INTRODUCTION
Bipolar I disorder (BP-I) in children and adolescents is associated with substantive psychosocial dysfunction and human suffering [1, 2]. Safe and effective treatments are needed to reduce symptomatology and improve quality of life for the vulnerable youngsters and families impacted by this illness.
Lithium is a benchmark treatment for adults suffering from bipolar illness [3], with evidence of benefit dating back almost 60 years [4]. However, there has been relatively little research regarding the use of lithium in the treatment of youths suffering from mania [5]. Given this paucity of information, a Written Request (WR) was issued by the Food and Drug Administration (FDA) under the auspices of the Best Pharmaceuticals for Children Act (BPCA) (FDA 2002) for the study of this agent in youths. In response, a contract was awarded to rigorously study lithium in children and adolescents with mania.
A key step in developing evidence-based dosing paradigms for any compound is the characterization of the drug's pharmacokinetics (PK) [6]. Therefore, two of the goals of this contract were to characterize the pharmacokinetics of lithium and to develop evidence-based dosing for lithium in children and adolescents [7].
Although many studies have examined the PK of lithium in adults [8–10], relatively little is known about the PK of lithium in pediatric patients. Vitiello et al. [11] described lithium PK in nine children (aged 9–12 years) with a DSM-III-R primary diagnosis of conduct disorder or adjustment disorder. Subjects received one single 300 mg dose of lithium carbonate. The disposition of lithium in these children appeared to generally be similar to that seen in adults. However, their elimination half-life and greater total lithium clearance were shorter than reported in adult studies.
The present study was performed in order to describe the first dose PK of lithium in children and adolescents. In addition to characterizing the disposition of lithium in children and adolescents with BP-I, we also explored whether patient-specific characteristics (e.g. age, gender and weight) influence the PK of lithium in this patient population.
MATERIALS & METHODS
The data presented herein were collected as part of an open-label clinical trial [7]. All procedures were approved by each participating investigator's Institutional Review Board for Human Investigation. The parents/guardians of all study subjects provided written informed consent and all youths provided written assent before participation.
Study Subjects
Youths aged 7 to 17 years who met DSM-IV (APA 1994) criteria for BP-I in a current manic or mixed state without active psychotic symptoms were eligible to participate. Subjects underwent a psychiatric interview by a child and adolescent psychiatrist. In addition, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (KSADS-PL) [12] was administered by a trained interviewer to confirm the clinician's diagnosis. Subjects also needed to receive a score of 20 or greater on the Young Mania Rating Scale (YMRS) [13] both at screening and at the initiation of lithium dosing. Subjects were required to be in good physical health and capable of swallowing study medication as whole lithium carbonate capsules.
Subjects with a current diagnosis of schizophrenia, schizoaffective disorder, a pervasive developmental disorder, anorexia nervosa, bulimia nervosa, substance dependence, or obsessive-compulsive disorder were excluded. Also, subjects with an intelligence quotient less than 70 based on the results of the Wechsler Abbreviated Scales of Intelligence (WASI) Vocabulary and Matrix Reasoning Subscales [14] were not included. Other exclusion criteria included positive screens for drugs of abuse during screening and at retest 1 to 3 weeks later, current general medical conditions and unstable medical illnesses that might be affected adversely by lithium or could influence the efficacy or safety of lithium, a previous trial with lithium lasting at least 4 weeks with trough serum concentrations between 0.8–1.2 mEq/L, or a history of an allergy or adverse reaction to lithium. Furthermore, subjects could not be taking: psychotropic agents other than stimulants within the preceding 2 weeks, stimulants within the preceding week, or fluoxetine or depot antipsychotics within the past month.
Potential subjects that had a psychiatric hospitalization within 1 month of screening for psychosis or serious homicidal/serious suicidal ideation or who were currently experiencing active hallucinations or delusions were also excluded. Youth with symptoms of mania that may be attributable to a general medical condition or secondary to use of medications were not eligible. Sexually active females who were not using adequate forms of birth control were not eligible. Additionally, female subjects were ineligible if they were currently pregnant or lactating.
The screening period to determine subject eligibility was 3–28 days in duration. Subjects currently receiving fluoxetine at the screen were able to have an extended screening period lasting up to, but not exceeding, 6 weeks.
Medication Dosing and Sample Collection
Prior to their first dose, subjects were required to fast for at least 8 hours. Eligibility criteria were reviewed and confirmed prior to receiving the first dose of medication. Subjects weighing less than 20 kg were to receive a single dose of 300 mg (arm I). Subjects weighing 20 kg or more were randomly assigned to receive either a single 600 mg (arm I) or 900 mg (arm II) dose of lithium. Randomization assignments were stratified by age (children: 7–11 years vs. adolescents: 12–17 years) and sex.
Blood samples for lithium serum levels were obtained pre-single dose, and at 0.5, 1, 1.5, 2, 4, 8, 12, and 24 hours post-dose. The patients were admitted to the clinical research center for the first 24 hours of the study. In addition, subjects were randomly assigned in a 1:1 ratio to return to the study site for collection of a single blood sample to assess lithium serum concentration at either 48 or 72 hours post-dose.
Safety Assessments
Prior to the subject receiving lithium, an electrocardiogram (ECG), a physical examination, and a determination of sexual maturation [15, 16] was performed. Blood pressure and pulse were measured prior to receiving lithium and at 2, 8, and 24 hours after the single dose.
Additionally, laboratory examinations, including a chemistry profile, complete blood count with differential, urine toxicology screen, urinalysis, thyroid stimulating hormone (TSH), triiodothyronin (T3), serum thyroxine (T4), anti-thyroid antibody, urine (on the day of dosing) and a serum (during the screening process) pregnancy test for females was obtained prior to subjects receiving their first dose of lithium carbonate. The collection of a 24 hour urine sample was also initiated immediately prior to the first dose of lithium in order to determine creatinine clearance. Spontaneously reported adverse events were recorded during the blood sample collection period.
Lithium Assays
Lithium concentrations in serum were measured using standard clinical chemistry methods available at each site. These included: the LI Flex reagent cartridge (Dade Behring) used on the Dimension Clinical Chemistry System, a lithium Ion-Specific Electrode in a DuPont Na/K/Li Analyzer, the colorimetric Vitros Li Slide method on a Vitros chemistry system at 3 sites, and a spectrophotometric method on the Beckman Coulter Synchron chemistry analyzer at 2 sites. Concentrations were reported in a format with 2 significant digits after the decimal point from centers 1, 2, and 4, and with one such significant digit from centers 3, 6, 7, and 8. The lower limit of quantification was 0.20 mEq/L for samples which were analyzed at centers 1, 3, 4, 7 and 8, and was 0.25 mEq/L at centers 2 and 6.
Pharmacokinetic Analyses
The creatinine clearance was both measured directly and calculated using the Schwartz method [17, 18] in subjects under 12 years of age. The Cockcroft-Gault method [19] was used for all other subjects.
Statistical Analyses
Nominal data are reported as frequencies and percents and continuous data are reported as means and standard deviations unless otherwise noted.
Population Pharmacokinetic Analyses
One-, two-, and three-compartment disposition models with first-order, zero-order, sequential zero- and first-order, and mixed-order absorption, with or without lag-time of oral absorption were considered. First-order, mixed-order, and parallel first-order and mixed-order elimination were assessed. Competing models were evaluated by their predictive performance assessed via visual predictive checks, NONMEM's objective function, and residual plots.
A model with two disposition compartments and first-order absorption and elimination was chosen as the structural model (base model). The amount of lithium in the absorption compartment (Agut) and initial condition is
where ka (h−1) is the absorption rate constant for lithium. The amount of lithium in the central compartment (Ac) is
where CL (L/h) is the apparent elimination clearance of lithium, CLic (L/h) is the apparent intercompartmental clearance, and Vc (L) and Vp (L) are the apparent volumes of distribution of the central and peripheral compartments. The total volume of distribution is described by Vc + Vp. The amount in the peripheral compartment (Ap) is
For the visual predictive check, the plasma concentration profiles were simulated for 20,000 subjects for each competing model and assessed for the whole dataset and for each dose separately. From these data the median, the nonparametric 80% prediction interval (10% to 90% percentile), and the nonparametric 50% prediction interval (25% to 75% percentile) were calculated for the predicted plasma concentrations. These prediction interval lines were then over-laid on the original raw data. If the model described the data adequately, then 20% of the observed data points should fall outside the 80% prediction interval at each time point and 50% of the data should fall outside the interquartile range. The median predicted concentrations and the prediction intervals were compared with the observed data. It was assessed whether the median and the prediction intervals mirrored the central tendency and the variability of the observed data for the various models.
The between subject variability (BSV) was estimated for all PK parameters with an exponential parameter variability model. The residual unidentified variability was described by a combined additive and proportional error model.
Possible relationships between patient specific covariates such as body size, age, gender, sexual maturation and renal function, and the individual pharmacokinetic parameter estimates were first explored by graphical analysis. The individual estimates for eta (deviation of the individual estimate from the population mean) of the respective pharmacokinetic parameters were plotted against the individual values of the covariate (eta-plots). Several body size descriptors such as total body weight, body mass index (BMI), and fat-free mass (FFM) [20] were tested. The effect of body size on the pharmacokinetic parameters was predicated on allometric scaling based on FFM as follows:
where Vi and Vpop are the group and populaton estimates of volume of distribution for all subjects with the same FFM, FFMi is the individual FFM, and FFMstd is a standard FFM chosen at 53 kg to enable comparisons with adult subjects. The CLi and CLpop are the group and population estimates of clearance for all subjects with the same FFM. Similar equations were used for allometric scaling based on a standard body weight of 70 kg.
After accounting for body size and body composition, the potential effect of other covariates was assessed. Covariates were introduced into the model in a stepwise fashion. Inclusion of a specific covariate in the final model was based on visual analysis of eta-plots, change in NONMEM's objective function, and the reduction in BSV.
Computation
The Laplacian estimation method with the interaction estimation option in NONMEM version VI level 1.1 (NONMEM Project Group, University of California, San Francisco, CA, USA) was used for population PK modeling and simulation. The Beal M3 method [21] was implemented with the F-FLAG option in NONMEM in order to consider concentrations below the quantification limit. The individual limits of quantification for each site were included.
RESULTS
Demographics
Thirty-nine subjects were enrolled into treatment arms I and II across seven study sites and received the single dose of study medication. No subjects who received study medication discontinued before the 48/72 hour visit. These 39 subjects (20 males and 19 females) received study medication, had evaluable pharmacokinetic data, and were included in this analysis.
The average age of the subjects was 11.8 years. None of the subjects received a dose of 300 mg of lithium, because no subject weighed less than 20 kg. Seventeen (9 children and 8 adolescents) received a starting dose of 600 mg of lithium and 22 (11 children and 11 adolescents) received a starting dose of 900 mg of lithium. Subject demographics are shown in Table 1.
Table 1.
Demographics of subjects with bipolar I disorder completing the study who received a single dose of lithium carbonate
| Mean (SD) | Children (n=20) | Adolescents (n=19) | Overall (n=39) |
|---|---|---|---|
| Age, years | 9.9 (1.4) | 14.0 (1.5) | 11.9 (2.5) |
| Sex | |||
| Male | 10 | 10 | 20 |
| Female | 10 | 9 | 19 |
| Race | |||
| Caucasian | 17 | 14 | 31 |
| African American | 2 | 4 | 6 |
| Other | 1 | 1 | 2 |
| Tanner Stage | |||
| 1 | 13 | 1 | 14 |
| 2 | 2 | 3 | 5 |
| 3 | 2 | 6 | 8 |
| 4 | 2 | 4 | 6 |
| 5 | 1 | 5 | 6 |
| Height, cm | 142.8 (13.9) | 159.6 (6.6) | 151.0 (13.8) |
| Weight, kg | 43.8 (14.8) | 59.9 (15.1) | 51.6 (16.9) |
| Age of onset of BP-I, years | 7.3 (2.4) | 10.3 (3.0) | 8.8 (3.1) |
| Length of illness, weeks | 133.1 (102.8) | 191.7 (129.0) | 161.6 (118.5) |
| Dosing Stratum N (%) | |||
| 600 mg | 9 (45.0%) | 9 (47.4%) | 18 (46.2%) |
| 900 mg | 11 (55.0%) | 10 (52.6%) | 21 (53.8%) |
| Creatinine clearance, mL/min* | 140.3 (40.3) | 125.3 (48.1) | 132.6 (44.5) |
n=18 children and 19 adolescents
Safety and Tolerability
No subjects discontinued subsequent prospective open-label lithium treatment due to any adverse event that occurred during this portion of the study. Twelve subjects reported adverse events through either 48 or 72 hours after the first dose. The most frequently reported adverse events were headache (33%), abdominal pain (17%), initial insomnia (17%), dizziness (17%) and nausea (17%).
Concentration-time data
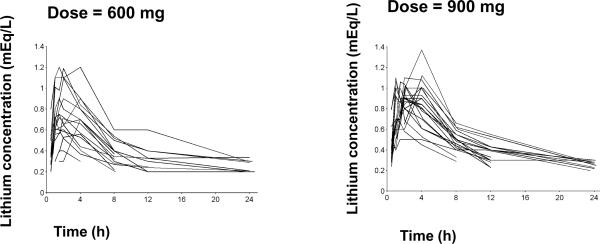
The individual lithium concentration-time curves for each dose level are shown in Figure 1. The concentration-time curves were typically bi-exponential. Lithium concentrations were below the quantification limit for one subject in the 600 mg dose group at 8 h, for five subjects in the 600 mg and four subjects in the 900 mg group at 12 h and for seven subjects in the 600 mg and eleven subjects in the 900 mg group at 24 h after the dose.
Figure 1.
Individual lithium concentration time profiles
Population PK analysis
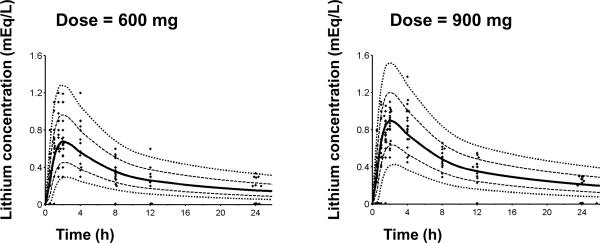
Figure 2 shows the array of lithium plasma concentrations for all subjects in the two dosing groups along with the results of the optimum population model predictions. A two-compartment model with first-order absorption provided optimum fitting based on the objective function, visual predictive checks, and residual plots. The visual predictive check shown in Figure 2 indicates suitable recapture of the central tendency in the data as approximately 50% of the observed data points fall below and 50% above the median prediction (solid line) at each time point. The variability was adequately predicted for the 600 mg dose. For the 900 mg dose at the later time points the variability in the observed data was slightly over-predicted, as ideally 20% of the observed data should fall outside the 80% prediction interval. Overall suitable predictive performance was achieved with the final model. Therefore this model qualified for general assessment of the pharmacokinetics in these subjects.
Figure 2. Visual predictive check for lithium plasma concentration versus time profiles.
The plots show the measured concentrations, the 80% prediction interval [10th – 90th percentile] and the interquartile range [25th – 75th percentile] based on the model including fat-free mass as a covariate. Lines are 10%, 25%, 50%, 75%, 90% percentiles of the predicted concentrations. The markers close to the x-axis represent concentrations below the quantification limit (BQL). At 12 and 24 h many subjects had BQL concentrations (below 0.20 or 0.25 mEq/L) which were taken into account in the model.
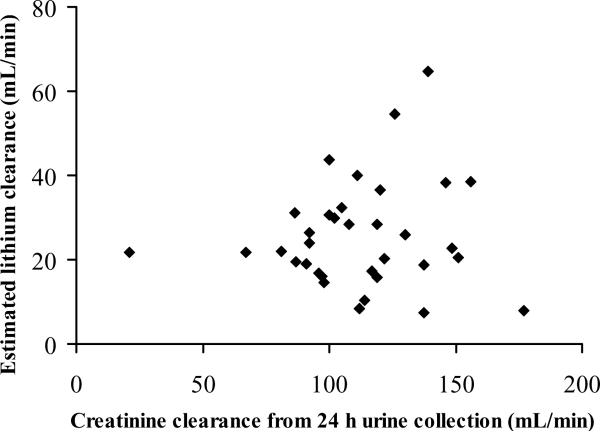
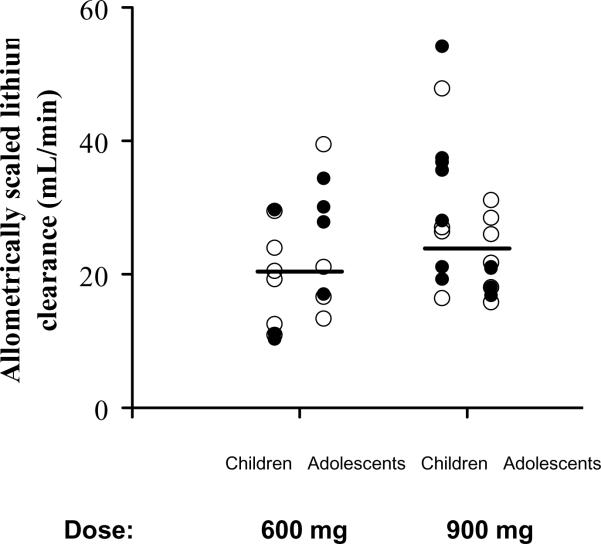
The population parameter estimates and BSV are listed in Table 2. According to the population analysis (eta-plots), clearance and volume of distribution terms were positively correlated with total body weight, FFM, BMI and height. Within the range of creatinine clearances present in the studied subjects, creatinine clearance based on 24 h urine collection or calculated by the Cockcroft and Gault and the Schwartz equations did not show correlation with the estimated lithium plasma clearance (Figure 3). The allometrically scaled estimated lithium plasma clearance was similar between the two doses, males and females, and children and adolescents (Figure 4). Inspection of the eta-plots did not reveal any relationships between PK parameters and age, race, gender, or sexual maturation state; however the sample size in this study was relatively small for detecting such possible effects. The inclusion of infants or age extended over decades is usually needed to detect an age factor.
Table 2.
Population pharmacokinetic estimates of lithium
| Parameter (unit) | Population estimate (between subject variability %) | ||
|---|---|---|---|
| Base modela | Allometric scaling by total body weightb | Allometric scaling by fat-free massc | |
| CL (L/h) | 1.34 (51.6) | 1.50 (44.5) | 1.79 (42.2) |
| V (L)d | 39.7 (8.84) | 63.4 (9.16) | 56.4 (5.51) |
| T1/2abs (min) | 29.3 (39.1) | 27.6 (39.5) | 29.9 (32.2) |
| Tlag (min) | 16.8 (78.7) | 17.5 (76.7) | 17.4 (82.6) |
| T1/2α (h)e | 2.66 (31.7) | 2.96 (10.9) | 2.43 (31.1) |
| T1/2β (h)f | 27.3 (41.0) | 36.2 (25.3) | 27.4 (28.4) |
| CVcp (%) | 38.1 | 39.4 | 38.6 |
| SDcp (mEq/L) | 0.1 (fixed) | 0.1 (fixed) | 0.1 (fixed) |
no covariate included
CL, V, and T1/2β allometrically scaled based on a standard total body weight of 70 kg to allow comparison to results from adult trials
CL, V, and T1/2β allometrically scaled based on a standard fat-free mass of 53 kg
V: total volume of distribution (Vc + Vp), average (%CV) calculated from individual parameter estimates of Vc and Vp
average (% coefficient of variation) half-life of the α-phase calculated from individual population parameter estimates
average (% coefficient of variation) terminal half-life calculated from individual population parameter estimates
Figure 3. Total lithium clearance estimated from the population pharmacokinetic analysis (base model) versus creatinine clearance.
An outlier with a measured creatinine clearance of 553 mL/min and lithium clearance of 22.8 mL/min was excluded. Measured creatinine clearance was not available for 4 subjects. The regression line (not shown) is: y = 0.040x + 21.2 and R2 = 0.008.
Figure 4. Lithium clearance from the population pharmacokinetic analysis allometrically scaled to 53 kg fat-free mass in all subjects in relation to administered dose.
Horizontal lines depict median values, open circles male and closed circles female subjects.
Inclusion of fat free mass, calculated according to Janmahasatian et al. [20], as a covariate for clearance and volume of distribution terms based on allometric scaling resulted in a decrease in BSV of 9.4% on CL, 8.4% on Vc, and 6.7% on CLic, and no decrease in BSV of Vp. Inclusion of total body weight as a covariate on clearance and volume of distribution terms based on allometric scaling resulted in a decrease in BSV of 7.1% on CL, 39% on CLic, and no decrease in BSV of Vc and Vp. Predictive performance according to visual predictive checks was considerably improved by including FFM or WT as a covariate compared to the base model. Also after inclusion of a measure of body size as covariate, no correlation between the estimated lithium clearance and creatinine clearance was identified. Figure 3 shows the estimated lithium clearances in most subjects in relation to measured creatinine clearances. The graph depicts considerable variability in lithium clearance in this group of young patients with mania.
The parameter estimates for the base model and the models including total body weight and FFM as covariates are shown in Table 2. Due to the large decrease in BSV of CL and Vc, and based on physiological considerations, the model including FFM as a covariate was chosen as the final model. Visual predictive checks for the model including FFM as covariate are shown in Figure 2. The predictive performance was similar for the model including WT. The estimates for the α- and β-half-life calculated from population pharmacokinetic parameter estimates (Table 2) described a fast initial decline of the concentrations with a half-life averaging 2.4 hours followed by a long terminal phase of about 27 hours.
DISCUSSION
The present report includes a population analysis which has the advantage of `borrowing' information for individual subjects from the entire group of profiles, consideration of BQL values, and simultaneously addresses issues such as the optimum structural model, the influence of covariates which might account for the variability in drug exposures among the subjects, and utilizes several statistical measures of optimum fitting and predictive performance such as visual predictive checks (Figure 2).
The population analysis revealed two phases in the disposition of lithium with an initial half-life of 2.4 hours and a later half-life of 27 hours. These phases are evident in Figures 1 and 2. It is important to note that neither of these phases determines the multiple-dose accumulation of lithium. Sahin and Benet [22] have recently pointed out that the `operational multiple-dose half-life' is a composite of various phases for drugs with absorption and polyexponential disposition and dependent on the dosing interval (τ) according to:
Multiple-dose simulations indicate that the T1/2op for lithium is13.1 hr for the QD, 14.0 hr for the BID, and 15.1 hr for the TID (q8h) dosing regimens. The terminal phase in the single-dose profiles accounts for only a part of the dose of drug and thus does not dominate in controlling elimination of lithium. Further, the multiple dose simulations suggested that a starting dose of 300 mg twice or three times daily for youths weighing 30 kg or more and a starting dose of 300 mg once daily for those weighing less than 30 kg appear to be appropriate based on safety margins for trough concentrations (data available on request).
The pharmacokinetic parameters of lithium reflect realistic clinical conditions as the measurements were obtained at the time of first dosing in manic children and adolescent patients. Thus the variability in the time-course profiles (Figure 1) and the 48% CV in apparent CL (Figure 4) is not unexpected. These findings argue for careful selection of initial dosages of the drug based on body weights and continued practice of therapeutic drug monitoring to assure a range of effective and non-toxic drug concentrations.
The use of FFM provided the best accounting for variability in CL and volumes in these patients. Lithium is a simple ion which largely distributes into body water spaces, which helps explain these results. However, lithium is appreciably cleared by the kidneys and the lack of correlation of lithium clearance with creatinine clearance was not expected (Figure 3). The reason or reasons for this are not clear. Incomplete urine collections do not account for these observations as both directly measured and calculated (from plasma values) creatinine clearances yielded such variability. The range of creatinine clearances observed in this study was limited as all patients had normal renal function. In this case observing a relationship between creatinine clearance and lithium clearance is more difficult. Also the number of patients in this study was substantially higher than in the previously published pediatric lithium study; however it is still relatively low for detecting covariate effects. The expectation of lithium clearance relating to GFR may serve best to anticipate disposition of the drug in older patients and those with renal failure.
Several studies explored lithium PK in adults [23–32]. The average reported clearance was between 1.32 and 2.15 L/h and half-life between 17.1 and 27.1 h (parameters allometrically scaled to 70 kg body weight where possible). The majority of these studies were analyzed by non-compartmental analysis (NCA).
Prior to our study, lithium pharmacokinetics had only been examined in 9 children, ages ranging from 10 to 12 years, weighing between 27 and 56 kg [11]. In this study, the average apparent lithium clearance from NCA was 1.58 L/h, which corresponds to 2.5 L/h when scaled allometrically to 70 kg body weight. Their average β-half-life from least square regression was 17.9 h. It was concluded that children had a shorter elimination half-life and greater clearance compared to adults. An NCA performed on their graphed average concentrations revealed a large extrapolated fraction of the AUC of 22%. Also the lowest measured lithium concentrations in this study were 0.03 mEq/L. NCA does not consider concentrations below the quantification limit, which might be a reason for the reported lower terminal half-life and higher clearance compared to reports from adults.
Our study also found that the terminal half-life from NCA appears shorter and the clearance scaled to 70 kg body weight higher (average 2.66 L/h) than those values previously reported in adults. The NCA provides similar results for clearance for our study and the Vitiello study [11]. Due to truncation of the concentration-time profiles, NCA leads to biased results towards shorter elimination half-lives and higher clearances, and to the misleading conclusion that lithium clearance would be higher in children than in adults. When taking into account the concentration time profiles of all subjects simultaneously and considering concentrations below the quantification limit by population PK analysis, the allometrically scaled clearance is within the range of values reported for adults. This suggests that the differences in lithium PK parameters between children and adults can be explained by including the effect of body weight. Therefore population PK modeling was an essential tool for this analysis.
Lithium PK in plasma and urine was modeled previously by population analysis in adults, utilizing a two compartment model. The estimate for renal clearance was 1.53 L/h [25]. In a population PK analysis in 79 adult patients [32], a clearance of 1.36 L/h and volume of 32.8 L was reported based on a one compartment model. In this study only trough concentrations were measured, and neither data nor fittings were shown. Lean body weight and creatinine clearance were identified as covariates for lithium clearance.
In a study in obese patients [33] a greater clearance compared to normal weight adults which correlated with total body weight but not creatinine clearance was reported. Volume of distribution correlated with fat-free mass, but volume per kg total body weight was lower in obese compared to normal weight subjects. These results agree with our conclusions that clearance and volume are correlated with total body weight and even better with fat-free mass. Our study also included obese patients (BMI greater than 24 in 12 out of 39 patients). For our study population including both lean and obese subjects, FFM explained even more of the variability than total body weight. Therefore it is important to also take into account body composition, especially in obese subjects. As obesity is commonly encountered in patients taking antipsychotic drugs, it is a useful finding that lithium clearance correlates well with fat-free mass.
Limitations of the present study are the relatively high limits of quantification of the clinical assays which lead to many samples being below the quantification limit and also the lack of measuring lithium amounts excreted in urine for determination of lithium renal clearance. Population PK analysis including the Beal M3 method for handling BQL data was applied as the most sophisticated method available in order to deal with this limitation [21, 34].
Additionally, the use of clinical laboratories, rather than a central laboratory, for lithium assay is a limitation of the present study. Despite this limitation, it was not anticipated that the results would be substantially affected, as the utilized lithium assays are standard and validated clinical methods. Similar commercial techniques for lithium assay were employed across the study sites. Specifically, all clinical laboratories used in this study analyzed the lithium samples as they were received, rather than batched for group analysis. Additionally, prior to implementation, Linearity and Precision were verified for lithium with very low coefficients of variation (typically below 6%). All clinical laboratories subscribe to College of American Pathologists (CAP) Linearity and Calibration standards. Furthermore, all clinical laboratories used in this study run quality control daily, per CAP standards, in order to ensure accuracy and precision between day and within day variation for the analyses.
In conclusion, linear elimination for lithium was found within the studied dosage regimen. Fat free mass was identified as the covariate which explained most of the variability in clearance and volume of distribution parameters. The difference in body size explains different values for the PK parameters in children compared to adults. Possible uses of the developed population pharmacokinetic model are to predict other dosage regimens, support scaling from adult to pediatric pharmacokinetics and support the design of future clinical trials.
ACKNOWLEDGEMENTS
This project has been funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN275200503406C. Additional support for clinical research centers used in this project has been funded by the National Institutes of Health, grant #s GCRC M01 RR000080 and RR025014. The authors would like to thank Brieana M. Rowles, M.A. for her technical assistance in the drafting of this manuscript.
Dr. Findling receives or has received research support, acted as a consultant and/or served on a speaker's bureau for Abbott, Addrenex, AstraZeneca, Biovail, Bristol-Myers Squibb, Forest, GlaxoSmithKline, Johnson & Johnson, KemPharm, Lilly, Lundbeck, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Sanofi-Aventis, Sepracore, Shire, Solvay, Supernus Pharmaceuticals, Validus, and Wyeth. Dr. Kafantaris has received research support from AstraZeneca, Eli Lilly & Co, Glaxo-Smith Kline, Janssen Pharmaceuticals, and Pfizer. Dr. Pavuluri's work unrelated to this manuscript is currently supported by NIMH, NICHD, Dana Foundation, NARSAD, AFSP, and Marshall Reynolds Foundation. Dr. Frazier receives or has received research support from Bristol-Myers Squibb, Eli Lilly & Co, Johnson & Johnson, Neuropharm, Otsuka America Pharmaceutical, and Pfizer Inc. Dr. Sikich has a current financial interest in that she receives research funding or participates in clinical trials with Janssen, Pfizer, Bristol Myers-Squibb, Neuropharm, Curemark and Seaside Pharmaceuticals, and received software for a computer intervention in schizophrenia from Posit Science; in the past, Dr. Sikich received research funding from Eli Lilly, Janssen, Pfizer, Otsuka, and Astra Zeneca, and has served as a consultant for Sanofi Aventis and ABT Associates. Dr. Kowatch receives or has received research support, acted as a consultant and/or served on a speaker's bureau for AstraZeneca, Forest, Medscale, National Alliance for Research on Schizophrenia and Depression, NICHD, NIMH, Physicians Postgraduate Press, and the Stanley Foundation.
Footnotes
DISCLOSURES The other authors have no financial ties to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18:1023–35. doi: 10.1017/S0954579406060500. [DOI] [PubMed] [Google Scholar]
- 2.McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:107–25. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 3.Muzina DJ, Calabrese JR. Maintenance therapies in bipolar disorder: focus on randomized controlled trials. Aust N Z J Psychiatry. 2005;39:652–61. doi: 10.1080/j.1440-1614.2005.01649.x. [DOI] [PubMed] [Google Scholar]
- 4.Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2:349–52. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- 5.Findling RL, Pavuluri MN. Lithium. In: Geller B, DelBello MP, editors. Treatment of Bipolar Disorder in Children & Adolescents. Guilford Press; New York: 2008. pp. 43–68. [Google Scholar]
- 6.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 7.Findling RL, Frazier JA, Kafantaris V, et al. The Collaborative Lithium Trials (CoLT): specific aims, methods, and implementation. Child Adolesc Psychiatry Ment Health. 2008;2:21. doi: 10.1186/1753-2000-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amdisen A. Serum level monitoring and clinical pharmacokinetics of lithium. Clin Pharmacokinet. 1977;2:73–92. doi: 10.2165/00003088-197702020-00001. [DOI] [PubMed] [Google Scholar]
- 9.Thornhill DP. Pharmacokinetics of ordinary and sustained-release lithium carbonate in manic patients after acute dosage. Eur J Clin Pharmacol. 1978;14:267–71. doi: 10.1007/BF00560460. [DOI] [PubMed] [Google Scholar]
- 10.Wing YK, Chan E, Chan K, et al. Lithium pharmacokinetics in Chinese manic-depressive patients. J Clin Psychopharmacol. 1997;17:179–84. doi: 10.1097/00004714-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Vitiello B, Behar D, Malone R, et al. Pharmacokinetics of lithium carbonate in children. J Clin Psychopharmacol. 1988;8:355–9. [PubMed] [Google Scholar]
- 12.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 14.Wechsler DA. Wechsler Abbreviated Scale of Intelligence Manual. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- 15.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–9. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr. 1984;104:849–54. doi: 10.1016/s0022-3476(84)80479-5. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106:522–6. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 19.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Janmahasatian S, Duffull SB, Ash S, et al. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 21.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 22.Sahin S, Benet LZ. The operational multiple dosing half-life: a key to defining drug accumulation in patients and to designing extended release dosage forms. Pharm Res. 2008;25:2869–77. doi: 10.1007/s11095-008-9787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obach R, Borja J, Prunonosa J, et al. Lack of correlation between lithium pharmacokinetic parameters obtained from plasma and saliva. Ther Drug Monit. 1988;10:265–8. doi: 10.1097/00007691-198803000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Hunter R. Steady-state pharmacokinetics of lithium carbonate in healthy subjects. Br J Clin Pharmacol. 1988;25:375–80. doi: 10.1111/j.1365-2125.1988.tb03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaillot J, Steimer JL, Mallet AJ, et al. A priori lithium dosage regimen using population characteristics of pharmacokinetic parameters. J Pharmacokinet Biopharm. 1979;7:579–628. doi: 10.1007/BF01061210. [DOI] [PubMed] [Google Scholar]
- 26.Yang YY, Yeh EK, Chang SS, et al. Maintenance lithium levels could be lowered: based on Taiwanese and Danish studies. J Formos Med Assoc. 1991;90:509–13. [PubMed] [Google Scholar]
- 27.Mason RW, McQueen EG, Keary PJ, et al. Pharmacokinetics of lithium: elimination half-time, renal clearance and apparent volume of distribution in schizophrenia. Clin Pharmacokinet. 1978;3:241–6. doi: 10.2165/00003088-197803030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann W, Kanarkowski R, Matkowski K, et al. Studies of lithium pharmacokinetics in patients with affective illness. Pol J Pharmacol Pharm. 1988;40:47–54. [PubMed] [Google Scholar]
- 29.Chen C, Veronese L, Yin Y. The effects of lamotrigine on the pharmacokinetics of lithium. Br J Clin Pharmacol. 2000;50:193–5. doi: 10.1046/j.1365-2125.2000.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turck D, Heinzel G, Luik G. Steady-state pharmacokinetics of lithium in healthy volunteers receiving concomitant meloxicam. Br J Clin Pharmacol. 2000;50:197–204. 3. doi: 10.1046/j.1365-2125.2000.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ku JS, Wu ZY, Fang YS, et al. Clinical study on the pharmacokinetics of lithium carbonate. Int J Clin Pharmacol Ther Toxicol. 1987;25:648–50. [PubMed] [Google Scholar]
- 32.Jermain DM, Crismon ML, Martin ES., 3rd Population pharmacokinetics of lithium. Clin Pharm. 1991;10:376–81. [PubMed] [Google Scholar]
- 33.Reiss RA, Haas CE, Karki SD, et al. Lithium pharmacokinetics in the obese. Clin Pharmacol Ther. 1994;55:392–8. doi: 10.1038/clpt.1994.47. [DOI] [PubMed] [Google Scholar]
- 34.Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11:371–80. doi: 10.1208/s12248-009-9112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]