ABSTRACT
Iatrogenic injury to the urinary tract during colorectal surgery can be a source of significant morbidity. Although most cases of ureteral injury occur in patients without significant risk factors, the incidence of urinary tract injuries increases in patients with prior pelvic operations, inflammatory bowel disease, infection, and in patients with extensive neoplasms causing distortion of normal surgical planes. The most commonly injured locations are the ureter, bladder, and urethra. Mechanisms of injury include ligation, transection, devascularization, and energy induced. Early identification of urinary tract injuries is paramount in minimizing morbidity and preservation of renal function. Anatomic considerations for preventing injuries, diagnostic techniques for localizing and staging injuries, as well as reconstructive techniques and principles of repair are discussed.
Keywords: Iatrogenic injury, repair, ureter, bladder, urethra
Iatrogenic injury to the urinary tract during operations within the pelvis and retroperitoneum occur most commonly to the ureters followed by injuries to the bladder and urethra. Although most cases of ureteral injury occur in patients without significant risk factors,1 the incidence of urinary tract injuries increases in patients with prior pelvic operations, inflammatory bowel disease, infection, and in patients with extensive neoplasms causing distortion of normal surgical planes. Unrecognized congenital anomalies such as a duplicated ureter (1/125 persons), retrocaval ureter, horseshoe or pelvic kidneys (1/400 persons) can present unfamiliar anatomy to the surgeon.2 In some cases, resection and reconstruction of a portion of the urinary tract is mandated by disease severity. The rate of urologic complications with the application of minimally invasive technologies (laparoscopy and robotics) for colorectal procedures has been relatively constant when compared with open surgical techniques. Use of the various energy-based tissue devices in close proximity to the urinary tract can cause a delayed presentation of a urinary injury. Early identification of urinary tract injuries is paramount in minimizing morbidity and preservation of renal function. Intraoperative repair of injuries to the urinary tract can be performed through consultation with a urologic surgeon or by the initial operating surgeon. Principles of repair for specific injuries are discussed herein.
URETERAL INJURIES
Incidence
Injury to the ureter is the most common urologic complication of pelvic surgery with an incidence of 1 to 10%.1,3,4,5,6,7,8 Urologic surgeons are not immune to iatrogenic ureteral injuries with urologic procedures accounting for up to 30% of injured ureters—most commonly due to intraluminal endoscopic procedures for stone disease.9,10 Gynecologic procedures account for the vast majority of ureteral injuries (up to 50% in some series). Surgical procedures involving the colon and rectum account for ∼5 to 15% of ureteral injuries, and this rate has remained stable even through the introduction and adoption of minimally invasive surgical techniques. Abdominoperineal resection followed by sigmoidectomy is most commonly associated with iatrogenic ureteral injuries in colorectal surgery.
Anatomy
The ureter is divided into three anatomic segments based on the position on an abdominal x-ray (kidney, ureter, bladder [KUB]):
Upper third ureter extending from the ureteropelvic junction (UPJ) to the upper border of the sacroiliac joint
Middle third ureter coursing over the bony sacrum
Lower third ureter from the inferior border of the sacroiliac joint to the ureterovesical junction (UVJ) within the bladder
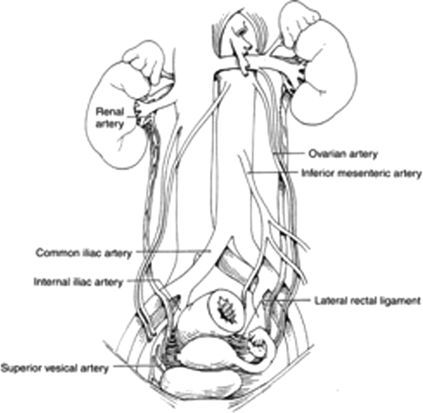
The ureter begins posterior to the renal artery at the ureteropelvic junction (UPJ) and courses along the anterior edge of the psoas muscle.11 The gonadal vessels cross ventral to the upper third ureter from medial to lateral. The ureter then passes over the iliac vessels normally at the location of the bifurcation of the common iliacs. In females, the ureter crosses dorsal to the ovary, and underneath the broad ligament within 2 centimeters of the uterine vessels. At this location is where a majority of iatrogenic injuries occur during gynecologic surgery. In the male, the vas deferens crosses ventral to the ureter (immediately proximal to the ureter entering the detrusor) as it courses from the midline prostate to join the gonadal vessels laterally near the internal inguinal ring. The ureter courses through the bladder wall (detrusor muscle) at an oblique angle to prevent reflux of urine. Although significant variability exists, the blood supply to the ureter originates through multiple small unnamed arterial branches of the renal, aorta, gonadal, internal iliac, and middle rectal vessels. The vessels approach the ureter from the medial aspect above the iliacs and from the lateral aspect inferior to the iliacs. This anatomic relationship is clinically important when mobilizing the ureter. The left ureter is crossed ventrally by the left colic or inferior mesenteric and sigmoidal vessels and is adjacent to the descending and sigmoid colon. The right colic, ileocolic vessels, and the root of the mesentery containing the superior mesenteric vessels cross the right ureter.1 The right ureter is adjacent to the cecum, terminal ileum, and appendix.
During surgery of the colon and rectum, iatrogenic ureteral injuries usually occur at three distinct locations: (1) at the takeoff of the inferior mesenteric artery, (2) where the infundibulopelvic ligament/uterine vessels cross the pelvic brim, and (3) between the lateral rectal ligaments (see Fig. 1).
Figure 1.
Anatomy of the ureter. Reproduced with permission from Delacroix, Winters.12 Copyright © 2009 Informa Healthcare Communications.
Prevention/Early Identification
The ureter will preferentially adhere to the peritoneum during mobilization of the descending colon rather than maintaining its normal position along the psoas muscle. Visualization of the ureter is crucial prior to transection of the colonic mesentery. It can be identified by visualization, or in the case of a healthy ureter, by the intrinsic peristaltic activity of the ureter. Gentle pressure applied to the ureter will frequently cause peristalsis—termed the Kelly sign.
Ureteral catheterization can be used to aid in identification of the ureters and to aid in identification of ureteral injuries, but they do not prevent ureteral injury. Nam et al assessed the clinical value of prophylactic ureteral catheter placement prior to 162 laparoscopic segmental left and right colectomies.3 There were no complications for ureteral catheter placement and no ureteral injuries. Operative time was increased by 11.3 minutes. Another small randomized trial compared sequential versus simultaneous ureteral catheterization and showed no increase in operative times when performed in a simultaneous fashion on patients undergoing complex or reoperative colorectal procedures.13 The authors concluded the simultaneous insertion allowed for more individualized patient selection after laparotomy but did not comment on the coordination difficulties this could create. Lighted ureteral stents are also commercially available and were placed in 66 patients prior to laparoscopic colectomy.5 The most common complication was self-limiting hematuria in 98.4% of patients with an average duration of 2.5 days for unilateral stenting and 3.3 days with bilateral stenting. Most colon and rectal surgical series addressing this question are performed at high-volume centers with a baseline low rate of ureteral injury. This low prevalence of injuries does not allow for statistically sound analysis regarding overall benefits.
It is our opinion that the choice for ureteral stenting is a surgeon's preference based on multiple variables including complexity of case, anatomy, and experience. This is especially true with minimally invasive techniques in a hostile abdomen. With greater experience, iatrogenic injury rates decrease.4 Diagnosis of a suspected injury can also be confirmed with an on-table intravenous pyelogram (IVP) or through retrograde injection of methylene blue or radiographic contrast through ureteral catheters. An IVP is performed by intravenous (IV) administration of 2.0 cc/kg of IV contrast (up to 150 cc maximum) and taking a plain x-ray of the abdomen. In complex patients at higher risk for urinary tract injury, placement of ureteral catheters may provide for more prompt identification of iatrogenic ureteral injuries thereby reducing morbidity through immediate intraoperative rather than delayed repair.
Mechanisms of Injury
Iatrogenic ureteral injuries can be classified based on the mechanism of injury: laceration, ligation, devascularization, and energy-related. Prompt identification and repair (if necessary) is optimal to avoid postoperative morbidity.
LACERATION
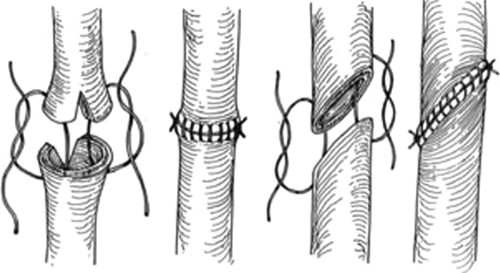
Transection or partial laceration of a ureter is repaired dependent on the location of the injury (see location specific repair). Crucial technical keys include spatulation of the ureter prior to repair, a tension-free anastomosis, and using only short- to moderate-term absorbable sutures.
LIGATION
For a ligation injury recognized intraoperatively, the clamp or tie should be removed and a ureteral stent can be placed for 4 to 6 weeks. Imaging with a renal ultrasound or contrast radiograph (computed tomography [CT] or intravenous pyelogram [IVP]) should be performed to detect a subsequent ureteral stricture. These injuries, with resultant ureteral stricture, can manifest without symptoms with resultant silent renal atrophy, thus follow up is prudent to confirm ureteral patency.
DEVASCULARIZATION
The devascularization injury is not usually apparent at the time of surgery and is more common after radiation therapy and vascular surgical procedures. The normal healthy ureter is very resistant to this type of injury due to the extensive collateral blood supply. These injures can present months after the initial surgery usually as obstruction due to a ureteral stricture.
ENERGY
Various energy sources are used for dissection and hemostasis during surgery and can be a source of injury to the urinary tract. These injuries can present in the early postoperative period with either fistula (urinoma) or stricture formation. Many laparoscopic surgeons use alternatives to monopolar cautery in an attempt to lower the energy spread, and thus injuries to surrounding structures. Even with these newer devices, collateral tissue damage can be induced dependent on the proximity, duration, and energy setting used.14 Energy-based injuries are caused from local devascularization and urothelial injury. If recognized intraoperatively, conservative treatment with a ureteral stent can be employed to decrease postoperative ureteral edema. Similar to a crush injury, these patients should be followed with either an x-ray or sonogram at least 3 months after stent removal to detect the development of ureteral stricture.
Location-Specific Ureteral Repair
General principles for repair include use of absorbable suture to prevent stone formation, a tension-free spatulated anastomosis over an indwelling ureteral stent, and placement of a closed suction drain in the area of repair. Elevated drainage after repair can be differentiated from peritoneal fluid by sending the drain contents for creatinine levels and comparing them to serum levels. If fluid creatinine levels are equal to serum levels, the drainage is not urine. In the case of a delayed repair, delineation of the length and location of injury are necessary in surgical planning and patient counseling. In cases of prolonged obstruction with renal parenchymal loss, a nuclear renogram can objectively assess each kidney's renal function. With substantial parenchymal loss and a poorly functional kidney, nephrectomy can be considered. The reparative procedure depends greatly on the location of the diseased/injured ureter. Repair of ureteral injuries can be performed through minimally invasive or open surgical techniques. Other than enteric interposition (ileal ureter) and autotransplantation, each of the described procedures in all segments of the ureter can be performed in the appropriate patient with minimally invasive surgical techniques (robotic or laparoscopic repair).15,16,17 Individualized treatment is based on location, stricture disease, integrity of the abdomen, and surgeon experience.
PROXIMAL ONE-THIRD
Injuries to the proximal one-third ureter account for 2% of ureteral injuries.10 Repairs to injuries of the proximal ureter depend on the length of the damaged segment. Simple spatulated ureteroureterostomy (U-U) with ureteral stent placement is the preferred method of repair (Fig. 2). Success rates for U-U are greater than 90% in most series. Mobilization of the kidney with fixation sutures to the psoas tendon (nephropexy) can allow for lengths of up to 4 cm to be repaired in a tension-free fashion with a ureteroureterostomy. Right-sided nephropexy provides more length than a left-sided nephropexy due to the shorter left renal vein. In cases of long segments of damaged ureters, a bowel interposition (ileum or appendiceal) can be used.18,19,20 Crohn disease, radiation enteritis, and a serum creatinine greater than 2.0 mg/dL are contraindications to performing an ileal ureter. Depending on the capacity/size of the bladder, a psoas hitch (Fig. 3) or Boari flap (Fig. 4) can sometimes be used to reach the upper ureter.21,22 These procedures are more commonly used for injuries of the middle and distal third ureter and will be discussed below. An individualized approach must be taken with very long proximal ureteral strictures. At specialized centers, autotransplantation with reanastomosis to the iliac vessels and native more distal ureter can also be performed.23
Figure 2.
Ureteroureterostomy with spatulated anastomoses of running absorbable suture. Reproduced with permission from Delacroix, Winters.12 Copyright © 2009 Informa Healthcare Communications.
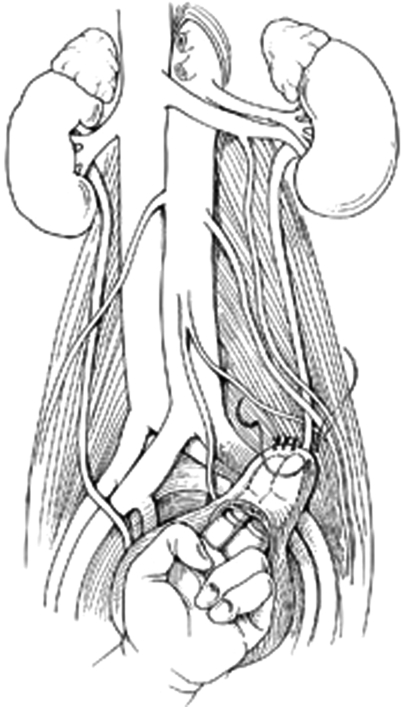
Figure 3.
Psoas hitch after transverse cystotomy and interrupted anchoring sutures placed through psoas tendon. Reproduced with permission from Delacroix, Winters.12 Copyright © 2009 Informa Healthcare Communications.
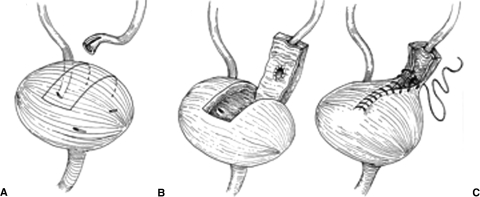
Figure 4.
Boari flap. (A) Incision site for posterior pedicle based bladder flap. (B) Ureteral reimplantation shown with transverse submucosal tunnel. (C) Tubularization of the bladder flap. Reproduced with permission from Delacroix, Winters.12 Copyright © 2009 Informa Healthcare Communications.
MIDDLE ONE-THIRD
Injuries to the middle one-third ureter account for 7% of ureteral injuries.10 If the injury is at the level of the iliac vessels or proximal, the preferred method of repair is ureteroureterostomy for short segments. For larger segments in which a tension-free anastomosis is not possible, a ureteroneocystostomy is performed with the aid of a psoas hitch or Boari flap.
For a psoas hitch (Fig. 3), the bladder is mobilized by ligating the superior vesical pedicle on the side contralateral to the injury. Localization of the contralateral ureter is prudent prior to this maneuver. The bladder can then be opened through a transverse anterior cystotomy and secured to the psoas tendon using several 2–0 simple absorbable sutures (SAS) through the psoas tendon. Care must be taken not to include the genitofemoral nerve positioned on the anterior surface of the muscle lateral to the common iliacs. The suture should be placed in the direction linear to the tendon to avoid entrapment of the femoral nerve running in the belly of the psoas muscle.24 The ureter can then be tunneled by passing a clamp from the lumen of the bladder (near the hitched area) through the muscle fibers and grasping the a stay stitch placed on the distal ureter. The ureter can then be passed through wall of the detrusor and a widely spatulated anastomosis can be completed using interrupted 3–0 to 4–0 SAS placed from mucosa to mucosa. Tunneling the ureter at an oblique angle through the wall of the detrusor can prevent urinary reflux, but may have a slightly higher stenosis/stricture rate. A ureteral stent can then be placed and the anterior cystotomy closed in a vertical fashion. A Foley catheter should be kept in place for 7 to 14 days with stent removal at 4 to 6 weeks from surgery.
For injuries spanning longer more proximal distances, a Boari flap technique can be utilized (Fig. 4). This is another effective, yet more complex method for replacement of an extensively damaged midureter. A flap of the anterior bladder wall is raised in a rectangular fashion and affixed to the psoas tendon as previously described. The vascular supply of this flap is based on the ipsilateral superior vesical artery. The ureter is tunneled through the most proximal portion of the flap and a neoorifice is created using the above technique. A ureteral stent is placed. The bladder flap is then tubularized and closed in a two layer fashion using running 3–0 SAS for mucosal approximation followed by running 2–0 SAS for seromuscular approximation. Again, a Foley catheter should be kept in place for 7 to 14 days with stent removal at 4 to 6 weeks from surgery. This procedure should not be performed in a patient with a small bladder capacity as functional capacity can be significantly reduced with resultant postoperative voiding dysfunction. In patients with prior pelvic irradiation, extensive bladder mobilization with Boari flap formation to the upper ureter can be fraught with complications. A well-vascularized bladder flap with a tension-free anastomosis is key to preventing complications such as recurrent stricture and urine leak.
Lastly, a transureteroureterostomy (TUU) can be performed by tunneling the injured ureter under the posterior peritoneum anterior to the bifurcation of the great vessels. The ureter can then be anastomosed end to side to the contralateral uninjured ureter with minimal mobilization to the recipient ureter. Absolute contraindications to TUU are insufficient ureteral length and a diseased contralateral ureter. Relative contraindications include a history of urothelial carcinoma, nephrolithiasis, irradiation, chronic infection, or retroperitoneal fibrosis. Due to the inherent risks of operating on both ureters, this procedure is not popular with reconstructive surgeons, but still does have a place in the armamentarium of reconstructive urology.
DISTAL ONE-THIRD
Injuries to the distal one-third ureter account for 91% of ureteral injuries.10 The repair of choice for distal ureteral injuries is a ureteroneocystostomy. This can be performed via primary anastomosis to the bladder for injuries to the very distal 2 to 3 centimeters. Open surgical techniques of passing the ureter through the bladder wall and anastomosis are applied. Principles of reconstruction mandate a tension-free anastomosis. If this cannot be obtained with primary reimplantation, previously described mobilization through transection of the contralateral superior vesical pedicle with or without a psoas hitch is indicated.
BLADDER INJURIES
Injuries to the bladder can result from any surgical procedure in the pelvis or as a result of trochar insertion during laparoscopy. If recognized at the time of injury, an isolated bladder injury is usually repaired without difficulty. Patients with prior radiation and inflammatory bowel disease are at an increased risk for entero/colovesical fistula formation after an injury. With the use of multilayer reconstruction and tissue flap interpositions (omental) these risks can be minimized in higher risk patients. Short or moderate absorbable sutures are always used (never permanent or long duration absorbable).
Anatomy
The cephalad and posterior portions of the bladder are covered with peritoneum whereas the ventral and lateral surfaces of the bladder are within the extraperitoneal space of Retzius. Posteriorly, the peritoneum meets the anterior rectal peritoneum forming the rectovesical space approximately 2 centimeters cephalad to the tips of the seminal vesicals. The capacity of an adult bladder varies but normal ranges from 350 to 600 milliliters when full. Upon filling, the bladder distends to a position outside of the true pelvis. Umbilical trochar insertion without drainage of the bladder is ill-advised.
The vascular supply to the bladder is based on the internal iliac artery (hypogastric artery) and the venous drainage through the internal iliac vein. Surgically, the vascular pedicles to bladder are divided based on the relation to the ureters: into the lateral pedicle (superior vesical pedicle, lateral to the ureters) and posterior pedicle (posteromedial to the ureters).
The bladder is innervated with multiple branches from the autonomic pelvic plexus. Autonomic efferent fibers from the anterior portion of the pelvic plexus (the vesical plexus) pass up the lateral and posterior ligaments to innervate the bladder. The bladder wall is richly supplied with parasympathetic cholinergic nerve endings and has abundant postganglionic cell bodies. Sparse sympathetic innervation of the bladder has been proposed to mediate detrusor relaxation.24 Although not a distinct surgical structure, damage to this plexus during abdominoperineal resection and low anterior resections can result in voiding dysfunction. Prevention through technical modifications as well as diagnosis will be discussed in a separate article on voiding dysfunction within this issue of Clinics in Colon and Rectal Surgery.
Diagnosis/Staging
Iatrogenic injuries to the bladder are staged as25
Grade 1: contusion, intramural hematoma or partial thickness laceration
Grade 2: extraperitoneal bladder wall laceration <2 cm
Grade 3: extraperitoneal >2 cm or intraperitoneal <2 cm
Grade 4: intraperitoneal bladder wall laceration >2 cm
Grade 5: intra- or extraperitoneal bladder wall laceration involving the trigone or bladder neck
Injuries to the bladder can present in a delayed fashion or at the time of surgery. Risk factors for bladder injury include previous operations, radiation, malignant infiltration, chronic infection, and inflammation. Intraoperative identification allows for immediate repair (cystorrhaphy). An unrecognized bladder injury will usually present clinically in the early postoperative period. Signs and symptoms can include drainage from a surgical incision, increased output from surgical drains, vaginal leakage, ileus, apparent oliguria, and urinary ascites with elevated BUN and serum creatinine due to reabsorption. Diagnosis can be made radiographically by a CT cystogram or fluoroscopic cystogram.26,27 Passive filling of the bladder with opacification from filtered contrast from the kidneys is not sufficient to diagnose a bladder injury and is not a true CT cystogram. A CT cystogram is performed with retrograde contrast instillation (through a Foley catheter) of 200 to 300 cc of water-soluble contrast prior to the exam. The Foley is clamped during the scan. Extraperitoneal injuries are identified by contrast extravasation being confined to the lateral pelvic side walls or within the space of Retzius.
The development of a colovesical or enterovesical fistula is a delayed complication of cystotomy.27,28 An abdominal-pelvic CT scan with oral and rectal water soluble contrast has a greater sensitivity than cystoscopy in accurately diagnosing and staging an enterovesical/colovesical fistula.12 Although not used for staging, the poppy seed test is the most sensitive and specific for diagnosis of a fistula.28 A 1.25-ounce container of poppy seeds is mixed into a 12-ounce beverage or a 6-ounce serving of yogurt and ingested by the patient. Urine is then visually inspected for 48 hours for identification of poppy seeds. The sensitivity and specificity of the test is 100%. This test does not provide anatomic information, but is much more cost effective as a screening test in patients with equivocal symptoms (US$5 vs $600).
Repair
All injuries recognized intraoperatively should be repaired. For small extraperitoneal injuries (grade 2) without complicating factors, treatment is a Foley catheter for 7 to 14 days. Late presentation of grade 3 to grade 5 injuries require operative repair. Closed suction drains should be left in place after repairs. Suprapubic tube placement is not necessary in most cases.
For injuries to the ventral bladder, dome, or posterior bladder away from the ureteral orifices, the mucosa is closed in a running fashion using 3–0 SAS followed by a seromuscular running suture of 2–0 SAS. The bladder can then be irrigated to ensure a watertight closure. A third layer in a Lembert fashion can be used in cases at high risk for fistula formation or when a leak is identified. In the laparoscopic setting, a one-layer closure is performed using 2–0 SAS to close all layers of the bladder. An additional layer can then be added using a 2–0 SAS in a Lembert fashion for more extensive injuries.
For injuries more posterior involving the trigone of the bladder or near the ureteral orifices, a thorough inspection for possible ureteral injuries is mandated. This can be accomplished by mobilization of the bladder anteriorly within the space of Retzius. An anterior cystotomy is then performed in the sagittal plane extending caudal to the pubic symphysis. This allows maximal exposure to the interior of the bladder and full inspection of the trigone. A self-retaining retractor is then placed with the retraction blades within the bladder lumen. Indigo carmine can be given through an intravenous access and can aid in identification of the ureteral orifices. Bilateral ureteral catheters or stents can be placed for injuries approaching the ureteral orifices. Closure of the posterior bladder injury is then performed from this anterior luminal exposure. The deep muscular layer is initially closed using 2–0 SAS followed by closure of the mucosal layer using 3–0 SAS. In patients that have received neoadjuvant radiotherapy, a more extensive dissection with interposition of omentum or perivesical fascia can minimize the risk of fistula formation.
URETHRAL INJURIES
The most common urethral injury in colon and rectal surgery is the traumatic Foley placement. Direct injuries to the urethra are rare, but can occur during extirpative surgery for extensive vaginal and anorectal malignancies. Many of these patients have a history of radiation therapy and are prone to fistula formation. These injuries and the postoperative sequelae (fistula formation) can be a source of significant morbidity and frustration.
Diagnosis/Staging
Urine leakage may not be evident within the surgical field due to bladder decompression with a Foley catheter. Retrograde injection of methylene blue tinted saline can aid in diagnosis. A l4-gauge Angiocath is placed in the urethral meatus next to the Foley catheter (do not remove the Foley) and 10 to 20 milliliters are injected. Of course, this is only helpful if the surgical field is still open. The most common presentation of a urethral injury is with fistula formation in the postoperative period, usually after Foley catheter removal. In these patients, accurate staging is paramount and will dictate the surgical options. Cystoscopy, retrograde urethrogram, exam under anesthesia (EUA), and CT scan of abdomen and pelvis with oral and rectal contrast are used in conjunction to help delineate the location and extent of fistulas. Retrograde urethrograms should always be performed in both the bilateral oblique as well as the anteroposterior position.
Repair
If recognized at the time of surgery, primary repair of iatrogenic urethral injuries is the method of choice. The urethra and periurethral tissue should be mobilized to obtain a tension-free repair. For small defects, the goal is a watertight repair with 3–0 or 4–0 SAS in multiple layers. If the patient has had neoadjuvant radiation therapy or has poor tissue compliance, placement of either an omental flap or local tissue flap can help reduce the risk of fistula formation. In the case of extensive urethral loss recognized at the time of surgery, tissue flaps may be used in reconstructing the urethra. In the female, a flap of the vaginal epithelium or a tubularized anterior bladder flap can be used and reinforced with a Martius flap.29 In the male, multiple flaps are available including a dartos flap from the scrotum, foreskin flap, or penile skin flap. Although chordee (curvature of penis with erection) is a concern with lengths longer than 2 centimeters, mobilization of the male urethra can provide a tension-free anastomosis for defects up to 3 centimeters. In severe injuries, primary repair may not be feasible. A suprapubic catheter is placed and repair can be performed after several months. The patient will require restaging. A multitude of complex reconstructive techniques are available with variable success rates and morbidity.30
Injuries not identified at the time of surgery can present postoperatively as urine drainage per rectum or perineum, pneumaturia, or fecaluria. Symptoms of bladder outlet obstruction can herald the development of a urethral stricture. Spontaneous closure of urethrorectal fistulas is very rare.
Urinary fistulas are staged according to location, size, and radiation history.31
Stage 1—low (<4 cm from the anal verge and nonirradiated)
Stage 2—high (>4 cm from the anal verge and nonirradiated)
Stage 3—small (<2 cm diameter irradiated fistula)
Stage 4—large (>2 cm diameter irradiated fistula)
Stage 5—large (ischial decubitus fistula)
Enteric diversion by means of a diverting colostomy or ileostomy is recommended for stage 3 through stage 5. Diversion is normally performed in advance of repair to allow the inflammation associated with the fistula to subside. For large radiated fistulas (grades 4–5), a distal end colostomy is not recommended as this can preclude abdominoperineal endoanal pull through (Turnbull–Cutait) as a reconstructive option of last resort. The choices for repair are diverse and depend on local tissue integrity and staging. For maximal drainage, a suprapubic catheter at the time of repair is recommended in addition to a Foley catheter.32 Transanal rectal flap advancement can be used for stage 1 fistulas or in combination with other techniques for higher stage fistuals.33 Principles of repair include transection and closure of fistulas, placement of local tissue flaps or grafts (buccal mucosa34) within the interspace between lumens, and avoidance of overlapping suture lines. Complete excision of the fistula is not always necessary or possible. For stage 3 through stage 5 fistulas, harvest and interposition of regional myofascial flaps (rectus abdominus, gracilis or gluteal) augment the repair.35,36 Operative approaches and techniques include
Transanal–transsphincteric approach with fistula closure and rectal advancement flap (dorsal lithotomy anterior sphincterotomy)39
York–Mason/transsphincteric approach with fistula closure and rectal advancement flap32,40,41 (jackknife posterior sphincterotomy)
Abdominoperineal pull-through with regional myofascial interposition flap (Turnbull–Cutait)42,43
Surgical selection should be on fistula stage and the experience of the reconstructive surgeon. Higher stage fistulas (grades 3–5) and recurrences normally require regional flap incorporation and sometimes even permanent surgical urinary diversion.44
Favorable outcomes for surgically corrected rectourethral fistulas are mostly dependent on stage and appropriate choice in initial surgical treatment. Success rates vary from greater than 90% for low-grade fistulas to 70% for higher-grade fistulas.31,32,33,35,36,37,38,39,40,41,42,43,44
CONCLUSIONS
Iatrogenic injuries to the urinary tract are an inevitable byproduct of colon and rectal surgery in patients with complex pathologies. Early identification and repair are key in minimizing morbidity. In cases with significant postoperative complications (i.e., large urethrorectal fistulas in radiated fields), a multidisciplinary approach to diagnosis, staging, and repair should be used.
REFERENCES
- 1.St Lezin M A, Stoller M L. Surgical ureteral injuries. Urology. 1991;38(6):497–506. doi: 10.1016/0090-4295(91)80165-4. [DOI] [PubMed] [Google Scholar]
- 2.Fröber R. Surgical anatomy of the ureter. BJU Int. 2007;100(4):949–965. doi: 10.1111/j.1464-410X.2007.07207.x. [DOI] [PubMed] [Google Scholar]
- 3.Nam Y, Wexner S. Clinical value of prophylactic ureteral stent indwelling during laparoscopic colorectal surgery. J Korean Med Sci. 2002;17(5):633–635. doi: 10.3346/jkms.2002.17.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larach S W, Patankar S K, Ferrara A, Williamson P R, Perozo S E, Lord A S. Complications of laparoscopic colorectal surgery. Analysis and comparison of early vs. latter experience. Dis Colon Rectum. 1997;40(5):592–596. doi: 10.1007/BF02055385. [DOI] [PubMed] [Google Scholar]
- 5.Chahin F, Dwivedi A, Paramesh A, et al. The implications of lighted ureteral stenting in laparoscopic colectomy. J Soc Laparoendosc Surg. 2002;6(1):49–52. [PMC free article] [PubMed] [Google Scholar]
- 6.Scala A, Huang A, Dowson H M, Rockall T A. Laparoscopic colorectal surgery—results from 200 patients. Colorectal Dis. 2007;9(8):701–705. doi: 10.1111/j.1463-1318.2006.01198.x. [DOI] [PubMed] [Google Scholar]
- 7.Fry D E, Milholen L, Harbrecht P J. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118(4):454–457. doi: 10.1001/archsurg.1983.01390040064013. [DOI] [PubMed] [Google Scholar]
- 8.Goodno J A, Jr, Powers T W, Harris V D. Ureteral injury in gynecologic surgery: a ten-year review in a community hospital. Am J Obstet Gynecol. 1995;172(6):1817–1820. discussion 1820–1822. doi: 10.1016/0002-9378(95)91417-x. [DOI] [PubMed] [Google Scholar]
- 9.Assimos D G, Patterson L C, Taylor C L. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152(6 Pt 2):2240–2246. doi: 10.1016/s0022-5347(17)31650-6. [DOI] [PubMed] [Google Scholar]
- 10.Selzman A A, Spirnak J P. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155(3):878–881. doi: 10.1016/s0022-5347(01)66332-8. [DOI] [PubMed] [Google Scholar]
- 11.Anderson J K, Kabalin J N, Cadeddu J A. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editor. Campbell-Walsh Urology. 9th ed., vol. 1. Philadelphia: Elsevier; 2007. Surgical anatomy of the retroperitoneum, adrenals, kidneys, and ureters. pp. 34–37.
- 12.Delacroix S E, Jr, Winters J C. In: Whitlow CB, Beck DE, Margolin DA, Hicks TC, editor. Improved outcomes in colon and rectal surgery. Vol. 1. New York: Informa; 2009. Urologic complications of colorectal surgery.
- 13.Pokala N, Delaney C P, Kiran R P, Bast J, Angermeier K, Fazio V W. A randomized controlled trial comparing simultaneous intra-operative vs sequential prophylactic ureteric catheter insertion in re-operative and complicated colorectal surgery. Int J Colorectal Dis. 2007;22(6):683–687. doi: 10.1007/s00384-006-0219-1. [DOI] [PubMed] [Google Scholar]
- 14.Emam T, Cuschieri A. How safe is high-power ultrasonic dissection. Ann Surg. 2003;237(2):186–191. doi: 10.1097/01.SLA.0000048454.11276.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil N N, Mottrie A, Sundaram B, Patel V R. Robotic-assisted laparoscopic ureteral reimplantation with psoas hitch: a multi-institutional, multinational evaluation. Urology. 2008;72(1):47–50. discussion 50. doi: 10.1016/j.urology.2007.12.097. [DOI] [PubMed] [Google Scholar]
- 16.Lee D I, Schwab C W, Harris A. Robot-assisted ureteroureterostomy in the adult: initial clinical series. Urology. 2009;75(3):570–573. doi: 10.1016/j.urology.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Schimpf M O, Wagner J R. Robot-assisted laparoscopic distal ureteral surgery. JSLS. 2009;13(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- 18.Boxer R J, Fritzsche P, Skinner D G, et al. Replacement of the ureter by small intestine: clinical application and results of the ileal ureter in 89 patients. Trans Am Assoc Genitourin Surg. 1978;70:99–102. [PubMed] [Google Scholar]
- 19.Goldwasser B, Leibovitch I, Avigad I. Ureteral substitution using the isolated interposed vermiform appendix in a patient with a single kidney and transitional cell carcinoma of the ureter. Urology. 1994;44(3):437–440. doi: 10.1016/s0090-4295(94)80112-6. [DOI] [PubMed] [Google Scholar]
- 20.Verduyckt F J, Heesakkers J P, Debruyne F M. Long-term results of ileum interposition for ureteral obstruction. Eur Urol. 2002;42(2):181–187. doi: 10.1016/s0302-2838(02)00266-x. [DOI] [PubMed] [Google Scholar]
- 21.Riedmiller H, Becht E, Hertle L, Jacobi G, Hohenfellner R. Psoas-hitch ureteroneocystostomy: experience with 181 cases. Eur Urol. 1984;10(3):145–150. doi: 10.1159/000463777. [DOI] [PubMed] [Google Scholar]
- 22.Warwick R T, Worth P H. The psoas bladder-hitch procedure for the replacement of the lower third of the ureter. Br J Urol. 1969;41(6):701–709. doi: 10.1111/j.1464-410x.1969.tb09981.x. [DOI] [PubMed] [Google Scholar]
- 23.Bodie B, Novick A C, Rose M, Straffon R A. Long-term results with renal autotransplantation for ureteral replacement. J Urol. 1986;136(6):1187–1189. doi: 10.1016/s0022-5347(17)45278-5. [DOI] [PubMed] [Google Scholar]
- 24.Brooks J D. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editor. Campbell-Walsh Urology. 9th ed., vol. 1. Philadelphia: Elsevier; 2007. Anatomy of the lower urinary tract and male genitalia. pp. 53–60.
- 25.Goor H van. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007;9(Suppl 2):25–34. doi: 10.1111/j.1463-1318.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 26.Deck A J, Shaves S, Talner L, Porter J R. Computerized tomography cystography for the diagnosis of traumatic bladder rupture. J Urol. 2000;164(1):43–46. [PubMed] [Google Scholar]
- 27.Jarrett T W, Vaughan E D., Jr Accuracy of computerized tomography in the diagnosis of colovesical fistula secondary to diverticular disease. J Urol. 1995;153(1):44–46. doi: 10.1097/00005392-199501000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Kwon E O, Armenakas N A, Scharf S C, Panagopoulos G, Fracchia J A. The poppy seed test for colovesical fistula: big bang, little bucks! J Urol. 2008;179(4):1425–1427. doi: 10.1016/j.juro.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 29.Tanagho E A. Urethrosphincteric reconstruction for congenitally absent urethra. J Urol. 1976;116(2):237–242. doi: 10.1016/s0022-5347(17)58762-5. [DOI] [PubMed] [Google Scholar]
- 30.Webster G D, Mathes G L, Selli C. Prostatomembranous urethral injuries: a review of the literature and a rational approach to their management. J Urol. 1983;130(5):898–902. doi: 10.1016/s0022-5347(17)51561-x. [DOI] [PubMed] [Google Scholar]
- 31.Rivera R, Barboglio P G, Hellinger M, Grousse A E. Staging rectourinary fistulas to guide surgical treatment. J Urol. 2007;177(2):586–582. doi: 10.1016/j.juro.2006.09.058. [DOI] [PubMed] [Google Scholar]
- 32.Fengler S, Abcarian H. The York Mason approach to repair of iatrogenic rectourinary fistulae. J Urol. 1998;173(3):213–217. doi: 10.1016/s0002-9610(96)00015-3. [DOI] [PubMed] [Google Scholar]
- 33.Dreznik Z, Alper D, Vishne T H, Ramadan E. Rectal flap advancement—a simple and effective approach for the treatment of rectourethral fistula. Colorectal Dis. 2003;5(1):53–55. doi: 10.1046/j.1463-1318.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- 34.Spahn M, Vergho D, Riedmiller H. Iatrogenic recto-urethral fistula: perineal repair and buccal mucosa interposition. BJU Int. 2009;103(2):242–246. doi: 10.1111/j.1464-410X.2008.08002.x. [DOI] [PubMed] [Google Scholar]
- 35.Bruce R, El-Galley R, Galloway N. Use of rectus abdominis muscle flap for the treatment of complex and refractory urethrovaginal fistula. J Urol. 2000;163(4):1212–1215. [PubMed] [Google Scholar]
- 36.Zmora O, Tulchinsky H, Gur E, Goldman G, Klausner J M, Rabau M. Gracilis muscle transposition for fistulas between the rectum and urethra or vagina. Dis Colon Rectum. 2006;49(9):1316–1321. doi: 10.1007/s10350-006-0585-3. [DOI] [PubMed] [Google Scholar]
- 37.Youssef A, Fath-alla M, El-Kassaby A. Perineal subcutaneous dartos pedicled flap as a new technique for repairing urethrorectal fistula. J Urol. 1999;161:1498–1500. [PubMed] [Google Scholar]
- 38.Visser B, McAninch J, Welton M. Rectourethral fistulae: the perineal approach. J Am Coll Surg. 2002;195(1):138–143. doi: 10.1016/s1072-7515(02)01207-3. [DOI] [PubMed] [Google Scholar]
- 39.Culkin D, Ramsey C. Urethrorectal fistula: transanal, transsphincteric approach with locally based pedicle interposition flaps. J Urol. 2003;169(6):2181–2183. doi: 10.1097/01.ju.0000057966.75714.01. [DOI] [PubMed] [Google Scholar]
- 40.Mason A Y. Surgical access to the rectum—a transsphincteric exposure. Proc R Soc Med. 1970;63(Suppl):91–94. [PMC free article] [PubMed] [Google Scholar]
- 41.Crippa A, Dall'oglio M F, Nesrallah L J, Hasegawa E, Antunes A A, Srougi M. The York-Mason technique for recto-urethral fistulas. Clinics (Sao Paulo) 2007;62(6):699–704. doi: 10.1590/s1807-59322007000600007. [DOI] [PubMed] [Google Scholar]
- 42.Cutait D E, Cutait R, Ioshimoto M, Hyppólito da Silva J, Manzione A. Abdominoperineal endoanal pull-through resection. A comparative study between immediate and delayed colorectal anastomosis. Dis Colon Rectum. 1985;28(5):294–299. doi: 10.1007/BF02560425. [DOI] [PubMed] [Google Scholar]
- 43.Remzi F H, El Gazzaz G, Kiran R P, Kirat H T, Fazio V W. Outcomes following Turnbull-Cutait abdominoperineal pull-through compared with coloanal anastomosis. Br J Surg. 2009;96(4):424–429. doi: 10.1002/bjs.6458. [DOI] [PubMed] [Google Scholar]
- 44.Elliott S, McAninch J, Chi T, Doyle S M, Master V A. Management of severe urethral complications of prostate cancer therapy. J Urol. 2006;176(6 Pt 1):2508–2513. doi: 10.1016/j.juro.2006.07.152. [DOI] [PubMed] [Google Scholar]