ABSTRACT
Enterocutaneous fistulas represent a challenging situation with respect to wound care and stoma therapy. An understanding of the principles of wound care and the various techniques and materials that are available is of vital importance to enhance patient comfort and recovery as well as facilitate fistula healing. Skin barriers, adhesives, dressings, pouches, and negative pressure dressings are all materials that are available in the armamentarium of the enterostomal therapist. Proper utilization of these items and appropriate modifications to their application requires an intimate knowledge of the characteristics of the fistula being treated. Wound care management is a key element in the overall care and healing of the enterocutaneous fistula.
Keywords: Enterocutaneous fistula, wound management, skin barriers, pouches, negative pressure dressings
Wound care management of the enterocutaneous fistula (ECF) is one of the greatest challenges in the surgical patient and can present a complex problem for the clinician. ECFs represent a demanding situation in terms of their management that often requires a multidisciplinary approach to facilitate patient care and fistula healing. Medical, surgical, nutritional, and enterostomal wound care services are frequently involved each with a vital role. By definition, a fistula is an abnormal, epithelialized connection between two body structures and can occur anywhere surgical wounds occur.1 Fistulas in the gastrointestinal (GI) system are classified according to site of origin and termination, volume and type of drainage, and etiology. An ECF is an epithelialized connection from the intestine to the skin.
An ECF may occur either spontaneously or related to surgery. It is estimated that ∼80% of ECFs occur as complications of abdominal surgery with an overall incidence of 0.8 to 2%.2 Most ECFs occur in the postoperative setting and are commonly associated with malnutrition and postsurgical sepsis. An ECF as a result of surgery can be seen related to cancer, inflammatory bowel disease, Crohn's disease, trauma, or lysis of adhesions. The fistula may develop as a result of breakdown of the primary anastomosis or an unidentified injury to the bowel during surgery. A spontaneous fistula can be associated with diverticular disease, ischemic bowel, perforated ulcerations, or appendicitis.3 Regardless of the initiating event that leads to formation of an ECF, management and control of the wound can be very difficult.
The most demanding aspects of the ECF are skin protection, drainage quantification, and drainage containment to prevent additional skin damage secondary to the corrosive effluent surrounding the aperture.4 The effects of continuous moisture from the ECF can severely damage the surrounding skin and risk development of infection. In addition, the effluent acts as a chemical irritant, depending on the origin of the fistula, and can compromise skin integrity. These factors pose significant problems in wound care related to the ECF and can be very difficult to manage.
GOALS
An effective ECF wound care management plan should achieve the following goals.5 Ideally, the goals can be achieved simultaneously, but prioritization may be necessary based on the individual patient with the ECF:
Skin protection
Patient comfort and mobility
Containment of drainage and odor
Accurate measurement of effluent
Cost containment
Skin Protection
Skin protection and prevention of further skin breakdown surrounding the ECF are key components in wound care. There are several causes of impaired skin integrity at the site of the ECF. The four most common causes are mechanical trauma, allergic responses, infections, and chemical irritants.6 Frequent dressing changes due to adhesives and pouching methods can contribute to the breakdown of the surrounding skin causing repeated mechanical trauma and prevent proper healing. Allergic reactions to the dressing or pouch materials may cause erythema, edema, or weeping skin, which can become more susceptible to infection. Infections secondary to entrapment of exudates against the skin, namely fungal, can result in chronic skin infections with erythema, papule and vesicle formation.
The most common chemical irritant is bowel contents. The enzymatic contents of the effluent are many times more detrimental to the skin integrity than the actual volume of the effluent. More proximal ECFs contain proteolytic digestive enzymes, which further damage the surrounding skin by entrapment, leaking and persistent moisture, which in turn jeopardizes skin integrity and healing. These digestive secretions exhibit a toxic effect on living tissue and correlates with our clinical observations that fistula effluent does indeed inhibit wound healing.7 Healing the surrounding skin, preventing further skin breakdown, and minimizing contamination are key components in the wound management of these patients and are of paramount importance. Without this, a futile cycle develops in which skin breakdown leads to failure of pouch adherence, further leakage, and worse skin maceration.
Patient Comfort and Mobility
Skin irritation and discomfort can seriously affect the patient if the wrong management technique is used. Certain pouches and/or appliances with the use of belts can help minimize and prevent unnecessary patient discomfort, which is a key psychosocial component of wound management in these patients. Ambulatory patients should not be restricted in their recovery and mobility should not be compromised by a wound management system. Therefore, individualization is an important part in devising the best care for a patient with an ECF.
Containment of Drainage and Odor
Drainage containment is a key essential to improving the surrounding skin integrity. Enteric contents can spill onto the surrounding skin leading to persistent tissue inflammation and infection, which if left untreated, can develop into sepsis. Containment of the effluent can be accomplished with pouching devices, suction devices, dressings or a combination of these management techniques. Dressing material that absorbs and retains caustic secretions is thought to be a major contributor in the delay of healing of ECFs. Therefore, continuous suction devices or stoma application has been advocated as an adjunct to wound care and skin preservation.8 However, the patient's overall clinical status may play a role in devising the best solution for that individual patient.
Though not commonly addressed, persistent odor from the ECF can be a tremendous source of anxiety and social concern for these patients. Odor control is best controlled with the use of a pouch. Most pouches have an odor-proof film and both internal and external deodorants are available and can help with odor elimination. Deodorants are available in tablet, liquid, or powder forms. There are certain medications that can be taken that may help with odor control including chlorophyll tablets, bismuth subgallate, and bismuth subcarbonate. In addition, special deodorizers can be placed in the pouch to assist with odor control such as 3% hydrogen peroxide and Hollister M9 odor-eliminating drops.
Accurate Measurement of Effluent
Measurement of the fluid and electrolyte balances in these patients is another important goal in wound care of the ECF patient. Leakage around catheters can give an inaccurate reading of actual fluid losses. Dressings can also give an inaccurate reading unless the dressings are weighed on a regular basis. These factors must be taken into account during wound management of ECF patients. Especially with short bowel and proximal ECFs, fluid losses can be up to multiple liters daily. Concomitant electrolyte abnormalities can lead to secondary effects ranging from cardiac arrhythmias to renal failure. Accurate measurement of the effluent is essential to help guide the fluid and nutritional needs in these often sick patients.
Cost Containment
Cost-effective medical care is also a principal component in the wound care of patients with ECFs. Attention must be applied not only to the products and materials used for wound care, but labor and time must also be considered. Hospital costs for ECFs are considerable and the average length of stay varies widely.9 In a critically ill patient with an ECF, costs can average approximately $10,000 per day.5 Skin care rituals that consume excessive time and expense without resulting in optimal patient outcomes must be eliminated. Therefore, cost containment is another essential goal in the treatment of ECF patients.
ASSESSMENT
The initial step after identification of an ECF consists of an overall assessment of the patient, nature of the fistula, and condition of the associated wound. Evaluation for infection and/or sepsis, electrolyte imbalances and nutritional needs are also crucial.
This assessment includes four factors that must be individualized for each ECF patient: (1) origin of the fistula tract, (2) location of the fistula opening at skin level, (3) type of effluent (i.e., enzyme and electrolyte constitution), and (4) skin integrity.
Origin of the Fistula Tract
First, identification of where the fistula is communicating with the bowel aids in the management of the wound. The location of the origin of the fistula is paramount to providing good wound care and healing of the surrounding skin. It has been identified that the small bowel is the most common site of origin in ECFs (Table 1).10 The enteric contents from the small bowel can be more challenging to deal with compared with colonic-cutaneous fistulas based on characteristics and type of effluent alone.
Table 1.
Site of Origin of Enterocutaneous Fistulas
| Fistula Location | Number of Fistulas | % |
|---|---|---|
| Adapted from Draus et al.10 | ||
| Stomach | 8 | 7.6 |
| Duodenum | 5 | 4.7 |
| Small bowel | 67 | 63.2 |
| Colon | 26 | 24.5 |
| Total | 106 | 100 |
Diagnostic studies can be used to help identify the location of origin of the fistula and therefore help plan wound care and/or stoma therapy. Although covered in depth by Drs. Lee and Stein in this issue, contrast studies, computed tomography scans, fistulograms, and even methylene blue can be used to provide anatomic information about the source, length, course and other characteristics of the ECF.11 Importantly, if diagnostic studies are not available, insight into the origin of the fistula can often be identified based on examining effluent characteristics alone. Odor, color, consistency, type and amount of effluent, and the effect on the surrounding skin can be used to determine the origin of the fistula. The fistula effluent can also be sent for laboratory analysis to determine the chemical make-up that may also give additional clues.
Location of the Fistulous Opening at the Skin Level
The location of the fistulous opening on the skin plays a significant role in wound management, product selection, and skin care. Identification of the aperture in relation to skin folds, bony structures (i.e., costal margin, anterior superior iliac spine), and open wounds play a key role in devising the best system for each individual patient. Irregular skin surfaces and defects may need to be corrected with skin barriers (covered below). In addition, convexity of the wound in relation to the skin surface needs to be taken into account for wound care, pouch selection, and devising an appropriate management system.
Single versus multiple openings may also play a factor in choosing the best route for wound care and management. Multiple fistulas in close proximity may be addressed with a single management technique. Widely spread fistulas, on the other hand, may need to be addressed separately and pouched or dressed separately. In addition, normal skin bridges should always be evaluated to determine size, condition and method of handling. Consideration in small bridges should be given for either proper protection versus opening to unify multiple adjacent openings into one area.
Type of Effluent
Characteristics of the ECF effluent can help to identify the source of the fistula if no diagnostic studies are available. The amount and nature of the effluent is a key factor in determining skin care methods and materials used to protect the surrounding skin. A high-output fistula is defined when the output is greater than 500 mL/d, moderate when the volume is 200 mL to 500 mL/d, and low when the output is less than 200 mL/d.12 If the fistula is considered a high-output fistula, then a pouching system may be more appropriate for that patient. If the output is low, then a simple skin barrier and/or dressing may be more appropriate. As evidence of this, patients with long-standing end colostomies technically have an ECF that, through a well-rehearsed bowel regimen, are often able to simply cover the stoma with a gauze bandage in between bowel movements.
The caustic nature of the effluent will also play a role in determining which skin care materials to use. More enzymatic effluent may require a pouching or suction device to divert the effluent from the surrounding skin and prevent further skin breakdown.
Skin Integrity
Assessment of the skin integrity around the fistula is also important. The initial condition of the surrounding skin is critical in determining the type of skin materials to be used in the wound management of these patients. If the skin is relatively healthy, skin barriers and other means to prevent any breakdown are essential. In most instances, the skin surrounding the fistula is denuded, either from the nature of the effluent or the constant presence of moisture, and therefore a management technique to heal and prevent further damage is crucial to the healing process. If the skin has either erythema or skin loss due to repeated trauma to the wound, then more aggressive skin care is necessary. Skin that is ulcerated or infected can be the most difficult wound to treat in the ECF patient.
MANAGEMENT
The materials selected for the wound care of the ECF patient depends on the characteristics of the fistula such as output, type of drainage, location, and perifistular skin integrity. Materials include skin barriers; adhesives; dressings, pouches and wound managers; and negative pressure dressings such as vacuum-assisted closure (V.A.C.; Kinetics Concepts Inc., San Antonio, TX).5,13,14
Skin Barriers
ECFs can often represent very difficult situations with respect to skin protection. Depending on the origin location of the fistula in the GI tract, fistula output could be acidic, alkaline, or contain proteolytic enzymes, all of which could be damaging to skin. As previously stated, the enzymatic nature of the effluent is more damaging to the skin than the actual volume.
Skin barriers are the mainstay in fistula management with respect to skin protection by forming physical barriers between effluent and the skin surface. Skin barriers are available in various forms; they include solid wafers, powder, paste, and sealants.
Solid wafers are pectin-based products that have their own adhesive surface.14 This surface melts on contact with the patient's warm skin and creates a seal thereby protecting the skin from direct contact with the effluent. Wafers are changed only when they loosen from wound edges or melt out. Additional sheets of wafer can be used to increase or build up the area available for adhesion.
Skin barrier powders are used in fistulas with associated skin wounds that are wet and weeping.5 Barrier powders [available from numerous manufacturers such as Hollister (Hollister, Inc., Libertyville, IL) and Coloplast (Humlebaek, Denmark)] are pectin or karaya-based, and are used to absorb the moisture and create a dry surface for subsequent dressing or pouch application. A thin layer of powder is applied to the denuded skin and allowed to dry before application of the barrier wafer or adhesive surface of a pouch system. Care must be taken not to apply excessive amounts of powder. These powders should be discontinued once the denuded skin is intact and re-examination of the wound performed.
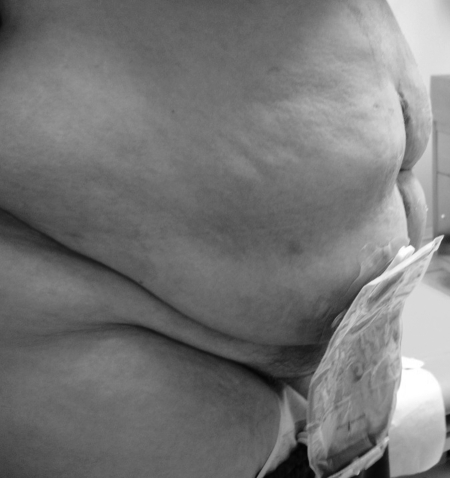
Skin barrier pastes are used to reinforce the inner edges of solid wafers to protect them from melt out and establish an improved seal. Pastes may also be used to smooth out irregularities in perifistular skin to create a flat surface for wafer application. This is crucially important in obese patients where skin surface irregularities and folds may severely limit proper pouching (Fig. 1).
Figure 1.
Obese ostomy patient with multiple skin folds.
Finally, skin sealants are used to protect the skin surface from adhesives and to create a tacky surface for improved adherence of pouches such as 3M Cavilon no-sting barrier (3M, St. Paul, MN). Sealants are also used to seal in powders on denuded skin surfaces and facilitate pouch placement. Skin sealants often contain alcohol and therefore can only be placed on intact skin. Sealants are available in numerous forms including wipes, sprays, gels, or brush-on liquid.
Adhesives
Adhesives are used to enhance or extend another product's adhesive surface or attach two surfaces when modifying a fistula collection system. Mobile and flaccid skin seems to benefit in particular from adhesive use to improve the seal and increase the wear time of a pouching system. Adhesives are available in three forms: liquid, aerosol, and double-faced adhesive sheet or disc. Liquid and aerosol adhesives contain solvents and must be applied slowly and evaporate before application of any additional products. Sheets of adhesives or double-faced adhesive discs can be used to create or increase the adhesive surface of a pouch when unusually shaped pouch apertures are required.
Dressings
Dressings are readily available and easy to implement for the appropriate patient. They provide a cost-effective method of wound care for simple ECFs and/or low-output fistulas, less than 200 mL/d. Overlying gauze dressings in combination with skin barriers can be used to effectively contain and manage ECF effluent. However, if fistula output is considered moderate or high or the dressings are being changed more frequently than every 4 hours, a pouching system should be implemented. In this instance, frequent dressing changes become impractical and do not offer the ability to accurately record fistula output. In addition, frequent tape changes associated with dressings can also jeopardize skin integrity especially in the setting of already compromised skin due to effluent-induced irritation.
Pouches
As mentioned earlier, one of the main goals of fistula management is drainage control and containment. Drainage control is very important as continued contact of effluent with the skin surface can lead to maceration and increased likelihood of breakdown and perifistular skin infection, namely fungal. Depending on the volume of drainage, this can be accomplished in a variety of manners.
Since the advent of enterostomal therapy (ET) nursing at the Cleveland Clinic in the 1950s, many draining wounds and fistulas have been managed with ostomy pouches. Effective pouching of a fistula can achieve all the goals of fistula management and various ostomy pouches are available today. The choice of pouch system to be used depends on the characteristics of the fistula being managed. Generally, high-output fistulas with very thin liquid effluent are best managed with pouches that have a urinary outlet system or spigot to allow for easy drainage and emptying.
Continuous drainage can be achieved with urinary outlet pouches by attaching drainage tubing to the urinary spigot. This can greatly enhance nursing efficiency by minimizing the need for pouch emptying and prevents pouch overfilling in situations where fistula output is very high. Alternative pouch outlets include fecal outlets with a drainable clip that is appropriate for fistulas with 24-hour outputs less than 1000 mL and wide tubular outlets or wound managers (Fig. 2).
Figure 2.

Wound management appliance.
Pouches are also available as one- or two-piece appliances (Figs. 3 and 4). One piece pouches are preferable as they lack an attachment ring and are therefore more pliable for application to irregular skin surfaces. Two-piece pouches, however, allow for access to the fistula without removing the entire pouch if interventions such as frequent irrigations are required. The majority of pouches available today have a skin barrier surface to enhance skin protection. Pouches with an adhesive surface are also available when surface barriers are being applied separately, as in complex wounds. Finally, pouches also vary based on capacity, which is predetermined by the size of the adhesive surface as well as odor-proof versus odor-resistant properties.
Figure 3.
One-piece stomal appliance.
Figure 4.
Two-piece stomal appliance.
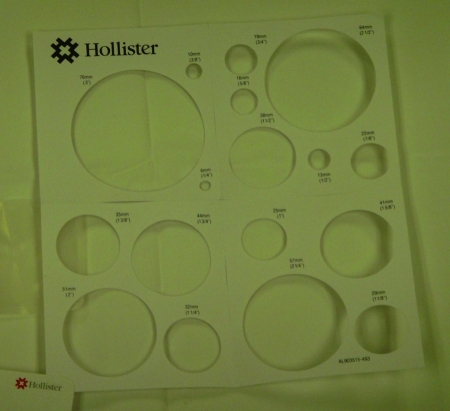
Proper application of a pouch and the implementation of modifications14 based on the individual wound characteristics are as equally important as selecting the right pouch for the wound. Care must be taken to size the orifice in the adhesive surface appropriately so as to ensure accommodation of the fistula with minimum exposure of the perifistular skin to the effluent (Fig. 5). Typically about 1 to 2 inch margin of skin is required to minimize exposure and provide maximum protection. Generally, an appropriately sized pouch should allow for a minimum of one inch of adhesive contact around the fistula.
Figure 5.
Sizing guide for stomas or fistulas.
POUCH MODIFICATIONS
Adhesive
Additional adhesives are occasionally required to attach two pouch surfaces, enhance the tack of an existing adhesive, or extend the adhesive surface on a pouch. Adhesives may also be needed when multiple applications of skin-barrier powder are required in the presence of severely denuded skin to improve the tack.
Trough Procedure
This is useful if the fistula is recessed within a wound that makes routine pouch application fail. The wound is covered with a transparent dressing in which a hole is cut in the most dependent portion of the wound over which a pouch is applied. To enhance adhesion of the transparent dressing and maximize skin protection from effluent, strips of barrier wafer can be cut and applied to the wound edge prior to application of the transparent dressing.
Silicon Molds
A complex pouch technique that can be used when the fistula orifice is recessed in an open wound or surrounded by numerous irregular skin surfaces. The mold fills the wound and creates a flat surface for pouch adherence.
Condom Catheter Fistula Pouch
Appropriate when skin surface contours or obstacles like retention sutures reduce the area available for pouch attachment. Using a flexible skin barrier and a condom catheter, a conduit for drainage can be created.
Bridging Technique
Complex wounds may occasionally have areas that require different care. This technique can be used to isolate one area of the wound that requires drainage containment from other areas that may require packing or dressing changes. Solid wafer skin barriers cut into small pieces and placed at the bridge location to help facilitate pouch placement.
Vacuum-Assisted Closure (V.A.C.) Dressings
V.A.C. dressings have been used extensively in the management of complex wounds due to proven enhancement in wound granulation and wound contracture.15,16 Occasionally, V.A.C. dressings have been applied to wounds with ECFs to contain fistula effluent and enhance wound healing. However, concerns do exist about suction occlusion with frequent need for dressing changes, impaired wound healing due to effluent trapped under the dressing sponge, and increased rates of wound infections. V.A.C. dressings can be tailored to individual wounds. Different sponges are available for use and include standard GranuFoam, WhiteFoam, and GranuFoam with silver. GranuFoam sponges have an open pore structure (400–600 microns) that allow for exudate removal and promote granulation formation. WhiteFoam sponges are less adherent with a higher tensile strength for easier placement and removal for tunnels and fistula tracks. GranuFoam with silver impregnation combine negative pressure technology with the antibacterial properties of silver delivered directly in the wound.
In most cases where the V.A.C. dressing was used, it enabled earlier discharge from the hospital and did enhance perifistular wound healing but did not lead to fistula closure.16 Unfortunately, the majority of this data remains limited to small series or chronic wounds such as diabetic and chronic venous ulcers. Yet, concerns about negative pressure increasing fistula output seem to be overstated. Modifications to this technique have shown great promise where the V.A.C. dressing is applied to the perifistular wound while skin barrier wafers are used to isolate the fistula itself.16 The fistula is then managed with the pouch applied on top of the V.A.C. dressing, thereby isolating fistula drainage from the remainder of the wound while taking advantage of all the benefits of a negative pressure dressing. The role that the V.A.C. plays in this algorithm for care of these wounds remains to be seen as larger series and further experience is gathered.
IMPLEMENTATION
Careful assessment of the wound and fistula is a key factor in determining the appropriate technique to be used for wound care and wound management. Once the characteristics of the wound have been identified, a wound care plan that includes all the stated goals can be implemented. Other considerations that are extremely important in designing a wound care plan are patient comfort, patient mobility, affordability, and discharge planning.
Wound preparation is also a vital factor to the success of any dressing strategy. The skin around a fistula needs to be clean, dry, and greaseless for effective pouch adherence. Any devitalized or necrotic tissue within a wound should be debrided prior to pouch and dressing application. Necrotic tissue impedes granulation formation and wound healing while acting as a culture medium for microbes. Each dressing or pouch change represents an opportunity to re-evaluate the wound and modify the wound care plan accordingly. It is extremely important to have a good wound care team for these very difficult patients.
SUMMARY
The wound management of an ECF is very demanding and often a frustrating challenge for the clinician and multidisciplinary team. The main objectives are to promote healing of the wound, containment of the effluent, and prevent further skin breakdown. A systematic approach to the skin and wound care management of an ECF patient is a key step in the control of the wound.
Today, there are many devices available to help aid in the wound management of the ECF patient. Individualization of a specific wound management system in the ECF patient is very feasible with the multitude of products available once the fistula has been assessed and goals identified. Once wound care is under control through the use of various products and methods discussed, then nutritional, medical, and possible surgical management can proceed for the benefit of the patient.
REFERENCES
- 1.Krasner D, Kane D. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals. Wayne, PA: Health Management Publications Inc; 1997. pp. 202–208.
- 2.Wainstein D E, Fernandez E, Gonzalez D, Chara O, Berkowski D. Treatment of high-output enterocutaneous fistulas with a vacuum-compaction device. A ten-year experience. World J Surg. 2008;32(3):430–435. doi: 10.1007/s00268-007-9235-8. [DOI] [PubMed] [Google Scholar]
- 3.Hwang R F, Schwartz R W. Enterocutaneous fistulas: current diagnosis and management. Curr Surg. 2000;57(5):443–445. doi: 10.1016/s0149-7944(00)00319-6. [DOI] [PubMed] [Google Scholar]
- 4.Schaffer D B. Closed suction wound drainage. Nursing. 1997;27(11):62–64. doi: 10.1097/00152193-199711000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Irrgang S, Bryant R. Management of the enterocutaneous fistula (continuous education credit) J Enterostomal Ther. 1984;11(6):211–228. doi: 10.1097/00152192-198411000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Lange M P, Thebo L M, Tiede S M, McCarthy B, Dahn M S, Jacobs L A. Management of multiple enterocutaneous fistulas. Heart Lung. 1989;18(4):386–390. [PubMed] [Google Scholar]
- 7.Leibovich S J, Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- 8.Orringer J S, Mendeloff E N, Eckhauser F E. Management of wounds in patients with complex enterocutaneous fistulas. Surg Gynecol Obstet. 1987;165(1):79–80. [PubMed] [Google Scholar]
- 9.Allardyce D B. Management of small bowel fistulas. Am J Surg. 1983;145(5):593–595. doi: 10.1016/0002-9610(83)90099-5. [DOI] [PubMed] [Google Scholar]
- 10.Draus J M, Jr, Huss S A, Harty N J, Cheadle W G, Larson G M. Enterocutaneous fistula: are treatments improving? Surgery. 2006;140(4):570–576. discussion 576–578. doi: 10.1016/j.surg.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Evenson A R, Fischer J E. Current management of enterocutaneous fistula. J Gastrointest Surg. 2006;10(3):455–464. doi: 10.1016/j.gassur.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Beck D. Intestinal fistulas. The Phoenix. 2005;Winter:58–61. [Google Scholar]
- 13.Dearlove J L. Skin care management of gastrointestinal fistulas. Surg Clin North Am. 1996;76(5):1095–1109. doi: 10.1016/s0039-6109(05)70499-0. [DOI] [PubMed] [Google Scholar]
- 14.Bryant R. Management of drain sites and fistulas. St. Louis: CV Mosby; 1992. In Acute and Chronic Wounds. pp. 259–281.
- 15.Cro C, George K J, Donnelly J, Irwin S T, Gardiner K R. Vacuum assisted closure system in the management of enterocutaneous fistulae. Postgrad Med J. 2002;78(920):364–365. doi: 10.1136/pmj.78.920.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goverman J, Yelon J A, Platz J J, Singson R C, Turcinovic M. The “Fistula VAC,” a technique for management of enterocutaneous fistulae arising within the open abdomen: report of 5 cases. J Trauma. 2006;60(2):428–431. discussion 431. doi: 10.1097/01.ta.0000203588.66012.c4. [DOI] [PubMed] [Google Scholar]