulation. Secondly, it may provide the cell with a direct means to sense and control the cellular concentration of a given factor. Thirdly, autoregulation of cell-type-specific factors could represent a form of memory that may contribute, or even define, a determined state. Fourthly, autoregulation of stimulus responsive factors may serve to amplify cellular signals transiently and, additionally, allow attenuation of the response irrespective of the presence or absence of a given inducer.
Analysis of the effects of both mutations and multimerisation of C/EBP recognition sequence in the xC/EBPα promoter (Fig. 5) show that, in contrast to the human gene but in common with the mouse and rat genes, the Xenopus homologue is subject to direct autoregulation. In addition, we have also identified a potential species-specific mode of action of Sp1 in the regulation of C/EBPα gene expression. For example, the mouse C/EBPα promoter contains a Sp1 consensus sequence between the –185 to –194 region that overlaps with a consensus C/EBP recognition sequence (21). A similar configuration also occurs in the rat promoter (22). Tang et al. (21) have shown recently that Sp1 competes for the binding of the C/EBPs to this site and, thereby, represses C/EBPα promoter activity. Because the expression of Sp1 was found to be higher in preadipocytes and to decrease during the early phases of differentiation, it was suggested that Sp1 may contribute, at least in part, to the repression of C/EBPα gene transcription prior to adipocyte differentiation (21). The decrease in Sp1 levels seen early in the differentiation programme may facilitate the access of other C/EBP family members to the C/EBP regulatory element and, thereby, cause derepression of the C/EBPα gene (21). This mechanism has been suggested to act in conjunction with the repressor AP2-α, the binding site of which is present in the human, rat and the xC/EBPα promoters, and its expression levels are also high in pre-adipocytes and decrease dramatically during differentiation (7,18–20). Our studies, on the other hand, demonstrate Sp1 as an activator of xC/EBPα gene transcription with deletion of its recognition sequence producing a drastic reduction in activity (Fig. 7). Such a mode of Sp1 action may also be the case for the human and the chicken promoters; the former contains a consensus Sp1 site but lacks C/EBP recognition sequence and the putative C/EBP and Sp1 sites present in the chicken gene are non-overlapping (23,34). Therefore, at least for the human, Xenopus and chicken C/EBPα genes, Sp1 may act as an activator and its regulation during adipogenesis may rely solely on the action of AP-2α.
In conclusion, we have demonstrated that the xC/EBPα gene is subject to direct autoregulation through the binding of the C/EBPs to a recognition sequence present in the proximal promoter region. In addition, we show that Sp1 activates its expression. Thus, the species-specific modulation of C/EBPα gene expression involves both autoregulation and the mode of action of Sp1.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Prof. Guntram Suske for the Sp1 expression plasmid, Bob Jones for the preparation of the figures and Scott Irvine for help with computer analysis. F.T.K. was supported by a PhD studentship from the University of Balikesir. D.P.R. acknowledges financial support from the Biotechnology and Biological Sciences Research Council (BBSRC).
DDBJ/EMBL/GenBank accession no. AJ250304
References
- 1.Yamanaka R., Lekstrom-Himes,J., Barlow,C., Wynshaw-Boris,A. and Xanthopoulos,K.G. (1998) CCAAT/enhancer binding proteins are critical components of the transcriptional regulation of hematopoiesis. Int. J. Mol. Med., 1, 213–221. [DOI] [PubMed] [Google Scholar]
- 2.Lekstrom-Himes J. and Xanthopoulos,K.G. (1998) Biological role of the CCAAT enhancer binding protein family of transcription factors. J. Biol. Chem., 273, 28545–28548. [DOI] [PubMed] [Google Scholar]
- 3.Descombes P. and Schibler,U. (1991) A liver-enriched transcriptional activator, LAP and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell, 67, 568–579. [DOI] [PubMed] [Google Scholar]
- 4.Ossipow V., Descombes,P. and Schibler,U. (1993) CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc. Natl Acad. Sci. USA, 90, 8219–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poli V. (1998) The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem., 273, 29279–29282. [DOI] [PubMed] [Google Scholar]
- 6.Darlington G.J., Ross,S.E. and MacDougald,O.A. (1998) The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem., 273, 30057–30060. [DOI] [PubMed] [Google Scholar]
- 7.Lane M.D., Tang,Q.-Q. and Jiang,M.-S. (1999) Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem. Biophys. Res. Commun., 266, 677–683. [DOI] [PubMed] [Google Scholar]
- 8.Umek R.M., Friedman,A.D. and McKnight,S.L. (1991) CCAAT-enhancer binding protein: a component of a differentiation switch. Science, 251, 288–292. [DOI] [PubMed] [Google Scholar]
- 9.Freytag S.O., Paielli,D.L. and Gilbert,J.D. (1994) Ectopic expression of the CCAAT/enhancer-binding protein-α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev., 8, 1654–1663. [DOI] [PubMed] [Google Scholar]
- 10.Lin F.-T. and Lane,M.D. (1992) Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev., 6, 533–544. [DOI] [PubMed] [Google Scholar]
- 11.Wang N.D., Finegold,M.J., Bradley,A., Ou,C.N., Abdelsayed,S.V., Wilde,M.D., Taylor,L.R., Wilson,D.R. and Darlington,G.J. (1995) Impaired energy homeostasis in C/EBPα knockout mice. Science, 269, 1108–1112. [DOI] [PubMed] [Google Scholar]
- 12.Flodby P., Barlow,C., Kylefjord,H., Ahrlund-Richter,L. and Xanthopoulos,K.G. (1996) Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein α. J. Biol. Chem., 271, 24753–24760. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D.E., Zhang,P., Wang,N.D., Hetherington,C.J., Darlington,G.J. and Tenen,D.G. (1997) Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl Acad. Sci. USA ., 94, 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rorth P. and Montell,D.J. (1992) Drosophila C/EBP: a tissue-specific DNA-binding protein required for embryonic development. Genes Dev., 6, 2299–2311. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q. and Tata,J.R. (1992) Characterization and developmental expression of Xenopus C/EBP gene. Mech. Dev ., 38, 79–81. [DOI] [PubMed] [Google Scholar]
- 16.Kousteni S., Tura-Kockar,F., Sweeney,G.E. and Ramji,D.P. (1998) Characterisation and developmental regulation of the Xenopus laevis CCAAT-enhancer binding protein-β gene. Mech. Dev., 77, 143–148. [DOI] [PubMed] [Google Scholar]
- 17.Christy R.J., Kaestner,K.H., Geiman,D.E. and Lane,M.D. (1991) CCAAT/enhancer binding protein gene promoter: Binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc. Natl Acad. Sci. USA, 88, 2593–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legraverend C., Antonson,P., Flodby,P. and Xanthopoulos,G. (1993) High level activity of the mouse CCAAT/enhancer binding protein (C/EBPα) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res., 21, 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q.-Q., Jiang,M.-S. and Lane,M.D. (1997) Repression of transcription mediated by dual elements in the CCAAT/enhancer binding protein α gene. Proc. Natl Acad. Sci. USA, 94, 13571–13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang M.-S., Tang,Q.-Q., Mclenithan,J., Geiman,D., Shillinglaw,W., Henzel,W.J. and Lane,M.D. (1998) Derepression of the C/EBPα gene during adipogenesis: identification of AP-2α as a repressor. Proc. Natl Acad. Sci. USA, 95, 3467–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Q.-Q., Jiang,M.-S. and Lane,M.D. (1999) Repressive effect of Sp1 on the C/EBPα gene promoter: role in adipocyte differentiation. Mol. Cell. Biol., 19, 4855–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rana B., Xie,Y., Mischoulon,D., Busher,N.L.R. and Farmer,S.R. (1995) The DNA binding activity of C/EBP transcription factors is regulated in the G1 phase of the hepatocyte cell cycle. J. Biol. Chem., 270, 18123–18132. [DOI] [PubMed] [Google Scholar]
- 23.Timchenko N., Wilson,D.R., Taylor,L.R., Abdelsayed,S., Wilde,M., Sawadogo,M. and Darlington,G.J. (1995) Autoregulation of the human C/EBPα gene by stimulation of upstream stimulatory factor binding. Mol. Cell. Biol., 15, 1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramji D.P., Vitelli,A., Tronche,F., Cortese,R. and Ciliberto,G. (1993) The two C/EBP isoforms, IL-6DBP/NF-IL6 and C/EBPδ/NF-IL6β, are induced by IL-6 to promote acute-phase gene transcription via different mechanisms. Nucleic Acids Res., 21, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatakos G., Davies,G.E., Grosse,M., Cryer,A. and Ramji,D.P. (1998) Expression of the genes encoding CCAAT-enhancer binding protein isoforms in the mouse mammary gland during lactation and involution. Biochem. J., 334, 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabatakos G., Kousteni,S., Cryer,A. and Ramji,D.P. (1998) Nucleotide sequence of ovine C/EBPɛ gene. J. Anim. Sci., 76, 2953–2954. [DOI] [PubMed] [Google Scholar]
- 27.Davies G.E., Sabatakos,G., Cryer,A. and Ramji,D.P. (2000) The ovine CCAAT-enhancer binding protein δ gene: cloning, characterization and species-specific autoregulation. Biochem. Biophys. Res. Commun., 271, 346–352. [DOI] [PubMed] [Google Scholar]
- 28.Gacesa P. and Ramji,D.P. (1994) Vectors: Essential Data. John Wiley and Sons, Chichester, UK.
- 29.Jones P. (1998) Manipulation of cloned DNA. In Jones,P. (ed.), Vectors: Cloning Applications. John Wiley and Sons, Chichester, UK, pp. 122–142.
- 30.Graham F.L. and van der Eb,A.J. (1973) A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology, 52, 456–467. [DOI] [PubMed] [Google Scholar]
- 31.Lincoln A.J., Monczak,Y., Williams,B.C. and Johnson,P.F. (1998) Inhibition of CCAAT/enhancer-binding protein α and β translation by upstream open reading frames. J. Biol. Chem., 273, 9552–9560. [DOI] [PubMed] [Google Scholar]
- 32.Kobel H.R. and Du Pasquier,L. (1986) Genetics of polyploid Xenopus. Trends Genet., 2, 310–315. [Google Scholar]
- 33.Ali A., Salter-Cid,L., Flajnik,M.J. and Heikkila,J.J. (1996) Molecular cloning of a cDNA encoding a Xenopus laevis 70-kDa heat shock cognate protein, hsc70.II. Biochim. Biophys. Acta, 1309, 174–178. [DOI] [PubMed] [Google Scholar]
- 34.Calkhoven C.F., Gringhuis,S.I. and Ab,G. (1997) The chicken CCAAT/enhancer binding protein α gene. Cloning, characterisation and tissue distribution. Gene, 196, 219–229. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Hu,H. and Atkinson,B.G. (1994) Characterization and expression of C/EBP-like genes in the liver of Rana catesbeiana tadpoles during spontaneous and thyroid hormone-induced metamorphosis. Dev. Genet., 15, 366–377. [DOI] [PubMed] [Google Scholar]
- 36.Quandt K., Frech,K., Karas,H., Wingender,E. and Werner,T. (1995) MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res., 23, 4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciliberto G., Colantuoni,V., DeFrancesco,R., DeSimone,V., Monaci,P., Nicosia,A., Ramji,D.P., Toniatti,C. and Cortese,R. (1993) Transcriptional control of gene expression in hepatic cells. In Karin,M. (ed.), Gene Expression: General and Cell-Type-Specific. Birkhauser, Boston, MA, pp. 162–242.
- 38.Ramji D.P., Cortese,R. and Ciliberto,G. (1993). Regulation of C-reactive protein, haptoglobin and hemopexin gene expression. In Mackiewicz,A., Kushner,M.D. and Baumann,H. (eds), Acute Phase Proteins: Molecular Biology, Biochemistry and Clinical Applications. CRC Press, Boca Raton, FL, pp. 162–242.
- 39.Ranjan M., Wong,J.M. and Shi,Y.B. (1994) Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J. Biol. Chem., 269, 24699–24705. [PubMed] [Google Scholar]
- 40.Bateman E. (1998) Autoregulation of eukaryotic transcription factors. Prog. Nucleic Acid Res. Mol. Biol., 60, 133–168. [DOI] [PubMed] [Google Scholar]
- 41.Tata J.R., Baker,B.S., Machuca,I., Rabelo,E.M.L. and Yamauchi,K. (1993) Autoinduction of nuclear receptor genes and its significance. J. Steroid Biochem. Mol. Biol., 46, 105–119. [DOI] [PubMed] [Google Scholar]