ABSTRACT
Bladder dysfunction following colorectal surgery may be related to extirpative procedures in the region of the pelvic autonomic plexus. The most common etiology is from autonomic disruption during abdominoperineal or low anterior resections. Contemporary technical modifications have allowed surgeons to achieve oncologic control while preserving the autonomic nerves that innervate the bladder and sexual organs. Although these modifications have resulted in a significant decrease in the incidence of postoperative bladder dysfunction, bladder dysfunction continues to be a source of significant morbidity after surgery. In this patient population, symptoms are not reliable for accurate diagnosis. The use of urodynamics provides objective measurements of bladder and outlet function and are paramount in providing an accurate diagnosis and in recommending treatments. Follow-up and treatment are highly individualized based on urodynamic findings, patient expectations, patient abilities, and family support. This article provides an overview of pertinent neuroanatomy, diagnosis, urodynamic interpretation, and treatment related to bladder dysfunction following pelvic colorectal surgery.
Keywords: Voiding dysfunction, colorectal surgery, urinary retention
Bladder dysfunction following colorectal surgery is most commonly related to extirpative procedures in the region of the autonomic pelvic plexus. The two procedures with the highest incidence of postoperative bladder dysfunction include abdominoperineal resections (approaching 50%) and low anterior resections (15–25%).1 Prompted by promising oncologic outcomes,2 surgical trends for rectal carcinoma have demonstrated a dramatic increase in low anterior resections (LAR) with a corresponding decrease in abdominoperineal resections (APR).3 Over the past 20 years, technical modifications have allowed surgeons to achieve oncologic control while preserving the autonomic nerves that innervate the bladder and genitalia.4 Performing a total mesorectal excision (TME) with autonomic nerve preservation (ANP) enables both local tumor control and preservation of autonomic nerves involved in urinary and sexual function.5,6,7,8,9,10,11,12,13,14 Oncologic and functional outcomes are superior when compared with previous nonanatomic dissections.15,16 Locally advanced tumors and neoadjuvant chemotherapy and radiation can make identification of the autonomic nerves and plexus more difficult and occasionally impossible.8 A study by Junginger8 analyzed the effects of TME with or without ANP. Identification of the pelvic autonomic nerves was complete in 72%, partial identification in 10.7%, and not at all in 17.3% of patients. Univariate analysis showed that the case number (experience), gender (males more than females), and T stage (T1–2 vs T3–4) exerted an independent influence on the achievement of complete pelvic nerve identification. Identification and preservation of the pelvic autonomic nerves (either bilateral or unilateral) was associated with low bladder dysfunction rates (4.5 vs 38.5%; p < 0.001). In this series of 150 patients with adenocarcinoma of the rectum, identification and preservation of the autonomic nerves was achieved in a majority of patients and led to the prevention of urinary dysfunction (4.5 vs 38.5%; p < 0.001). This article reviews the pathophysiology of voiding dysfunction after pelvic colorectal surgery.
NEUROANATOMY
The autonomic and somatic nervous systems mediate the function of the lower urinary tract (storage and voiding of urine). The detrusor is densely innervated by four groups of nerves: parasympathetic, sympathetic, somatic, and sensorimotor nerves.17
Contraction of the detrusor is performed primarily through the activation of the parasympathetic nerves and the release of acetylcholine at the neuromuscular junction (Table 1). These parasympathetic fibers originate from the spinal cord at the S2–S4 level and travel within the pelvic nerve to the pelvic plexus. The main trunks to the bladder and proximal urethra course in the visceral pelvic fascia, also called the posterior endopelvic fascia.8,18 Multiple pelvic preganglionic nerves pass laterally from the pelvic floor over the rectal fascia investments en route medially to the bladder.8 These preganglionic autonomic fibers course alongside the superior vesical vasculature to synapse with postganglionic autonomic fibers within the bladder wall.
Table 1.
Innervation to the Lower Urinary Tract
| Neurotransmitter | Location | Function | |
|---|---|---|---|
| NANC, nonadrenergic noncholinergic neurotransmitter. | |||
| Parasympathetic | Acetylcholine | Pelvic plexus (Pelvic nerve, S2–S4) | Bladder contraction |
| Sympathetic | Norepinephrine | Pelvic plexus (Hypogastric nerve & caudal sympathetic chain, T10–L2) | Closure of bladder neck and proximal urethra |
| Somatic | Acetylcholine | Pudendal nerve (S2–S4) | Closure of external sphincter |
| Sensory | NANC | Pelvic plexus | Sensation to urinary tract |
The sympathetic component of the autonomic nervous system helps to cause relaxation of the bladder body (increasing compliance for storage) and contraction of the trigone and bladder neck during storage. Sympathetic innervation to the bladder originates at the level of T10–L2 and arrives at the pelvic autonomic plexus via branches from the hypogastric nerve or through direct branches from the caudal sympathetic chain. Although controversial, sympathetic nerves may also contribute to smooth muscle contraction of the urinary sphincter.
Somatic motor innervation to the striated pelvic floor musculature and sphincter arises from the S2–S4 level and travels via the pudendal nerve through Alcock's canal. The perineal branches of the pudendal nerve follow the perineal artery into the superficial pouch to supply the ischiocavernosus, bulbospongiosus, and transverse perinei muscles. Some branches continue anteriorly to supply sensation to the posterior scrotum and perineum. Additional perineal branches pass deep to the perineal membrane to supply the levator ani and striated urethral spincter.18
NORMAL VOIDING
The function of the normal bladder can be simplistically divided into two phases of micturition: storage (filling) and emptying (voiding) phases (Table 2). When diagnosing and treating patients with voiding dysfunction, categorization of problems within each phase of bladder function allows systematic and logical review of even the most complex patients.
Table 2.
Activity of Motor Neurons to the Lower Urinary Tract
| Storage Phase | Emptying Phase | |
|---|---|---|
| –, silent (not active);+, active (releasing neurotransmitter). Storage Phase = filling phase; Emptying Phase = voiding phase. | ||
| Parasympathetic | – | + |
| Sympathetic | + | – |
| Somatic | + | – |
During the normal urinary storage phase, the bladder fills with urine without a perceivable rise in pressure. This property of the normal bladder is termed bladder compliance (Δ volume/Δ pressure). The normal bladder is highly compliant due to the intrinsic viscoelastic properties of the extracellular matrix of the bladder.19 The parasympathetic innervation to the bladder is silent during bladder storage while the sympathetic innervation is active. The lack of neurotransmitter release from the parasympathetic nerves facilitates low pressure bladder storage without inappropriate (unstable) contractions. On the other hand, the sympathetic nervous system is active during the storage phase, resulting in a closed bladder neck, increased tone of the proximal urethra, and aids in augmenting bladder relaxation. The somatic nervous system (innervating the striated sphincter) is also active during the storage phase and provides tonic contraction to the majority slow twitch fibers of the rhabdospincter.18 Sensory afferent fibers provide sensation to the bladder as the bladder fills.
During the normal emptying phase, the somatic nerve impulses cease and allow relaxation of the striated sphincter. Next, the sympathetic nerve impulse cease, which allows the bladder neck and proximal urethra to open resulting in decreased urethral resistance. Finally, the release of acetylcholine from parasympathetic nerve endings cause sustained contraction of the detrusor muscle and normal expulsion of urine. The sphincter and bladder neck should stay relaxed until parasympathetic contraction of the bladder has ceased.
BLADDER DYSFUNCTION
The most common sequela from autonomic nerve damage during surgery of the colon and rectum is detrusor denervation (parasympathetic), resulting in impaired contractility of the bladder. This usually results in contractions that are not sufficient to evacuate the bladder adequately (failure to empty). In this scenario, clean intermittent catheterization is the desired management if the dexterity and condition of the patient will allow. This results in continued “cycling” of the bladder, which has several desired effects. Foley catheter placement or suprapubic tube placement are other options of continuous bladder drainage that may be employed. However, it is recommended to institute intermittent catheterization if at all possible. A majority of these patients will regain the ability to empty the bladder, but may require up to 6 months to recover. Although the initial presentation is likely as a failure to empty, the most serious complications are abnormalities in urinary storage, which may lead to small capacity, poorly compliant, high-pressure bladders. In some patients with denervation injury, return of normal bladder function may not occur, and patients may persist with decentralized, noncompliant bladders with high storage pressures. Left unmanaged, these storage alterations can lead to deleterious changes in upper urinary tract function. This may predispose to hydronephrosis, urinary reflux, pyelonephritis, and declining renal function. Thus, all patients with abnormal bladder function should undergo a urodynamics assessment to rule out the development of a noncompliant, high-pressure storage bladder. Maintenance of a low-pressure storage reservoir (bladder) is necessary to prevent serious complications. Thus, in patients with documented bladder dysfunction after pelvic surgery, a urodynamics assessment is recommended at 3 to 6 months depending on symptomatology. This may be done sooner if symptoms of infection, incontinence, or retention persist.
As discussed above, bladder dysfunction is categorized based on the two normal phases of voiding into failure to store and failures to empty.19 Failures of storage or emptying can be the result of pathology within the bladder, the outlet (urethra/prostate/sphincter), or a combination of both. Thus, it is important for the clinician to systematically review voiding dysfunction in this manner and understand the function (or dysfunction) of the bladder and urethra during urinary storage and emptying. Urodynamic studies in the form of multichannel pressure-flow studies are the most effective way to obtain this information.
DIAGNOSIS
Successful voiding after catheter removal is not synonymous with normal bladder function.1 A sustained increase in residual urine volumes can be found in patients undergoing low anterior or abdominoperineal resections. Although the clinical significance of elevated residual urine (>200 mL) is controversial, these patients should be followed closely for the development of significant bladder dysfunction or urinary retention. Patients with urinary retention after colorectal surgery are encouraged to perform clean intermittent catheterization (CIC). This insures continued “cycling” of the bladder (intermittent distention and evacuation), which maintains the unique compliance of the bladder and minimizes the chance of bladder contraction around a chronic indwelling catheter. Therefore, initially after the acute surgical event it is recommended to instruct the patient on CIC technique. CIC is performed with a 12 to 14 French low friction catheter every 4 to 6 hours. If the patient remains unable to void, they are evaluated using pressure-flow urodynamic studies at approximately 2 to 3 months postoperatively. The use of urodynamics testing allows more precise determination of lower urinary tract dysfunction, and the CIC protocol can be adjusted based on the storage pressures and bladder capacity at the time of urodynamic evaluation. It may take up to 6 months for bladder function to return. For patients with hypocontractile bladders, there are no drugs with acceptable pharmacokinetics and side-effect profiles that have been shown to clinically increase contractility in the bladder. Many drugs developed for urinary retention are targeted to the outlet (prostate) as the source for elevated residual urine volumes and the inability to void. Although up to 40% of male patients undergoing pelvic colorectal procedure may have concomitant outlet abnormalities (BPH), the most common source of dysfunction is pelvic denervation to the bladder (not the outlet).
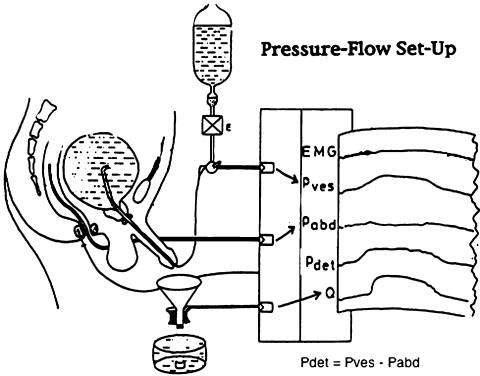
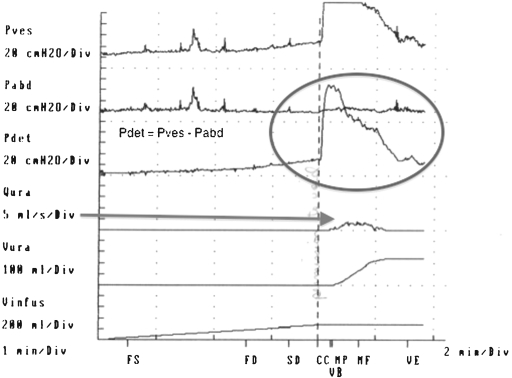
In complex patients, delineation of specific abnormalities cannot be reliably made based on symptoms alone. Some patients undergoing pelvic surgery may have a history of lower urinary tract pathology (BPH, stress incontinence), which can make diagnosis difficult. Integration of medical and surgical history, symptoms, residual urine volumes, and findings from urodynamic evaluations are used to diagnose and aid in selecting appropriate treatments. Urodynamic studies are controlled pressure-flow studies that evaluate bladder function in the storage and emptying phases (Fig. 1). A pressure-sensitive catheter is placed in the rectum (abdominal pressure) and the bladder (vesical pressure). The functional pressure (detrusor pressure) is determined by subtraction of the abdominal pressure from the vesical pressure. These studies provide the only objective functional studies of the lower urinary tract and are a valuable adjunct in patients with lower urinary tract dysfunction.17
Figure 1.
Multichannel urodynamics. Pressure sensitive catheters placed in the rectum and bladder. Pdet=Pves-Pabd Flow rate is determined by flowmeter collection of urine passed by the urethra into a collecting device.
URODYNAMIC FINDINGS
Failure to Store
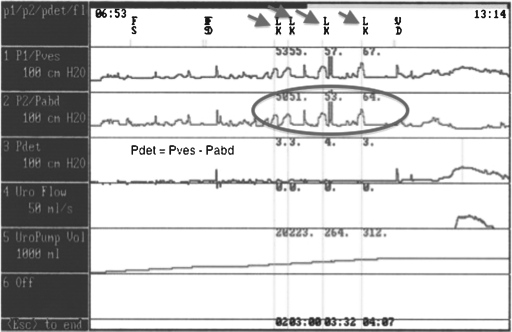
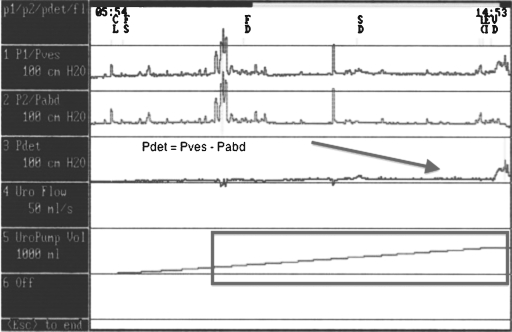
Storage failures result from either bladder or outlet abnormalities (or a combination). Bladder abnormalities can include involuntary bladder contractions (intermittent phasic contractions), low bladder compliance (tonic involuntary bladder contractions), or sensory abnormalities. These conditions usually result in involuntary contractions of the bladder, which may cause overactive bladder incontinence. On urodynamics, detrusor overactivity with involuntary rises in bladder pressure can be seen—usually associated with leakage (Fig. 2). Outlet abnormalities resulting in a failure to store usually include a decrease in outlet resistance. Denervation procedures may result in insufficient urethral closure. This reduction in bladder neck and urethral resistance may result in the occurrence of leakage during periods of increased abdominal pressure (cough, sneeze, bed transfer, bending over). This condition is known as stress urinary incontinence, where the resistance of the sphincteric complex is insufficient to withstand increases in intraabdominal pressure. On urodynamics studies, one would see an increase in leakage of urine with increased intraabdominal pressure in the absence of a detrusor contraction (Fig. 3). This condition is known as intrinsic sphincteric deficiency. Damage to the pudendal nerve or its branches from Alcock's canal can result in weakening of the striated urinary sphincter with resultant stress urinary incontinence (failure to store).
Figure 2.
Multichannel urodynamics–detrusor overactivity. Circle: Shows involuntary bladder contraction. Arrow: Urinary leakage.
Figure 3.
Multichannel urodynamics—stress urinary incontinence/intrinsic sphincter deficiency. Arrows: Urinary leakage occurrences. Circle: Increased abdominal pressure (without increased Pdet) corresponding to urinary leakage with stress urinary incontinence.
Failure to Empty
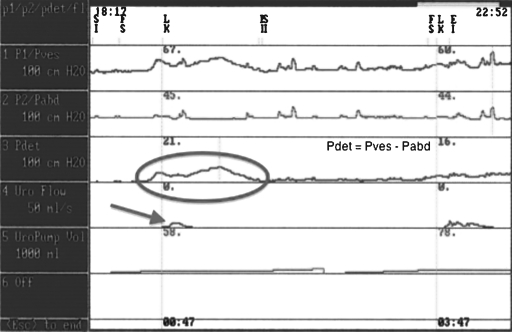
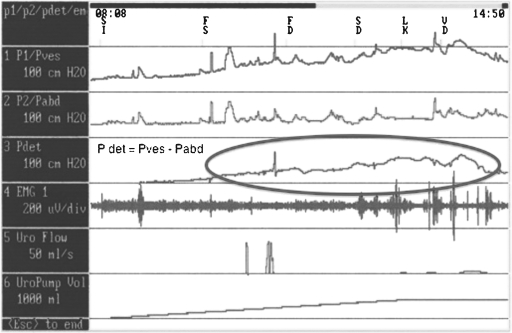
Emptying (voiding) failures can result from a bladder, outlet, or combination abnormality. Bladder abnormalities include inadequate bladder contractions for emptying. This occurs usually as a result of impaired contractility, which results in a bladder with insufficient contractile force to adequately empty the bladder. This is the most common abnormality following radical pelvic surgery. On urodynamic assessment, one would see low pressure, weak, intermittent contractions of the detrusor muscle with incomplete bladder emptying (Fig. 4). Other outlet abnormalities include obstruction (i.e., BPH, stricture) or dyssynergia of the external sphincter or bladder neck. In obstructed patients, the urodynamic findings are usually high-pressure contractions of the bladder associated with a very low velocity of urine flow (Fig. 5). In patients with detrusor external dyssynergia, the urodynamics studies usually reveal involuntary detrusor contractions with associated spasticity of the pelvic floor external sphincter complex. This is only seen in conditions involving neurogenic disorders—radical pelvic surgery may rarely create this condition.
Figure 4.
Multichannel urodynamics—hypocontractile bladder. Urodynamic findings after parasympathetic denervation following pelvic surgery. Arrow showing low pressure insufficient bladder contractions despite bladder volume approaching one liter (rectangle).
Figure 5.
Multichannel urodynamics—outlet obstruction. Urodynamic findings showing high pressure detrusor contraction (circle) with only low urinary flow (arrow 5 mL/s) resulting from outlet obstruction (prostate, bladder neck, or urethra).
TREATMENT
Comprehensive discussion regarding the multifaceted treatment of these various conditions is beyond the scope of this article. It is most important to note that treatment of postoperative bladder and urethral dysfunction is based on individualized management and can change over time. Injuries that are confirmed with urodynamics as having hypocontractile bladders are treated with long-term CIC. Concomitant outlet obstruction may be identified, but in the absence of bladder contractility usually does not warrant treatment, as there will be no emptying in the presence of a poorly contractile bladder. Other options in patients unable or unwilling to perform CIC via the urethra include catheterizable stoma formation, noncontinent ileovesicostomy, or suprapubic tube urinary diversion. Long-term follow-up of these patients will be necessary. An emerging technology with some promise is neuromodulation. In patients with nonobstructive retention, neuromodulation has demonstrated improved bladder function and significant reductions in the rate of retention.20 This is currently approved in patients with nonneurogenic bladder dysfunction, but studies are ongoing in select patients with neurogenic bladder (i.e., denervation injuries).
Patients with compliance abnormalities (failure to store) can be treated with a combination of medications and surgery. In the postoperative setting, these patients may present after extensive, partial bladder resection from their extirpative procedure or as a result of neurogenic alteration (Fig. 6). The use of anticholinergic medications can decrease the pressures within the bladder during storage as well as marginally increase bladder volumes. Patients with high-pressure bladders (low compliance) that are refractory to anticholinergic medications have a variety of options to treat the overactive bladder dysfunction. First, maximizing the doses of anticholinergic drugs or combined drug therapy should be considered. Usually high doses of these medications can achieve a degree of symptom reduction, but the adverse effects of these drugs must be monitored—as they increase with dose escalation.21 If these medical strategies fail, the use of neuromodulation for refractory overactive bladder may be considered. In patients with refractory nonneurogenic bladder overactivity, this technology has been demonstrated to achieve significant improvement in symptoms. As in patients with retention, this technology is U.S. Food and Drug Administration (FDA) approved for nonneurogenic patients only, and is undergoing further study in select neurogenic patients. Also, botulinum toxin has been injected into the detrusor muscle in patients with refractory detrusor overactivity with acceptable preliminary results.22,23 This therapy is not yet approved in the United States. For patients refractory to all treatment options, enterocystoplasty (bladder augmentation with bowel) is an excellent option with very high cure rates (see “Bladder Reconstruction and Diversion during Colorectal Surgery” in this issue of the Clinics). This operation may be done using multiple bowel segments, and may also incorporate an abdominal, continent catheterizable stoma for ease of postoperative catheterization.
Figure 6.
Multichannel urodynamics—noncompliant bladder. High pressure bladder during storage phase of micturition. Medical or surgical treatments can be applied. If left untreated, this patient could develop upper tract (renal) injury.
Patients may also have insufficient urethral resistance, and there are several options to treat men and women with intrinsic sphincteric deficiency. In women, the hallmark of treatment is the pubovaginal sling procedure.24 In women with severe ISD due to neurogenic dysfunction, an autologous fascia bladder neck sling is a very effective operation. In cases of concomitant abnormalities in bladder storage, this may be combined with procedures mentioned above. In women with lesser degrees of ISD with predominant anatomic abnormalities, a synthetic midurethral sling may be chosen. This should be done with some degree of caution, as excessive tension on these slings absolutely must be avoided to prevent mesh erosion. In men with ISD, a male version of the sling also is an option to increase sphincteric resistance.25 It should be mentioned that these procedures are best for mild to moderate degrees of sphincteric weakness. For more severe degrees of ISD in men, the artificial urinary sphincter remains the “gold standard” treatment.26,27 This procedure involves placement of a self-filling urethral “cuff” around the urethra to close the bulbous urethra during periods of intended urinary storage. This is a prosthetic device placed through a perineal or penoscrotal approach, and requires a scrotal pump to open the cuff and allow voiding. Erosions of the cuff into the urethra may occur, and the use of indwelling Foley catheters in patients with artificial urinary sphincters is to be avoided. Lastly, for both men and women with severe urethral incompetence, bladder neck closure procedures may be done through perineal or suprapubic approaches.28 Of course, alternative methods of bladder drainage will be needed for these complex patients.29
CONCLUSION
Bladder dysfunction after pelvic colorectal surgery is common. In this patient population, symptoms are not reliable for diagnosing and recommending treatments. The most common etiology is from autonomic disruption during APR or LAR with a resultant hypocontractile bladder. A majority of these cases will resolve within the first 6 months with no long-term sequelae, but these patients must be monitored for the development of a noncompliant hostile bladder. The use of urodynamics in these complex patients allows for objective measurements of bladder and outlet functions and can identify those patients at risk for upper tract damage. Treatment and follow-up are highly individualized based on urodynamic findings, patient expectations, patient abilities, and family support.
REFERENCES
- 1.Chaudhri S, Maruthachalam K, Kaiser A, Robson W, Pickard R S, Horgan A F. Successful voiding after trial without catheter is not synonymous with recovery of bladder function after colorectal surgery. Dis Colon Rectum. 2006;49(7):1066–1070. doi: 10.1007/s10350-006-0540-3. [DOI] [PubMed] [Google Scholar]
- 2.Wolmark N, Fisher B, National Surgical Adjuvant Breast and Bowel Project An analysis of survival and treatment failure following abdominoperineal and sphincter-saving resection in Dukes' B and C rectal carcinoma. A report of the NSABP clinical trials. Ann Surg. 1986;204(4):480–489. doi: 10.1097/00000658-198610000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingo P A, Guest J L, McGinnis L, et al. Patterns of inpatient surgeries for the top four cancers in the United States, National Hospital Discharge Survey, 1988-95. Cancer Causes Control. 2000;11(6):497–512. doi: 10.1023/a:1008944209648. [DOI] [PubMed] [Google Scholar]
- 4.Chessin D B, Guillem J G. Abdominoperineal resection for rectal cancer: historic perspective and current issues. Surg Oncol Clin N Am. 2005;14(3):569–586, vii. doi: 10.1016/j.soc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Enker W E, Havenga K, Polyak T, Thaler H, Cranor M. Abdominoperineal resection via total mesorectal excision and autonomic nerve preservation for low rectal cancer. World J Surg. 1997;21(7):715–720. doi: 10.1007/s002689900296. [DOI] [PubMed] [Google Scholar]
- 6.Hendren S, O'Connor B, Liu M, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242(2):212–223. doi: 10.1097/01.sla.0000171299.43954.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollabaugh R S, Jr, Steiner M S, Sellers K D, Samm B J, Dmochowski R R. Neuroanatomy of the pelvis: implications for colonic and rectal resection. Dis Colon Rectum. 2000;43(10):1390–1397. doi: 10.1007/BF02236635. [DOI] [PubMed] [Google Scholar]
- 8.Junginger T, Kneist W, Heintz A. Influence of identification and preservation of pelvic autonomic nerves in rectal cancer surgery on bladder dysfunction after total mesorectal excision. Dis Colon Rectum. 2003;46(5):621–628. doi: 10.1007/s10350-004-6621-2. [DOI] [PubMed] [Google Scholar]
- 9.Kim N K, Aahn T W, Park J K, et al. Assessment of sexual and voiding function after total mesorectal excision with pelvic autonomic nerve preservation in males with rectal cancer. Dis Colon Rectum. 2002;45(9):1178–1185. doi: 10.1007/s10350-004-6388-5. [DOI] [PubMed] [Google Scholar]
- 10.Moriya Y. Function preservation in rectal cancer surgery. Int J Clin Oncol. 2006;11(5):339–343. doi: 10.1007/s10147-006-0608-z. [DOI] [PubMed] [Google Scholar]
- 11.Moriya Y, Sugihara K, Akasu T, Fujita S. Importance of extended lymphadenectomy with lateral node dissection for advanced lower rectal cancer. World J Surg. 1997;21(7):728–732. doi: 10.1007/s002689900298. [DOI] [PubMed] [Google Scholar]
- 12.Saito N, Koda K, Nobuhiro K, et al. Nerve-sparing surgery for advanced rectal cancer patients: special reference to Duke's C patients. World J Surg. 1999;23(10):1062–1068. doi: 10.1007/s002689900624. [DOI] [PubMed] [Google Scholar]
- 13.Shirouzu K, Ogata Y, Araki Y. Oncologic and functional results of total mesorectal excision and autonomic nerve-preserving operation for advanced lower rectal cancer. Dis Colon Rectum. 2004;47(9):1442–1447. doi: 10.1007/s10350-004-0618-8. [DOI] [PubMed] [Google Scholar]
- 14.Yamakoshi H, Ike H, Oki S, Hara M, Shimada H. Metastasis of rectal cancer to lymph nodes and tissues around the autonomic nerves spared for urinary and sexual function. Dis Colon Rectum. 1997;40(9):1079–1084. doi: 10.1007/BF02050933. [DOI] [PubMed] [Google Scholar]
- 15.Heald R J, Husband E M, Ryall R D. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg. 1982;69(10):613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 16.MacFarlane J K, Ryall R D, Heald R J. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 17.Chapple C R, MacDiarmid S A. Urodynamics. London: Churchill Livingstone; 2002.
- 18.Yoshimura N, Chancellor M B. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editor. Campbell-Walsh Urology. 9th ed., vol. 3. Philadelphia: Elsevier; 2007. Physiology and pharmacology of the bladder and urethra. pp. 1922–1972.
- 19.Wein A. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editor. Campbell-Walsh Urology. 9th ed., vol. 3. Philadelphia: Elsevier; 2007. Pathophysiology and classification of voiding dysfunction. pp. 1973–1985.
- 20.Kerrebroeck P E van, Voskuilen A C van, Heesakkers J P, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178(5):2029–2034. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 21.MacDiarmid S A. Overactive bladder: improving the efficacy of anticholinergics by dose escalation. Curr Urol Rep. 2003;4(6):446–451. doi: 10.1007/s11934-003-0025-z. [DOI] [PubMed] [Google Scholar]
- 22.Apostolidis A, Dasgupta P, Denys P, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119. doi: 10.1016/j.eururo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Drake M J. Mechanisms of action of intravesical botulinum treatment in refractory detrusor overactivity. BJU Int. 2008;102(Suppl 1):11–16. doi: 10.1111/j.1464-410X.2008.07822.x. [DOI] [PubMed] [Google Scholar]
- 24.Chaikin D C, Rosenthal J, Blaivas J G. Pubovaginal fascial sling for all types of stress urinary incontinence: long-term analysis. J Urol. 1998;160(4):1312–1316. [PubMed] [Google Scholar]
- 25.Comiter C V. The male perineal sling: intermediate-term results. Neurourol Urodyn. 2005;24(7):648–653. doi: 10.1002/nau.20166. [DOI] [PubMed] [Google Scholar]
- 26.Comiter C V. Surgery Insight: surgical management of postprostatectomy incontinence—the artificial urinary sphincter and male sling. Nat Clin Pract Urol. 2007;4(11):615–624. doi: 10.1038/ncpuro0935. [DOI] [PubMed] [Google Scholar]
- 27.Webster G D, Perez L M, Khoury J M, Timmons S L. Management of type III stress urinary incontinence using artificial urinary sphincter. Urology. 1992;39(6):499–503. doi: 10.1016/0090-4295(92)90001-d. [DOI] [PubMed] [Google Scholar]
- 28.Bergman J, Lerman S E, Kristo B, Chen A, Boechat M I, Churchill B M. Outcomes of bladder neck closure for intractable urinary incontinence in patients with neurogenic bladders. J Pediatr Urol. 2006;2(6):528–533. doi: 10.1016/j.jpurol.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Gauthier A R, Jr, Winters J C. Incontinent ileovesicostomy in the management of neurogenic bladder dysfunction. Neurourol Urodyn. 2003;22(2):142–146. doi: 10.1002/nau.10093. [DOI] [PubMed] [Google Scholar]