ABSTRACT
In this article, the authors review both benign and malignant ovarian masses, as the colorectal surgeon who encounters an adnexal mass at the time of surgery should be aware of the steps necessary for surgical staging and optimal tumor resection.
Ovarian tumors—most of which are benign—are divided into three major categories, in order of frequency: epithelial, germ cell, and sex cord-stromal tumors. Nonneoplastic conditions of the ovary that may present as adnexal masses include the following, according to World Health Organization (WHO) classification: pregnancy luteoma, hyperplasia of ovarian stroma, hyperthecosis, massive edema, solitary follicle cysts and corpus luteal cysts, multiple follicle cysts, and endometriosis.
Epithelial ovarian tumors arise from the surface epithelium and can be benign or malignant. Histologic types are serous, mucinous, endometrioid, clear cell, or Brenner. Germ cell tumors are more likely to appear in females under 20 years, accounting for 70% of ovarian tumors in this age group. Approximately 3% are malignant. Teratomas are the most common germ cell tumors. Malignancies, in addition to malignant teratomas, include dysgerminomas, endodermal sinus tumors, and embryonal carcinomas. The more common sex cord-stromal tumors include granulosa stromal cell tumors, Sertoli–Leydig cell tumors, and gynandroblastomas.
Surgical staging and optimal tumor resection are also addressed, with a focus on epithelial malignancies, as they are the most relevant to colorectal surgeons.
Keywords: Adnexal masses, ovarian cancer, ovarian cysts
The purpose of this article is to review benign and malignant pathologies of the ovary. Although specific histology of ovarian masses will be discussed, this review is intended to familiarize the colorectal surgeon with the up-to-date management strategies for adnexal masses, especially the importance of optimal surgical resection for ovarian cancer. Management of the three main categories of ovarian tumors will be discussed, as will the anatomy of the normal ovary.
Ovarian tumors are divided into three major categories: epithelial, germ cell, and sex cord-stromal tumors. Nonneoplastic conditions of the ovary that may present as adnexal masses include the following, according to World Health Organization (WHO) classification: pregnancy luteoma, hyperplasia of ovarian stroma, hyperthecosis, massive edema, solitary follicle cysts and corpus luteal cysts, multiple follicle cysts (polycystic ovaries), multiple luteinizing follicle cysts and/or corpora lutea, endometriosis, surface epithelial inclusion cysts (germinal inclusion cysts), simple cysts, inflammatory lesions, and paraovarian cysts.1
Most ovarian tumors are benign. Epithelial ovarian tumors arise from the surface epithelium of the ovary, which has its origin from coelomic epithelium. These surface epithelial tumors can either be benign, of low malignant potential (borderline), or malignant. Histologic variants for these epithelial tumors include serous, mucinous, endometrioid, germ cell, and Brenner.
Germ cell tumors, the second most common type of ovarian tumor, are more likely to be found in the first two decades of life. Germ cell tumors account for 70% of ovarian tumors in this age group. Overall, they account for 20 to 25% of all tumors, benign as well as malignant, of the ovary. Approximately 3% are malignant.1,2 Malignant germ cell tumors include dysgerminomas, endodermal sinus tumors, embryonal carcinomas, and nongestational choriocarcinomas. Teratomas, which can contain tissue from all three germ cell layers (ectoderm, mesoderm, and neuroderm), are the most common germ cell tumors. Benign mature teratomas contain mature elements. Malignant teratomas contain immature elements and are graded according to the amount of immature neural tissue. Teratomas can also be monodermal, such as struma ovarii, which has mature thyroid tissue, or carcinoid tumors of the ovary.
The third most common ovarian tumors are those of a sex cord-stromal origin. The more common sex cord-stromal tumors include granulosa stromal cell tumors, Sertoli-stromal cell tumors, Sertoli–Leydig cell tumors, and gynandroblastomas.
SCREENING TESTS
Screening tests such as CA-125 and transvaginal ultrasound have been evaluated in nonrandomized and randomized clinical trials. Although having the ability to detect early stage disease, these studies have not been shown to improve mortality.3 Bailey et al and van Nagell et al have shown that 3 to 5% of asymptomatic postmenopausal women will have adnexal masses on transvaginal ultrasound.4 The complexity of an ovarian cyst can be a predictor of malignancy. These complexities vary from unilocular cysts to multilocular cysts to complex ovarian tumors with solid components or papillary projections within the cyst. Granberg and colleagues found an incidence of ovarian cancer of 0.3% in unilocular ovarian cysts, although multilocular cystic solid tumors or predominantly solid tumors have an incidence of malignancy of 36% and 39%, respectively.5 Although the incidence of ovarian cancer in unilocular cysts is very rare, removal of the adnexa at the time of laparotomy for colon and rectal surgery is warranted. This eliminates the need for continued surveillance with ultrasound and CA-125. Oophorectomy at the time of surgery also eliminates the risk of adnexal torsion and, thus, additional surgery. Total abdominal hysterectomy and bilateral salpingo-oophorectomy should be performed in postmenopausal women. Unilateral salpingo-oophorectomy or cystectomy would be the appropriate surgical step in a young woman who desires fertility.
The most recent United States screening trial—the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial—showed a positive predictive value of 1 to 1.3. In this study, 67% of the malignancies were detected during screening, but 72% had stage III or IV disease (Table 1).3 Eighty-nine percent of those detected by an abnormal CA-125 alone were stage III or IV, whereas 29% detected by ultrasound alone were stage III or IV. The number of surgeries-to-detection of cancer ratio was 19.5:1 in this 4-year screening trial. Patients in this study will be followed for 13 years to determine whether screening tests lower the mortality rate of ovarian cancer patients.3
Table 1.
International Federation of Gynecology and Obstetrics Staging for Primary Carcinoma of the Ovary
| From Greene, FL, ed. AJCC Cancer Staging Manual, Sixth Edition. New York: Springer-Verlag; 2002. Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. | |
| Stage I | Growth limited to the ovaries. |
| Stage Ia | Growth limited to one ovary; no ascites containing malignant cells. |
| Stage Ib | Growth limited to both ovaries; no ascites containing malignant cells. No tumor on the external surfaces, capsules intact. |
| Stage Ic | Tumor either Stage Ia or Ib, but with tumor on the surface of one or both ovaries; or with capsule ruptured; or with ascites present containing malignant cells or with positive peritoneal washings. |
| Stage II | Growth involving one or both ovaries with pelvic extension. |
| Stage IIa | Extension and/or metastases to the uterus and/or tubes. |
| Stage IIb | Extension to other pelvic tissues. |
| Stage IIc | Tumor either IIa or IIb, but with tumor on the surface of one or both ovaries; or with capsule(s) ruptured; or with ascites present containing malignant cells or with positive peritoneal washings. |
| Stage III | Tumor involving one or both ovaries with peritoneal implants outside the pelvis and/or positive retroperitoneal or inguinal nodes. Superficial liver metastasis equals stage III. Tumor is limited to the true pelvis, but with histologically proven malignant extension to small bowel or omentum. |
| Stage IIIa | Tumor grossly limited to the true pelvis with negative nodes but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces. |
| Stage IIIb | Tumor of one or both ovaries with histologically confirmed implants of abdominal peritoneal surfaces, none exceeding 2 cm in diameter. Nodes negative. |
| Stage IIIc | Abdominal implants >2 cm in diameter and/or positive retroperitoneal or inguinal nodes. |
| Stage IV | Growth involving one or both ovaries with distant metastasis. If pleural effusion is present, there must be a positive cytologic test results to allot a case to stage IV. Parenchymal liver metastasis equals stage IV. |
TUMOR MARKERS
Tumor markers for ovarian tumors depend on whether they are of an epithelial, germ cell, or stromal origin. The tumor marker for most malignant epithelial ovarian cancer is CA-125. Mucinous cystadenocarcinoma of the ovary can have an elevated carcinoembryonic antigen. Tumor markers for germ cell tumors include α-fetoprotein, β-human chorionic gonadotropin (β-hCG), placental alkaline phosphatase, and lactate dehydrogenase. Tumor markers for sex-cord stromal tumors can include steroid hormones, depending on the histologic subtype (Table 2).
Table 2.
Serum Markers in Ovarian Tumors
| CA-125 | AFP | β-hCG | E2 | Inhibin | TEST | LDH | |
|---|---|---|---|---|---|---|---|
| AFP, α-fetoprotein; β-hCG, β-human chorionic gonadotropin; E2, estradiol; TEST, testosterone; LDH, lactate dehydrogenase; EST, endodermal sinus tumor. | |||||||
| Epithelial ovarian cancer | + | − | − | − | − | − | − |
| Dysgerminoma | − | − | ± | − | − | − | − |
| EST | − | + | − | − | − | − | + |
| Immature teratoma | − | ± | − | ± | − | − | ± |
| Choriocarcinoma | − | − | + | − | − | − | ± |
| Embryonal | − | ± | + | ± | − | − | ± |
| Granulosa cell | ± | − | − | ± | + | − | − |
| Sertoli–Leydig | − | − | − | − | ± | + | − |
NORMAL OVARY
Prior to a full discussion of ovarian pathology, a description of normal ovarian anatomy is helpful. Ovaries are paired, light gray, and approximately the size of a large almond. Previous ovulations cause indentation of the epithelial surface. In women of reproductive age, the ovaries weigh 3 g to 6 g and measure ∼1.5 cm × 2.5 cm × 4 cm. With age, the ovaries become smaller and more firm. During the menstrual cycle, several ovarian follicles are recruited monthly. The dominant follicle releases the egg mid-cycle, thus forming the corpus luteum. If the corpus luteum persists through menstruation, it may be identified as an ovarian mass.6
The following benign ovarian masses are those most commonly found in women and therefore most likely to be encountered by the colorectal surgeon. For appropriate intraoperative management, it is important to remember that the most common ovarian masses in menstruating women are benign functional cysts, and in postmenopausal women benign neoplasms such as cystadenomas. However, the risk of malignancy is much greater in postmenopausal women.7 Frozen section should be used to allow appropriate surgery, which may include conservative surgery in women of childbearing age and full surgical staging when indicated.
FUNTIONAL (PHYSIOLOGIC) OVARIAN CYSTS
Follicular cysts are the most common cystic structures found in the ovaries. They are translucent, thin walled, and filled with a watery clear fluid. They can range in size from 2 to 15 cm and are often found in multiples. They are most likely to be seen in young, menstruating women. Recurrence rates of up to 40% are reported when these cysts are treated intraoperatively with simple drainage, so cystectomy is the recommended treatment.
Corpus luteum cysts are a less common functional cyst. They range from 3 to10 cm, with 4 cm the average. They have a smooth surface and are purplish red to brown, depending on whether the cyst contains an acute or chronic hemorrhage. This bleeding occurs within the cyst postovulation and is usually absorbed. When the hemorrhage is excessive, the cyst can increase in size and rupture may occur. Cystectomy again is the treatment of choice, which preserves the remaining ovary.
Theca lutein cysts are the least common of the physiologic cysts of the ovaries. Unlike the follicular or corpus luteum cysts, they are almost always bilateral, with moderate to severe ovarian enlargement (Fig. 1). They are caused by prolonged or excessive ovarian stimulation from either endogenous or exogenous gonadotropins or by increased ovarian sensitivity to gonadotropins. Therefore, they are most often related to pregnancies, especially molar pregnancies (50%) and choriocarcinomas (10%). Interestingly, they can also be present in newborn infants due to transplacental effects of maternal gonadotropins. Grossly, the ovaries may be massive in size, with multiple thin-walled cysts and straw-colored or hemorrhagic fluid, producing a honeycomb appearance. They should be handled very delicately, and drainage or puncture should not be attempted due to the risk of hemorrhage. Most patients with these cysts are asymptomatic; therefore, the management is conservative because the cysts gradually regress with the decrease or withdrawal of gonadotropin stimulation.
Figure 1.
An ovary showing theca lutein cysts.
BENIGN OVARIAN NEOPLASMS
Epithelial Ovarian Neoplasms
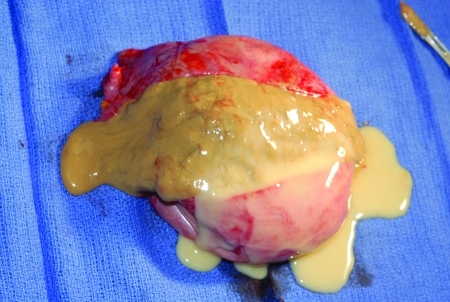
Benign serous (Fig. 2) and mucinous (Fig. 3) cystadenomas are the most common of the epithelial ovarian neoplasms. They can occur at any age, but peak incidence is between ages 20 and 45. Approximately 15% of serous cysts are bilateral, but mucinous cysts rarely are. They are both typically unilocular with a smooth glistening surface, filled with serous or mucinous fluid. Mucinous cystadenomas tend to be larger than their serous counterparts, often >10 cm, though they can grow up to 30 to 40 cm.
Figure 2.
A benign serous cystadenoma.
Figure 3.
A benign mucinous cystadenoma.
The remaining epithelial tumors—edometrioid, Brenner, and clear cell—are rare. Benign endometrioid tumors are typically found as mixed tumors with a predominant serous or mucinous component. Benign Brenner tumors are generally asymptomatic, incidental findings, often in association with a concurrent serous or mucinous cystadenoma. They are smooth, solid tumors, usually <5 cm; some are microscopic. They usually occur in women ages 40 to 60 and are unilateral 85 to 95% of the time.
Nonepithelial Ovarian Neoplasms
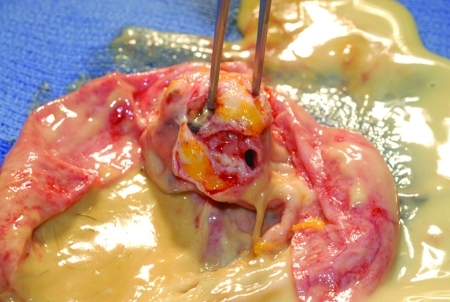
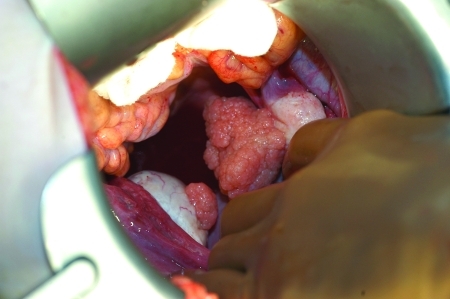
Benign cystic teratomas (dermoid cysts, mature teratomas) are the most common germ cell tumors of the ovary (90%) and are among the most common benign ovarian neoplasms. They can occur from infancy to the postmenopausal years but are most common in the first three decades of life. They are bilateral in up to 15% of cases. Teratomas range from a few millimeters to 25 cm in diameter and contain cystic and solid components. These solid areas appear as pelvic calcifications on x-rays in 50% of teratomas; they are often found incidentally during radiographic investigation of the gastrointestinal or genitourinary tract. The surface of the cyst is smooth, shiny, and opaque white; when opened, it contains a thick sebaceous liquid and often hair (Fig. 4). The cyst wall may also contain cartilage or teeth (Fig. 5). Rupture or slow leak of the sebaceous material into the peritoneal cavity can produce severe chemical granulomatous peritonitis. Operative management of a benign teratoma is cystectomy with preservation of as much normal ovarian tissue as possible, with copious irrigation of any sebaceous material that has leaked from the cyst.
Figure 4.
A benign cystic teratoma. The surface of the cyst is smooth, shiny, and opaque white. When opened, it contains a thick sebaceous liquid and often hair.
Figure 5.
A benign cystic teratoma, with the cyst wall containing cartilage.
Fibromas, which are classified as stromal tumors, are the most common solid benign neoplasms of the ovary. The average age of a woman with a fibroma is 48 years, and in premenopausal woman they are often misdiagnosed as leiomyomas prior to surgery. Grossly, they are solid, grayish-white, and well encapsulated. The cut surface shows homogeneous white solid tissue with a whorled or trabeculated appearance. They are slow growing, with an average size of 6 cm, but they can grow to huge tumors weighing 50 lbs. Meigs' syndrome is the association of an ovarian fibroma with ascites and hydrothorax; it occurs in <2% of fibromas and is directly related to the size of the tumor. The ascites forms as a transudate of fluid from the ovarian fibroma, which then crosses into the pleural space via the lymphatics of the diaphragm. Management of this solid tumor is surgical excision, which leads to resolution of symptoms, including ascites and hydrothorax if present.
NONNEOPLASTIC ADNEXAL MASSES
Endometriosis is defined by the presence and growth of glands and stroma identical to the lining of the uterus in an aberrant location. Cystic areas of endometriosis in the ovary are termed endometriomas and occur in approximately two-thirds of women with endometriosis. Endometriomas are one of the most common causes of enlargement of the ovary due to the prevalence of the disease, and most are asymptomatic. Ovarian endometriomas vary in appearance from blue-black implants a few millimeters in size to large, multiloculated hemorrhagic cysts up to 20 cm, but typically they are <10 cm. When ruptured or opened, endometriomas contain chocolate-like fluid, representing old hemorrhage. Endometriomas are often associated with dense adhesions and involvement of adjacent pelvic structures, including the rectosigmoid colon and rectum. Management of endometriomas can be medical or surgical depending on size, patient age, presence or absence of symptoms, and patient's future reproductive desires.
Tuboovarian abscesses are a consequence of pelvic inflammatory disease (PID) and are typically encountered in reproductive-aged females. Patients will usually be symptomatic with pelvic pain, adnexal tenderness, fever, and leukocytosis, but chronic sterile abscess may present with pain but no acute signs of infection. After diagnosis by ultrasound, initial management uses intravenous antibiotics, often with computed tomography-guided drainage of the abscess. If there is no clinical response to conservative therapy, surgical intervention is indicated and often requires a total abdominal hysterectomy with bilateral salpingo-oophorectomy, as the tuboovarian abscess typically encompasses the entire pelvis and posterior cul de sac, including the colon and rectum, with dense inflammatory adhesions. More conservative surgery with preservation of the uterus and contralateral adnexa is appropriate if they are salvageable for future childbearing, should that be the patient's desire.
Hydrosalpinx is the dilatation of an occluded fallopian tube, which often encompasses the ovary. The etiology is most often inflammatory from pelvic infections but can also be surgical from adhesions after previous tubal, uterine, bowel, or appendiceal surgery; therefore, it can be seen in women of any age. On imaging studies, it is often described as a sausage-shaped cystic mass. The adjacent ovary may or may not be visible, often leading to the question of a possible ovarian etiology. Management involves salpingectomy, with removal of the adjacent ovary based on its involvement and the patient's age and future child-bearing desires.
Ectopic pregnancy is the implantation of the blastocyst anywhere other than the endometrial lining of the uterus. It occurs most commonly in the fallopian tube but can also be found in the cornu of the uterus, the ovary, or the intraabdominal cavity. If ruptured or leaking, it can result in mild to severe hemoperitoneum. It is treated by excision of the tube and/or ovary or by salpingostomy with tubal conservation if future childbearing is desired and the tube appears salvageable. It can be easily ruled out by a negative serum β-hCG.
MALIGNANT OVARIAN TUMORS
Epithelial Carcinoma of the Ovary
Epithelial carcinoma of the ovary is the second most common gynecologic malignancy and has the highest mortality rate of gynecologic malignancies because >70% of patients present with stage III or IV disease. Because these carcinomas are the most relevant to colorectal surgeons, we detail their surgical management in greater detail after the discussion of ovarian masses.
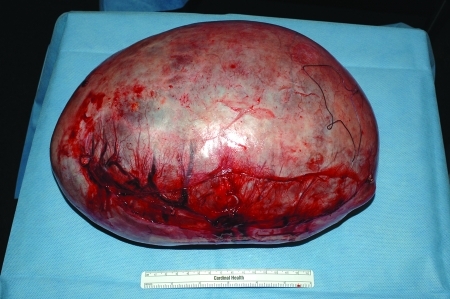
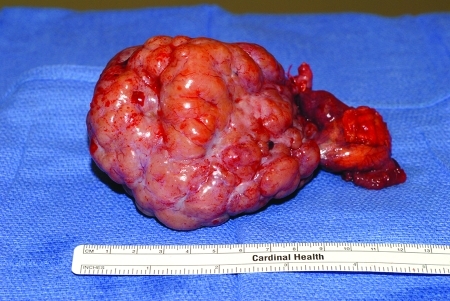
Histologic subtypes of epithelial adenocarcinoma of the ovary, as defined by WHO, include serous (Fig. 6), mucinous, endometrioid, clear cell, Brenner, and undifferentiated. The majority are serous. Mucinous and endometrioid account for ∼10% each, while clear cell, Brenner, and undifferentiated cancer each represent <1% of cases.8,9 Prior studies have not shown a difference in survival by histologic subtype, other than clear cell, which has the worst prognosis.10,11
Figure 6.
A serous epithelial adenocarcinoma, with papillations on the surface.
According to the American Cancer Society, for 2010, there will be an estimated 21,500 new cases and 14,600 deaths.12 The most recent Surveillance, Epidemiology and End Results (SEER) Program data reflect information analyzed from 1988 to 2001; 90% of all cancers of the ovary were adenocarcinomas. Women ages 60 to 69 were the most commonly affected age group, accounting for 21.7% of cases. The 70 to 79 age group ranked second (20.4%), and the 50 to 59 group accounted for 19.4%.13
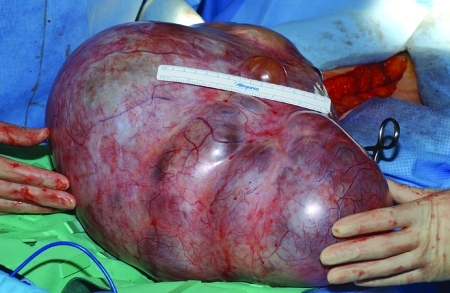
Epithelial adenocarcinoma (Fig. 7) of the ovary is most often found in postmenopausal women. In the recent SEER data, stage III and IV disease accounts for 35% and 32%, respectively, stage I accounts for 22%, and stage II accounts for 8%. In 3% of cases, the patients' stage was unknown or they were not staged. Survival decreases with advancing stage, with stage I patients' 5-year survival rates at 92%, and stage III and IV patients having survival rates of 33% and 18%, respectively.13
Figure 7.
An epithelial adenocarcinoma.
The recent SEER data showed mucinous carcinoma of the ovary to have the highest 5-year survival, of 77%, but this was due to a high percentage of these patients being diagnosed with stage I disease compared with the other epithelial histologic subtypes. In our experience, mucinous tumors of the ovary, both malignant and benign, can measure 40 cm or more. Pathologic grade plays an important role in prognosis and postoperative management in early stage disease.
Tumors of Low Malignant Potential
Tumors of low or borderline malignant potential account for 10% to 15% of ovarian epithelial tumors. Tumors of low malignant potential are defined as tumors with areas of cytologic atypia without invasion into the stroma. The highest frequency of tumors of low malignant potential of the ovary occurs in those 15 to 29 years old. Staging for ovarian tumors of low malignant potential is the same as for invasive ovarian cancer. However, more of these patients present with early-stage disease: 70% will present as stage I, 10% as stage II, 19% as stage III, and <1% as stage IV.14
Controversy surrounds the role of surgical stage of tumors of low malignant potential. Surgical staging allows for the best estimate of recurrence and survival. Survival data from SEER show excellent 5- and 10-year prognoses for tumors of low malignant potential of the ovary: stage I has 99% and 97% survival, respectively; stage II, 98% and 90%, respectively; stage III, 96% and 88%, respectively; and stage IV, 77% and 69%, respectively.15 The presence of residual disease left behind at laparotomy is another factor that determines survival.
Because tumors of low malignant potential occur in a young age group that generally desires fertility, unilateral oophorectomy is the appropriate surgical management. Otherwise, a total abdominal hysterectomy with bilateral salpingo-oophorectomy should be performed.
In addition, we recommend comprehensive surgical staging when ovarian tumors of low malignant potential are found at the time of initial laparotomy: The information obtained from surgical staging is important in counseling the patient on 5- and 10-year survival rates. Another advantage to surgical staging is that should an invasive carcinoma be found, an additional laparotomy for staging purposes is not needed. An important aspect of ovarian tumors of low malignant potential may be the finding of peritoneal implants, especially if these are invasive. Surgical removal of all visible implants is important for long-term survival. There is no role for postoperative chemotherapy in early stage disease, and there is questionable benefit for those with advanced stage disease. However, chemotherapy usually is recommended for those with invasive implants.16
Malignant Germ Cell Tumors
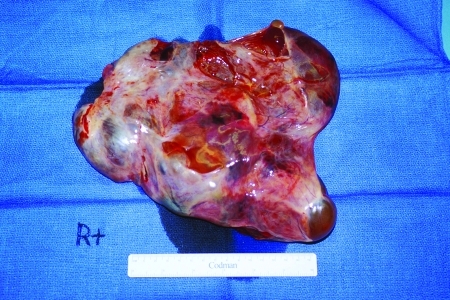
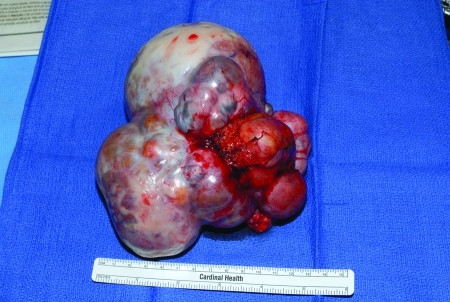
Dysgerminomas (Fig. 8) are typically solid masses arising from the ovary. They account for ∼50% of all malignant germ cell tumors of the ovary. Eighty percent occur in patients in their second and third decades. They are grossly bilateral in ∼10% of cases. Microscopic involvement of a normal ovary can occur in 10%.17
Figure 8.
A dysgerminoma.
Bilateral ovarian involvement with the other germ cell tumors and sex-cord stromal tumors is very unusual. Surgical management for dysgerminomas includes unilateral salpingo-oophorectomy and a thorough inspection of the contralateral ovary. If the contralateral ovary appears normal, additional surgical management such as wedge biopsy is not necessary. The surgical staging of these patients is similar to that of patients with epithelial carcinoma of the ovary. Sampling of the pelvic and paraaortic lymph nodes is important, as dysgerminomas can metastasize to lymph nodes, which has a direct bearing on the need for postoperative chemotherapy. Resection of enlarged, fixed nodes may not be possible to perform safely. Dysgerminoma is a very chemosensitive tumor, and this is a better choice for management than attempted resection. Patients with tumors greater than stage I-A receive a multidrug chemotherapy regimen that includes bleomycin, etoposide, and cisplatin.
Less common germ cell tumors of the ovary include yolk sac tumors, immature teratomas, embryonal carcinomas, and choriocarcinomas. The surgical management for these tumors of the ovary is similar to that of dysgerminoma. Again, surgical staging is vital, and any obvious metastatic disease should be resected or biopsied to document the extent of disease. All but very early stage immature teratomas require postoperative chemotherapy, as they have otherwise a very high risk of recurrence.
Stage distribution of germ cell tumors is different from epithelial tumors. Approximately 60 to 70% will be stage I. Stage III disease accounts for 25 to 30% of tumors, whereas stages II and IV are relatively uncommon.18 As these tumors occur primarily in young women, with a peak incidence in their early 20s, conservative management with a unilateral salpingo-oophorectomy and staging is usually adequate, as these tumors are rarely bilateral.
Ovarian Sex Cord-Stromal Tumors
Granulosa cell tumors of the ovary are the most common of the malignant sex cord-stromal tumors. These tumors can be responsible for excessive estrogen production, resulting in either postmenopausal bleeding in the adult woman or precocious puberty in a child. These tumors are staged similarly to epithelial ovarian cancer. Surgical management in a young patient who desires further fertility would be a unilateral salpingo-oophorectomy. For the adult woman, usual management is a total abdominal hysterectomy and bilateral salpingo-oophorectomy.
Other rare sex cord-stromal tumors of the ovary include Sertoli–Leydig cell tumors. These patients can present with virilization features—such as deepening of the voice, hirsutism, clitoromegaly, amenorrhea, and temporal hair loss—caused by testosterone produced by these tumors. They can present in young women, so conservative management with unilateral salpingo-oophorectomy is performed. Total abdominal hysterectomy and bilateral salpingo-oophorectomy should be performed in women who have completed childbearing.
SURGICAL MANAGEMENT OF EPITHELIAL OVARIAN MALIGNANCIES
The colorectal surgeon who encounters an adnexal mass at the time of surgery should be aware of the steps necessary for surgical staging and optimal tumor resection. This is especially important in the setting where a gynecologic oncologist is not available. Findings at the time of laparotomy that may be concerning for an ovarian cancer include ascites, multicystic ovarian masses, complex solid and cystic masses, and other solid masses. Excrescences or papillary-like projections on the surface of the ovary can also be a sign of a malignancy. Papillations found on the inside of a cyst are concerning for ovarian cancer. Goff et al, in a review of patterns of surgical care in the United States of patients with presumed early-stage disease, found that 21% had only an oophorectomy whereas 21% had an omentectomy performed as a partial staging operation. Forty-one percent had surgical management that included an omentectomy, debulking, and lymph node sampling. Similar rates of incomplete staging are seen in prostate, breast, and lung cancer in elderly, low-income, and minority patients.19
Patients with apparent stage I and II ovarian cancer referred for treatment who undergo additional surgery for staging purposes will often be found to have more advanced disease. Young et al showed that 31% were upstaged, with 77% of those being stage III.20 Surgical staging should be performed in the absence of omental, diaphragmatic, or upper abdominal metastatic disease. The steps of surgical management are outlined in Table 3.
Table 3.
Operative Steps in Ovarian Cancer
| 1. Midline incision |
| 2. Aspirate ascites or perform cytologic washing with 100 mL normal saline from each paracolic gutter, the cul de sac, and each diaphragm |
| 3. Exploration of abdomen, including palpation of each diaphragm, peritoneal surfaces, small and large intestine, omentum, pelvic and paraaortic nodes, and intraabdominal organs |
| 4. In the absence of upper abdominal disease >2 cm, obtain peritoneal biopsies from both paracolic gutters, anterior and posterior cul de sac, perform an omentectomy and sampling of pelvic and paraaortic lymph nodes. Perform tumor debulking, including total abdominal hysterectomy, bilateral salpingo-oophorectomy, and resection of metastatic disease (e.g., bowel resection) as necessary to attempt to optimally debulk to residual disease no greater than 1 cm. |
| 5. In the presence of upper abdominal disease ≥2 cm, perform total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and resection of metastatic disease that may include bowel resection. Attempt to optimally debulk to residual disease no greater than 1 cm. |
Surgical management may include resection of small and large intestines, as well as peritoneal and diaphragmatic resection. Optimally debulked patients are individuals with no remaining tumor nodules >1 cm.
Additional surgical management of ovarian cancer in addition to a total abdominal hysterectomy or bilateral salpingo-oophorectomy includes omentectomy, appendectomy, and selective pelvic and para-aortic lymph node sampling. In the absence of ascites, cytologic washings should be performed with instillation of 100 mL of normal saline along each paracolic gutter, the pelvis, and each diaphragm. In the absence of upper intraabdominal disease, omentectomy and biopsies of the peritoneum from both paracolic gutters and the anterior and posterior cul de sac should be performed.
Improvement in survival benefits with optimal debulking has been reported in retrospective or nonrandomized studies.21,22 Benefits of optimal resection of ovarian cancer have become more evident recently due to a randomized clinical trial involving stage III ovarian cancer or primary peritoneal cancer patients who were optimally debulked to ≤1 cm residual. These patients were randomized to intravenous cisplatin and paclitaxel versus intravenous paclitaxel, intraperitoneal cisplatin, and intraperitoneal paclitaxel. This study showed a 25% reduction in death from ovarian cancer and an improvement in median survival of ∼15 months. However, those receiving intraperitoneal chemotherapy had more toxicities, such as abdominal pain, fatigue, and metabolic and/or neurologic side effects. Additionally, their quality of life was worse during treatment but the same at 12 months after completing treatment.23
If the patient has been optimally debulked, a peritoneal catheter should be placed at the time of closing the abdomen. We use a 9.6 French single-lumen silicone catheter with implanted subcutaneous reservoir. This reservoir is placed in the right lower chest wall, and the catheter should be tunneled through the subcutaneous tissue and enter into the abdomen ∼6 cm lateral to the umbilicus. Approximately 10 cm of catheter should be left behind in the abdominal cavity. This technique described by Makheja resulted in a decrease in catheter complication rates.24
CONCLUSION
We hope that this review helps to increase colorectal surgeons' awareness of the adnexal masses they may come across during surgery, particularly epithelial masses, benign and malignant, as well as the standards for surgical staging and optimal tumor resection for the various types of masses.
REFERENCES
- 1.Scully R E. Classification of human ovarian tumors. Environ Health Perspect. 1987;73:15–25. doi: 10.1289/ehp.877315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berek J S, Hacker N F. In: Berek JS, Hacker NF, editor. Practical Gynecologic Oncology. Philadelphia: Lippincott Williams & Wilkins; 2005. Nonepithelial ovarian and fallopian tube cancers. pp. 511–541.
- 3.Partridge E, Kreimer A R, Greenlee R T, et al. PLCO Project Team Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113(4):775–782. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagell J R van, DePriest P D. Management of adnexal masses in postmenopausal women. Am J Obstet Gynecol. 2005;193(1):30–35. doi: 10.1016/j.ajog.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 5.Granberg S, Wikland M, Jansson I. Macroscopic characterization of ovarian tumors and the relation to the histological diagnosis: criteria to be used for ultrasound evaluation. Gynecol Oncol. 1989;35(2):139–144. doi: 10.1016/0090-8258(89)90031-0. [DOI] [PubMed] [Google Scholar]
- 6.Stenchever M A, Droegemueller W, Herbst A L. Mishell Dr. Comprehensive Gynecology. Maryland Heights, MO: Mosby; 2001.
- 7.Koonings P P, Campbell K, Mishell D R, Jr, Grimes D A. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989;74(6):921–926. [PubMed] [Google Scholar]
- 8.Scully R E, Young R H, Clement P B. In: Scully RE, Young RH, Clement PB, editor. Atlas of Tumor Pathology, Third Series, Fascicle 23. Washington, DC: Armed Forces Institute of Pathology; 1998. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. pp. 27–50.
- 9.Berek J S. In: Berek JS, Hacker NF, editor. Practical Gynecologic Oncology. Philadelphia: Lippincott Williams & Wilkins; 2005. Epithelial ovarian cancer. pp. 443–509.
- 10.Silverberg S G. Prognostic significance of pathologic features of ovarian carcinoma. Curr Top Pathol. 1989;78:85–109. doi: 10.1007/978-3-642-74011-4_5. [DOI] [PubMed] [Google Scholar]
- 11.Ludescher C, Weger A R, Lindholm J, et al. Prognostic significance of tumor cell morphometry, histopathology, and clinical parameters in advanced ovarian carcinoma. Int J Gynecol Pathol. 1990;9(4):343–351. doi: 10.1097/00004347-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M J. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 13.Kosary C L. Cancer of the ovary. In: SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics [T-098] Pub. No. 07–6215. Bethesda, MD: National Cancer Institute; 2002:133–144. Available at: http://Seer.cancer.gov/publications/survival/surv_ovary.pdf. Accessed January 31, 2010. Available at: http://Seer.cancer.gov/publications/survival/surv_ovary.pdf
- 14.Tinelli R, Tinelli A, Tinelli F G, Cicinelli E, Malvasi A. Conservative surgery for borderline ovarian tumors: a review. Gynecol Oncol. 2006;100(1):185–191. doi: 10.1016/j.ygyno.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Trimble C L, Kosary C, Trimble E L. Long-term survival and patterns of care in women with ovarian tumors of low malignant potential. Gynecol Oncol. 2002;86(1):34–37. doi: 10.1006/gyno.2002.6711. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Berek J S. In: Basow DS, editor. UpToDate. Waltham, MA: UpToDate; 2009. Ovarian tumors of low malignant potential.
- 17.Scully R E, Young R H, Clement P B. In: Scully RE, Young RH, Clement PB, editor. Atlas of Tumor Pathology, Third Series, Fascicle 23. Washington DC: Armed Forces Institute of Pathology; 1998. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. pp. 239–266.
- 18.Williams S D, Gershenson D M, Horowitz C J, Silva E. In: Hoskins WJ, Perez CA, Young RJ, editor. Principles and Practice of Gynecologic Oncology. Philadelphia: Lippincott Williams & Wilkins; 2000. Ovarian germ-cell tumors. pp. 1059–1073.
- 19.Goff B A, Matthews B J, Wynn M, Muntz H G, Lishner D M, Baldwin L M. Ovarian cancer: patterns of surgical care across the United States. Gynecol Oncol. 2006;103(2):383–390. doi: 10.1016/j.ygyno.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Young R C, Decker D G, Wharton J T, et al. Staging laparotomy in early ovarian cancer. JAMA. 1983;250(22):3072–3076. [PubMed] [Google Scholar]
- 21.Hacker N F, Berek J S, Lagasse L D, Nieberg R K, Elashoff R M. Primary cytoreductive surgery for epithelial ovarian cancer. Obstet Gynecol. 1983;61(4):413–420. [PubMed] [Google Scholar]
- 22.Piver M S, Lele S B, Marchetti D L, Baker T R, Tsukada Y, Emrich L J. The impact of aggressive debulking surgery and cisplatin-based chemotherapy on progression-free survival in stage III and IV ovarian carcinoma. J Clin Oncol. 1988;6(6):983–989. doi: 10.1200/JCO.1988.6.6.983. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong D K, Bundy B, Wenzel L, et al. Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 24.Makhija S, Leitao M, Sabbatini P, et al. Complications associated with intraperitoneal chemotherapy catheters. Gynecol Oncol. 2001;81(1):77–81. doi: 10.1006/gyno.2000.6108. [DOI] [PubMed] [Google Scholar]