SUMMARY
The feasibility of hepatic homotransplantation has been clearly established in principle inasmuch as several animals are still alive almost 3 years after complete hepatectomy and liver replacement. Both orthotopic and auxiliary operations are complicated surgical techniques. Nevertheless, the results in dogs are comparable to those which can be obtained with homotransplantation of the kidney.
In man the problem is more difficult. In patients who have a need for such operations, there is invariably a metabolic disorder more complex than that caused by renal failure. In addition, the new organ must function efficiently from the beginning since its complete functional failure leads to death within a few hours. There is no recourse to an artificial liver to maintain life until the reversal of an injury which is caused by either ischemia or rejection.
Nevertheless, research of several kinds may soon make possible the successful use of hepatic transplantation procedures for the definitive treatment of human liver disease as exemplified by the reports in this symposium concerning new techniques of organ preservation, histocompatibility analysis, and immunosuppression.
INTRODUCTION
In this discussion, we will allude to some of the significant advances which have been made in the field of liver transplantation. The history of these efforts is of relatively recent origin dating from the first descriptions of whole organ auxiliary liver transplantation in the dog by Welch in 1955 (14) and of orthotopic canine homotransplantation by Moore in 1959 (9). Nevertheless, the problems associated with both kinds of operation have been studied in such detail, as completely reviewed elsewhere (12), that the experimental background is comparable to that which is available for renal transplantation.
ORTHOTOPIC TRANSPLANTATION
Technical considerations in the dog
The operation includes complete extirpation of the recipient’s liver and its replacement with a homograft obtained from a nonrelated mongrel dog. In the cooled donor animal, the first step is dissection of all the structures entering and leaving the liver. A segment of aorta may be removed in continuity with the hepatic artery (Fig. 1) for ultimate anastomosis to the recipient aorta. Alternatively, the homograft hepatic artery may be prepared for attachment to the hepatic artery or to the right renal artery (after nephrectomy) of the recipient. With the development of better immunosuppressive regimens, the small-vessel arterial anastomoses have become the preferable techniques of rearterialization for reasons which will be described subsequently.
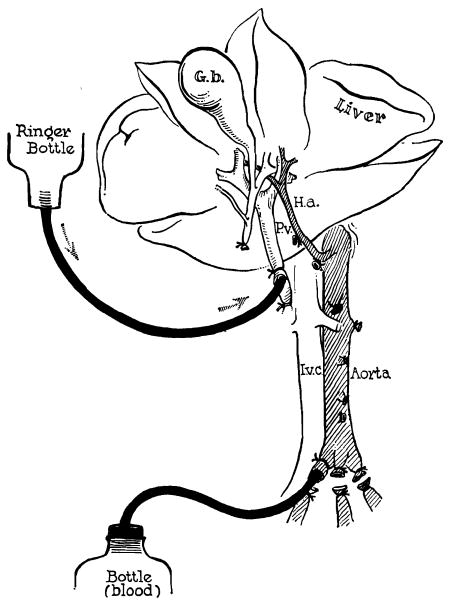
Figure 1.
Method of further cooling a liver homograft just before its removal. Donor animals are operated on with total body hypothermia of 29–31°C. Cold lactated Ringer’s solution is infused through the portal vein at the same time the donor animal is exsanguinated. (By permission of Surgery, Gynecology and Obstetrics, 1960, 111: 733.)
Before being sacrificed, the donor animal is exsanguinated. During this time the liver is cooled to 10–15°C by perfusion through the portal vein with chilled lactated Ringer’s solution (Fig. 1). It is then transplanted to the empty hepatic fossa from which the recipient liver has been removed. All of its major vascular channels are reconstructed and biliary drainage is provided with a cholecystoenterostomy (Fig. 2).
Figure 2.
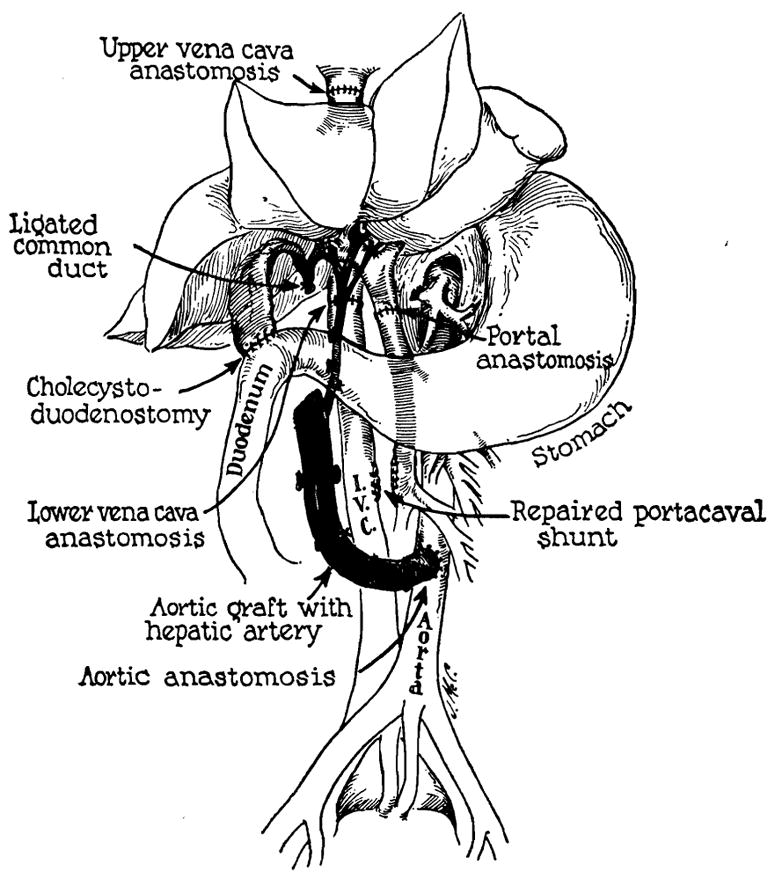
Reconstruction after orthotopic liver homotransplantation. Internal biliary drainage is with a cholecystoduodenostomy. In this preparation the aorta has been transplanted in continuity with the hepatic artery of the homograft. Alternatively, the homograft hepatic artery may be connected to the recipient hepatic or right renal artery. (By permission of Surgery, 1965, 58: 131.)
A crucial requirement in the dog is the prevention of venous hypertension during the part of the operation when both the inferior vena cava and the portal vein are occluded. These two venous beds can be individually decompressed with external bypasses as described by Moore. We prefer to first place the portal and vena caval systems in communication by means of a temporary portacaval shunt. During occlusion, it is then possible to protect both beds with a single external bypass (Fig. 3). As soon as the venous channels to the liver have been reconstructed, the temporary portacaval shunt is sectioned and the resulting defects are closed with lateral suture. Recently, Fonkalsrud (3) has described an ingenious alternative technique, designed to avoid the need for external bypasses, but the mortality from the procedure did not seem to have improved in comparison to that of the older methods.
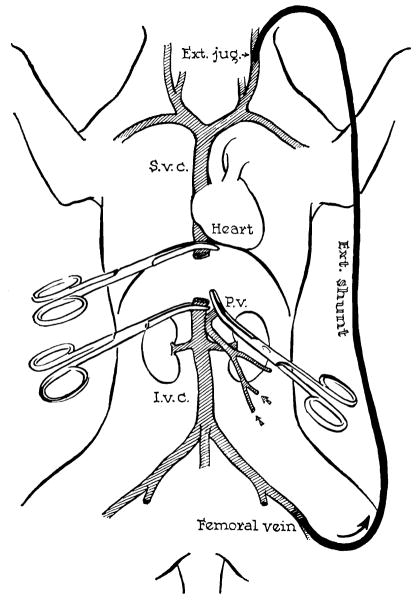
Figure 3.
Method for decompression of inferior vena caval and splanchnic systems during removal of recipient liver and replacement with a homograft. Note that a preliminary portacaval shunt has been placed. By means of this temporary anastomosis, the two venous systems are connected, allowing their decompression with a single external bypass. (By permission of Surgery, Gynecology and Obstetrics, 1960, 111: 733.)
Rejection in the nontreated animal
The function of the graft is usually satisfactory for several days. The animals may eat and appear quite normal. After 3 or 4 days, however, there is an inexorable rejection with elevations of bilirubin, alkaline phosphatase, and the serum transaminases. In our laboratories, survival in nontreated animals has been observed for as long as 21 to 31 days, but in the vast majority of cases the dogs can be expected to die within 10 days; the mean survival in 32 control experiments was 7.0 ± 3 (SD) days.
The histologic characteristics of rejection are similar to those with homotransplantation of other organs. The invasion of the homograft by mononuclear cells is massive. Many of these cells with pyroninophilic cytoplasm concentrate in the portal tracts and around the centrilobular veins. With electron microscopy, there is some evidence that they adhere to and presumably damage the sinusoidal endothelium.
It is possible that these findings are comparable to those described in the peritubular capillaries by Kountz and Dempster (5) and by Porter (11) during the rejection of renal homografts. Studies in our laboratories by Dr. Carl Groth and his associates have shown that there is a sharp reduction in both portal and hepatic arterial flow at this time. Examination of the host lymphoid tissues at comparable postoperative intervals reveal proliferation of the same pyroninophilic cells in the lymph nodes, spleen, and thymus.
Treatment with azathioprine
From 1962 to 1964 a number of efforts were made in our laboratories to prolong survival with the use of azathioprine. The administration of this drug poses a specific problem in canine liver homotransplantation since azathioprine is highly hepatotoxic in the dog. In spite of this handicap a substantial number of long term survivors were obtained after orthotopic transplantation.
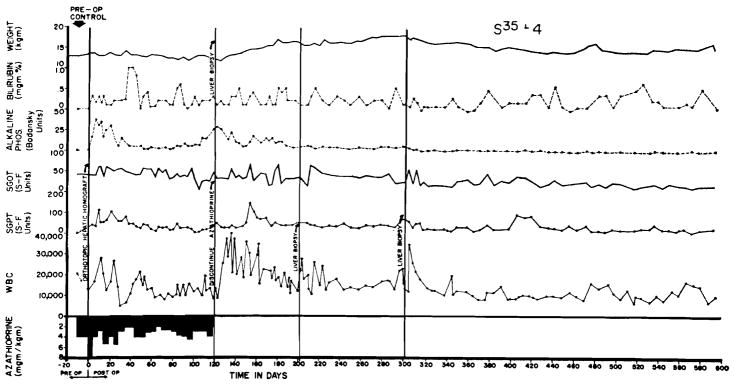
The evolution of the postoperative course was extremely variable. In more than 100 experiments, a course similar to that seen in Figure 4 was observed in 1/5 of the cases. This dog received a graft in March, 1964, 2 years and 10 months ago. Although there was good function from the beginning the animal was sick as long as immunosuppressive therapy was given, and did not begin to gain weight until discontinuance of azathioprine after 4 months. During the ensuing 21/2 years he has had excellent health. Homograft biopsies after 4, 8, and 12 months were interpreted as normal both with light and electron microscopy. It has become evident that results such as these were probably the consequence of a fortuitously good histocompatibility match between donors and recipients.
Figure 4.
Course of an animal which never had any clinically evident homograft rejection. Note rapid weight gain following cessation of therapy at 4 months. The pronounced leukocytosis after withdrawal of immunosuppression was commonly seen. This animal is still alive after 2 years and 10 months, having received no immunosuppressive therapy for 21/2 years of this time.
At the opposite end of the spectrum were approximately 1/3 of the experiments in which azathioprine did not prevent crushing rejection. The appearance of jaundice was delayed for several days or weeks, but all the recipient animals died of hepatic failure within 7 to 40 days after operation. Apparently, the chance histocompatibility match in these cases was poor.
Finally, approximately half the animals had a course between these two extremes. There was clinical and biochemical evidence of rejection, often to a severe degree, but this proved to be a reversible process. It is important to emphasize that no supplementary therapy was instituted in these animals at the time of their rejection crises. Treatment with azathioprine was continued in approximately the same doses as before. The latter observations, made in more than 40 animals, emphasize the important principle that rejection is a phenomenon which tends to be spontaneously reversible. It also emphasizes the caution that is necessary in attributing benefit to other therapeutic maneuvers carried out at this critical time.
The pathologic observations made in these animals correlated well with the foregoing clinical events. In brief, the principal destructive consequences of rejection were invariably observed during the first several weeks after transplantation, and were similar to those described above for nontreated animals. In dogs which survived the onslaught of the first several weeks, the mononuclear cells which had invaded the graft tended to disappear, leaving behind large necrotic areas. From this time onward, the abnormalities were predominately those caused by repair and regeneration.
Early, there was collapse of the reticulin network in the regions of the portal tracts and centrilobular veins. Later these same areas underwent fibrosis resulting in some cases in the development of a pseudolobular appearance (Fig. 5). In many animals with chronic survival, the homografts contained an appreciable number of the pyroninophilic mononuclear cells which are classically associated with acute rejection but which now appeared to be relatively well tolerated.
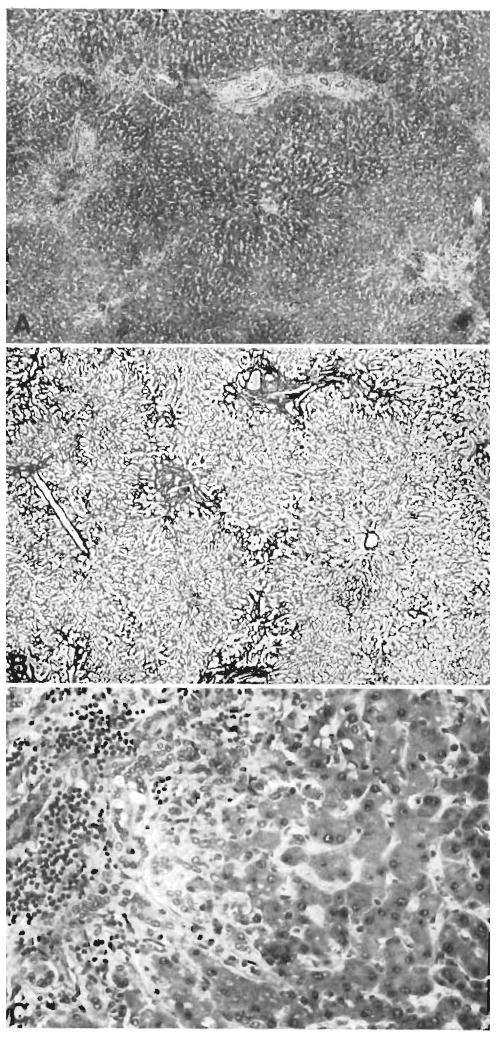
Figure 5.
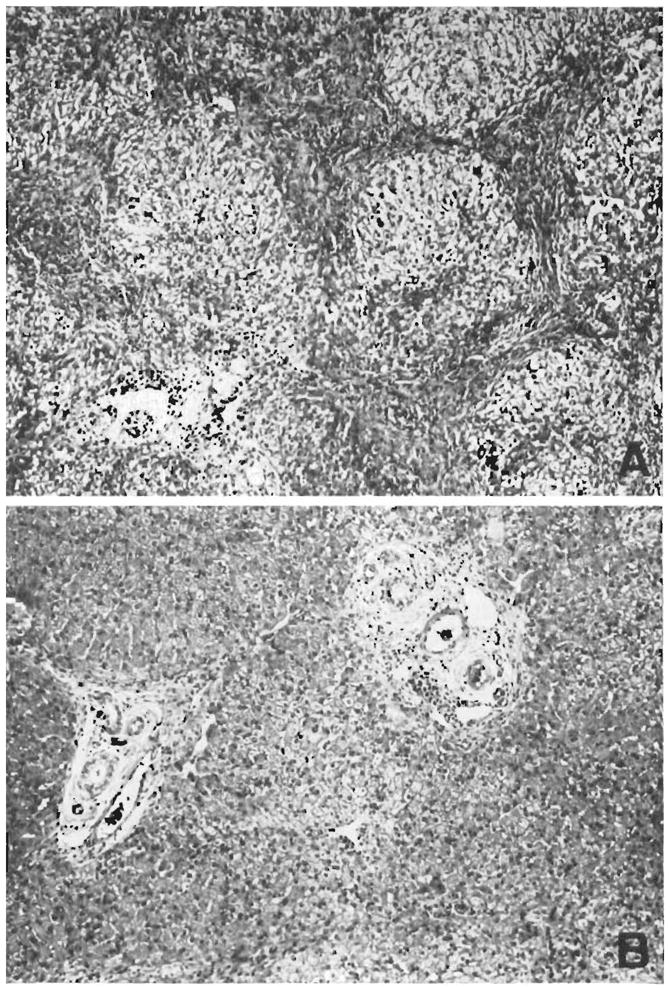
Two biopsies from a hepatic homograft. The first (A) was taken after the host had been receiving azathioprine for 121 days. The lobular architecture is distorted by thick bands of connective tissue which link portal tracts to each other and to central veins. Hepatocytes in the pseudolobules of regenerating liver contain much lipid. Azathioprine therapy was then discontinued, and 77 days later, 198 clays after transplantation, the second biopsy (B) was taken. There has been a striking improvement in the general liver architecture. Connective tissue bands are no longer so obvious and the liver cells look more healthy. This animal is still alive 21/2 years after operation. (By permission of Surgery, 1965,58: 131.)
Clinical experience
These laboratory studies have clearly demonstrated that orthotopic transplantation of the liver is feasible, but, unfortuantely, efforts to apply this method for the treatment of human disease have not yet been successful. In Denver, 5 such attempts were made in the spring and summer of 1963 (12). Similar cases have been reported from Boston (10) and Paris (2). All of these patients died within 22 days. Analysis of the causes for failure has materially influenced subsequent research.
First, difficulties were encountered in obtaining adequately preserved cadaveric organs. Immediately after death, efforts were made not only to cool but to perfuse the liver in situ by means of an extracorporeal pump oxygenator into which a heat exchanger had been incorporated. Access to the circulation of the cadaver was obtained through cannulas which were introduced immediately after death into the great vessels of the abdomen via the femoral vessels. After the homograft had been removed, it was further perfused with cold lactated Ringer’s solution.
In spite of these efforts, all of the human livers used in Denver and elsewhere were more or less seriously damaged by anoxia as indicated by acute increases in the peripheral levels of SGOT, SGPT, LDH, and alkaline phosphatase. These were almost certainly due to ischemic injury rather than to rejection. Changes in serum bilirubin usually followed the same curve but at a later time. Improved conditions of cadaveric organ procurement and preservation are clearly necessary. Progress in this direction has been reported by Mikaeloff (8) who was able to carry out successful orthotopic transplantation with dog livers which had been perfused for several hours.
A second problem was posed by changes in blood coagulation which preceded operation in most of these patients and which were aggravated at the time of transplantation. Previous canine studies had shown that fibrinolytic activity was an important contributing factor to a bleeding diathesis which often developed in the dog during the anhepatic interval; the same phenomenon was noted in man. Unfortunately, it had not been realized from the laboratory studies that a state of hypercoagulability often followed shortly after operation if there was good function of the new liver. In 3 of our patients, the euglobulin lysis time became prolonged to as much as 24 hours, indicating a virtual absence of fibrinolysins. During this period, these patients developed clots in the inferior vena cava from which serious or fatal pulmonary emboli originated.
The delayed hypercoagulability just described has subsequently been documented in dogs after orthotopic transplantation but to a far lesser degree. The situation was aggravated in the human cases by intraoperative administration of thrombogenic agents such as epsilon aminocaproic acid (EACA), human fibrinogen and fresh blood or plasma. In addition, a mechanical factor appeared to have been contributed in that the origin of the clots seemed to be related to trauma from a plastic external bypass catheter which was inserted into the inferior vena cava via the femoral venous system.
Lessons for future trials have evolved from these and other clinical observations. It has subsequently been learned that the suprarenal vena cava and portal vein can be safely occluded in patients with liver disease for an hour or more, making the dangerous external bypass unnecessary. Further, iatrogenic manipulation of the coagulation process seems contraindicated for amelioration of the bleeding diathesis during and just after the transplantation, unless this becomes absolutely necessary to prevent a fatal hemorrhage.
The latter possibility is more than a theoretical one, not only because of poor clotting, but also because the technical problems of this operation in the human are greater than those in the normal dog. Most of the patients who have been candidates for orthotopic transplantation have had advanced cirrhosis and portal hypertension. The majority also had primary carcinomas of the liver which contributed to the bulky size of the specimens. The difficulty of obtaining adequate hemostasis under these circumstances has been formidable. Two of the first 7 attempts at orthotopic homotransplantation of the liver failed because of uncontrollable hemorrhage (2, 12).
In spite of the fatal issue in all of these cases, pathologic studies of the homografts did not contravene the hope that liver transplantation could be accomplished in man under the appropriate circumstances. The livers examined at autopsy after residence in the host for 6–22 days were relatively well preserved with few signs of rejection (Fig. 6). In all the patients, fatal extrahepatic complications were present including pulmonary emboli and acute gastrointestinal ulceration. In addition, infection was an important feature of the terminal course in each case, caused by either pyogenic organisms or by unusual fungi or protozoa.
Figure 6.
Case 2 of Colorado series: treated human orthotopic hepatic homograft at 22 days. A, B, and C show typical appearance of the tissue. A, centers of lobules appear dark because the hepatocytes contain excess lipofusein. Increase of portal connective tissue was probably present before homotransplantation. There is patchy cellular infiltration, particularly in smaller portal tracts. Hematoxylineosin; ×20. B, lobular architecture is essentially normal. Reticulin stain; ×20. C, portal tract is infiltrated by mononuclear cells. There is proliferation of small bile ducts. Hematoxylineosin; ×250.
The problem of immunosuppression
In the clinical cases described above, immunosuppression was provided with azathioprine and prednisone, the two most important drugs in the therapeutic regimen which has been widely used for clinical renal homotransplantation. These agents are also of proven value for prevention of liver homograft rejection in dogs. However, the universal presence of infection in the human recipients of liver homografts suggests that the margin of safety is too slender to permit consistent success unless important improvements are made.
One such adjustment could involve the techniques of histocompatibility analysis to which several other participants in this symposium have directed their attention. With the ability to select the biologically most suitable donor, it might be possible to treat with smaller doses of both azathioprine and prednisone.
The most pressing need, however, is for better methods of immunosuppression. Of the various agents now being tested in research laboratories, none has excited more interest than antilymphocyte serum (ALS) and its globulin derivative (ALG). In our laboratories, it has been shown that both ALS and ALG prolong the functional life of liver homografts (13).
Eighteen dogs received orthotopic transplantation, 9 with sole treatment by antilymphocyte serum, and 9 others with immune globulin. The mean survival of these dogs was more than a month using for calculation an arbitrary statistical limitation of survival for any dog of 70 days. In reality, however, 4 of these 18 animals lived for more than 4 months, the longest followup being more than 9 months in an animal which received ALS for only the first 3 postoperative weeks. Prolongation of survival was not well correlated with the degree of lymphopenia produced by the ALS or ALG.
Additional experiments have been carried out using antilymphocyte globulin in combination with azathioprine, or with azathioprine and prednisone. Using either regimen of multiple drug therapy, there appeared to be greatly improved immunosuppression which, however, introduced an added technical problem. These dogs had better hepatic function during their early postoperative period than any previously treated group of animals and apparently as a consequence, there was a 70% incidence of thrombosis of the aortic grafts. Subsequently, we have used the hemodynamically more satisfactory but somewhat more difficult anastomoses of the homograft hepatic artery to the recipient hepatic or right renal artery. With this change in technique more than two thirds of animals are achieving chronic survival.
These laboratory observations, combined with the encouraging early results using globulin therapy as an adjuvant drug for clinical renal transplantation (13), have increased the hope that clinical liver transplantation will become a reality. One attempt has been made at human liver transplantation using the therapeutic combination of azathioprine, prednisone, and ALG. The recipient, a 28-year-old man with a primary hepatoma, received a cadaveric homograft from a 73-year-old donor on November 8, 1966. The donor was accepted in spite of his advanced age because of an unusual degree of antigen compatibility demonstrated by van Rood’s and Amos’s tissue typing teams which were working in Denver at that time. The interval from donor death to revascularization of the homograft was 99 minutes. Unfortunately, a very severe ischemic injury to the homograft had occurred and good liver function was not obtained. The patient died of hepatic failure after 7 days.
AUXILIARY TRANSPLANTATION
An alternative approach to liver transplantation is the use of an auxiliary organ. Such operations are attractive in concept, at least at first thought. The magnitude of the surgical procedure is less than that of organ replacement. In addition, it is not necessary to deprive the patient of the residual function of his own liver, an important consideration if one is considering therapy for patients with non-neoplastic liver disease.
With auxiliary transplantation, as described by Welch, the liver is placed in the pelvis or one of the paravertebral gutters where it receives its arterial supply from the aorta or iliac artery and its portal venous inflow from the inferior vena cava or an iliac vein (Fig. 7A). The biliary tract is drained by means of a cholecystoenterostomy.
Figure 7.
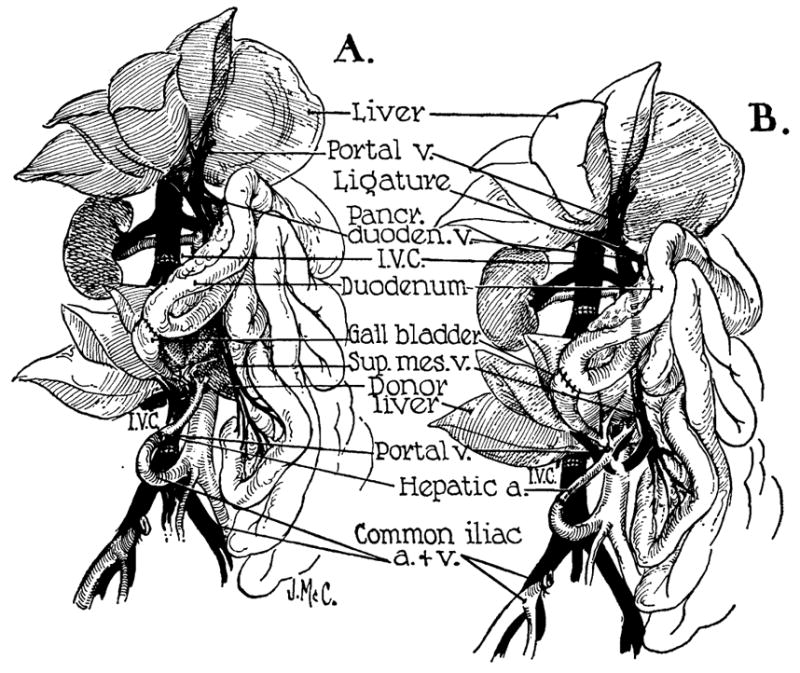
Auxiliary liver transplantation. A, method of Welch. Note that portal venous inflow is from the inferior vena cava. The homograft undergoes rapid atrophy. B, modification of Welch method in which nonhepatic splanchnic flow is diverted through the homograft. With this preparation, the homograft retains its size and the animal’s own liver shrinks. It is usually more convenient to bring the hepatic artery behind rather than in front of the portal vein as depicted. (By permission of Surgery, Gynecology and Obstetrics, 1965, 17: 121.)
Unfortunately, the homograft in this preparation undergoes striking atrophy which begins during the first few postoperative weeks in dogs being treated with azathioprine. The atrophy involves principally the hepatocytes, very often with selective preservation of the biliary duct system. In such livers, the signs of rejection are sometimes minimal or even absent.
From the beginning, it seemed highly likely that the bizarre behavior of the auxiliary homografts must be explained by other than immunologic mechanisms. In our laboratories, strong evidence has been obtained that this is the case. Using a preparation which does not involve transplantation, one or the other of its principal branches was detached from the portal vein of the dog and revascularized by an end-to-end anastomosis to the suprarenal inferior vena cava (6). By so doing, one portion of the liver received its venous inflow from the splanchnic bed, and the other was supplied with systemic venous blood. The volume of flow reaching each of two fractions of liver was approximately the same. Within a few weeks, that part supplied by systemic venous blood underwent striking atrophy. The other portion either remained normal or hypertrophied. These results suggested that there was a qualitative difference in the content of the splanchnic as opposed to systemic venous blood, a difference which influenced hepatic structure and function. That portion of liver tissue which had first access to portal venous blood operated at a physiologic advantage; the other portion atrophied.
This concept was applied to the planning of different methods of auxiliary liver transplantation (7). The technique previously used is shown in Figure 7A. In Figure 7B, the operation was changed in order to direct portal blood in a retrograde fashion through the transplant. This modification prevented atrophy of the homograft and the shrinkage now afflicted the autologous liver. In every successfully conducted experiment, the weight of the homograft exceeded that of the animal’s own organ. These experiments have done much to clarify the physiologic conditions which are important for successful auxiliary transplantation. Coexisting livers are each apparently capable of injuring the other by virtue of their competition for some essential substrate or substrates present in selective concentration in the splanchnic venous blood.
Use of the improved method of auxiliary transplantation described above would probably not be practical for clinical transplantation because of important differences in the intra-abdominal anatomy of the dog and man. Consequently, a number of compromise methods were evaluated in the laboratory, which might be technically feasible in humans. In the course of these investigations, it was found that various maneuvers which injure the host liver tend to favor the well-being of the homograft. For example, if an Eck fistula is imposed upon the autologous liver, or if its common duct is ligated the homograft atrophy can be reduced, although not completely prevented.
Clinical trials
The foregoing information was applied to the planning of the type of operation shown in Figure 8, which was first shown to be practical in dogs and then used in 2 patients who were dying of Laennac’s cirrhosis (4). In both cases, there had been a gastrointestinal hemorrhage requiring a first stage portacaval shunt for control of the bleeding. At the time of homotransplantation, from 1–3 days later, the auxiliary livers were placed in the right lower abdomen, revascularizing the hepatic artery from the aorta or one of the pelvic vessels, and the portal vein from the inferior vena cava or one of the iliac veins (Fig. 8). Thus, the homograft immediately enjoyed the advantage of having a double, albeit physiologically imperfect, blood supply as compared to the autologous liver which had only an arterial inflow. The metabolic situation of the homograft was further favored by the morbid state of the patient's diseased liver with its consequent reduced capacity for competition.
Figure 8.
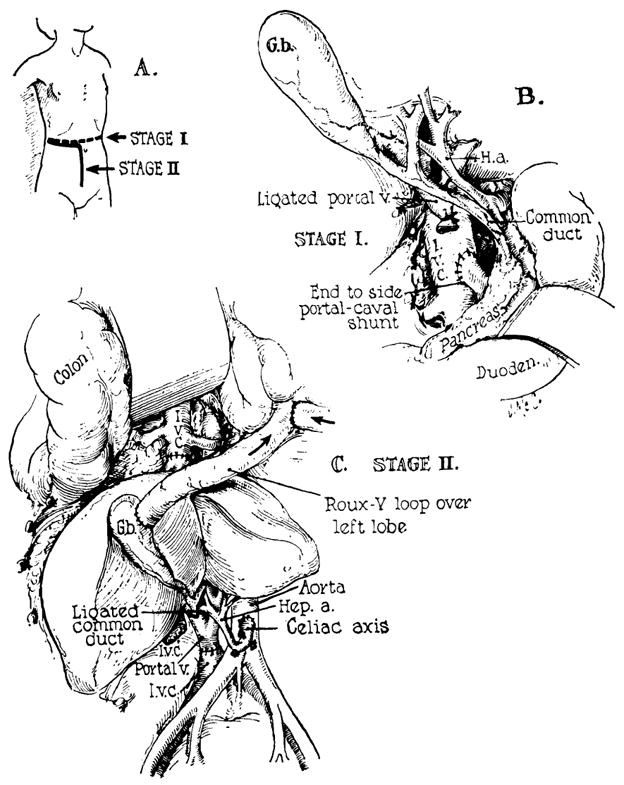
Method of auxiliary liver transplantation which has received a clinical trial. The procedure has been used twice at the University of Colorado Medical Center in patients with terminal Laennec’s cirrhosis. A, B, first-stage portacaval anastomosis is performed. This will usually be necessary for control of variceal hemorrhage. C, revascularization of the auxiliary liver in the right paravertebral gutter. (By permission of Pediatric Clinics of North America, 1966, 13: 381.)
There was unequivocal evidence of function of the auxiliary homografts. Pre-existing bilirubinemia fell from 40 to 30 mg/100 ml to 10 mg/100 ml or less in both cases. The depressed prothrombin times were increased to 50–100%. Both of these patients who were treated with azathioprine, prednisone, and actinomycin C eventually died of sepsis, after 23 to 35 days.
As with the orthotopic human livers, the transplanted organs were not seriously damaged from rejection, at the time of autopsy. The infectious complications were multiple. Both patients had pyogenic pneumonitis. In addition, one had infestation of the lungs with pneumocystic carinii and the other had generalized cytomegalic inclusion disease. The patient who lived for 35 days had had a continuous gastrointestinal hemorrhage for the last 10 days of his life. This was found to be due to ulcerative moniliasis involving almost the entire gastrointestinal tract.
In a third attempt at auxiliary transplantation at the University of Colorado, a cadaveric homograft was placed in the splenic fossa of a child with biliary atresia. The hepatic artery was anastomosed to the splenic artery and the portal vein to the splenic vein as originally described by Absolon (1). The vascular outflow was through the homograft vena cava which was connected to the side of the recipient’ inferior vena cava. The hepatic artery thrombosed a few minutes after revascularization necessitating removal of the organ.
Footnotes
Aided by grants AM 06283, AM 06344, HE 07735, AM 07772, AI 04152, FR 00051 and FR 00069 from the United States Public Health Service, and by a grant from the Medical Research Council of Great Britain.
References
- 1.Absolon KB, Hagihara PF, Griffin WO, Jr, Lillehei RC. Rev Int Hepat (Lyon) Tome. 1965;15:1481. [PubMed] [Google Scholar]
- 2.Demirleau Noureddine, Vignes Prawerman, Reziciner Larraud, Louvier Med Acad Chir (Paris) 1964;90:177. [PubMed] [Google Scholar]
- 3.Fonkalsrud EW, Shafey OA, Ono H, Longmire WP., Jr S Forum. 1966;17:215. [PubMed] [Google Scholar]
- 4.Halgrimson CH, Marchioro TL, Faris TD, Porter KA, Peters GN, Starzl TE. A M A Arch Surg. 1966;93:107. doi: 10.1001/archsurg.1966.01330010109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kountz SL, Williams MA, Williams PL, Kapros C, Dempster WJ. Nature (London) 1963;199:257. doi: 10.1038/199257a0. [DOI] [PubMed] [Google Scholar]
- 6.Marchioro TL, Porter KA, Illingworth BI, Faris TD, Herrman TJ, Sudweeks A, Starzl TE. S Forum. 1965;16:280. [PMC free article] [PubMed] [Google Scholar]
- 7.Marchioro TL, Porter KA, Dickinson TC, Faris TD, Starzl TE. Surg, Gynec & Obst. 1965;121:17. [PMC free article] [PubMed] [Google Scholar]
- 8.Mikaeloff P, Dureau G, Rassat JP, Chabert M, Dumont L, Belleville J, Tronchon J, Malluret J. Mem Acad Chir (Paris) 1965;91:286. [PubMed] [Google Scholar]
- 9.Moore FD, Smith LL, Burnap TK, Dallenbach FD, Dammin GJ, Gruber VF, Shoemaker WC, Steenberg RW, Ball MR, Belko JS. Transplant Bull. 1959;6:103. doi: 10.1097/00006534-195901000-00041. [DOI] [PubMed] [Google Scholar]
- 10.Moore FD, Birtch AG, Dagher F, Veith F, Krisher JA, Order SE, Shucart WA, Dammin GJ, Couch NP. Ann New York Acad Sci. 1964;120:729. doi: 10.1111/j.1749-6632.1964.tb34765.x. [DOI] [PubMed] [Google Scholar]
- 11.Porter KA, Joseph NH, Rendall JM, Stolinski C, Hoehn RJ, Calne RY. J Lab Invest. 1964;13:1080. [PubMed] [Google Scholar]
- 12.Starzl TE, Marchioro TL, Porter KA. In: Advances in Surgery. Welch C, editor. Yearbook Medical Publishers, Inc; Chicago: 1966. p. 295. [Google Scholar]
- 13.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. Surg, Gynec & Obst. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 14.Welch CS. Transplant Bull. 1955;2:54. [Google Scholar]