During the interval from 15 to 2½ months ago, 45 patients were treated at the University of Colorado Medical Center with renal homografts obtained from living volunteer donors. As a consequence of this experience, many aspects of renal homo-transplantation have come into focus, including the early41 and late39, 40 behavior of homografts, the events and reversibility of rejection,39, 40 the importance of technical factors in both donor22 and recipient,42 operations in obtaining good early renal function, the influence of blood type incompatibility upon results,41, 43 and the value of variant techniques for the treatment of infants and children.44
Despite the fact that the majority of patients survived operation and are presently in good health, there has been a persistently high mortality which has tended to occur in certain types of cases under recurrently similar circumstances. In this paper, an attempt will be made to analyze the factors which seemed to have had a demonstrable influence on the outcome of renal homotransplantation. Furthermore, the mechanisms that caused death will be documented in the unsuccessful cases, and suggestions made for changes in management which may, in the future, help avoid the previously encountered lethal complications.
Finally, note will be made of several unusual clinical syndromes which have been observed after renal homotransplantation, including the apparent development of host tolerance to specific strains of pathogenic bacteria, the appearance of unexplained fever or diffuse pulmonary disease late in the course of recovery; the coexistence of eosinophilia and fever in cases of atypical rejection, and the demonstration of physiologic events during rejection which suggest the important role of a vascular lesion in causing functional deterioration at this time.
METHODS
The general techniques described by Küss20 and Murray and Harrison28 were used for transplantation, with modifications of detail.42 A transabdominal operation was employed for children.44 All 45 patients had splenectomy,38 usually at the same time as homotransplantation and bilateral nephrectomy.42 Eight of the early patients had preliminary thymectomy,38 a procedure which is of unproved value. The temperature of all homografts was lowered during the period of ischemia either by total body hypothermia of the donor or by perfusion with chilled electrolyte solution immediately after removal.22, 43
The immunosuppressive regimen, as adapted from that of Calne and Murray and their associates,2–5, 29, 30 was previously described.39, 40 Whenever possible, the administration of azathioprine was begun 8 to 10 days before operation and used indefinitely thereafter. Azathioprine was thus employed in a fundamentally different way than other agents described below, in that it was used continuously rather than in response to specific indications of rejection. Furthermore, it has not been discontinued in any case.
With the onset of rejection, an event which was observed in 42 of the 45 patients, therapy with the use of daily doses of prednisone and intermittent doses of actinomycin C was begun.39 In 3 patients already receiving small quantities of prednisone, the doses were increased. After reversal of the rejection crisis, actinomycin C was discontinued, and the doses of prednisone were reduced as rapidly as possible.
From the beginning of azathioprine therapy, close scrutiny was accorded the hematologic picture. White counts were obtained on each day during hospitalization. Although the white blood counts gave no indication of the effectiveness of azathioprine in the prevention or the treatment of rejection, they provided an index of the toxicity of the drug and a direct, clear warning of overdosage. From the beginning, the dose was fixed at whatever level was possible without causing leukopenia.
Total body irradiation was used in only one case.40 Local irradiation of the homograft was employed in 4 cases as described subsequently, with the use of the general technique outlined by Hume.17
Numerous aspects of postoperative care are of vital importance in the avoidance of troublesome or lethal complications, but will not be described further here. These include the management of the acute postoperative diuresis,41, 42 careful bacteriologic control of all antibiotic therapy,33, 34 and vigorous supportive treatment (including postoperative dialysis if necessary) of the hypertension, fluid and sodium retention, and electrolyte abnormalities which accompany a rejection crisis.39
RESULTS
Survival statistics
Of the 45 patients, 27 are alive on Feb. 12, 1964. The average postoperative interval is 190.9 days, with a range of 70 to 448 days. Fifteen cases have been followed for 6 months or longer, and 3 for more than a year. All but one patient is living at home; the only exception is a 6-year-old child who suffered a cerebrovascular accident in December, 1963, and who has since remained semicomatose. He has good renal function 200 days following operation.
For the 18 patients treated unsuccessfully there was an average survival of 58 days, ranging from 1 to 208 days. Only 2 patients who lived for 3 months or longer subsequently died, both of late septic complications at 113 and 208 days. Their courses will be more carefully considered below.
Effect of consanguinity
Mother-to-off-spring donations have been most successful (Table I); 11 of 13 such patients are now alive 84 to 448 days after operation (average 174.6 days). The only deaths were of 21-and 25-year-old sons who succumbed of pneumonia 48 days postoperatively, and of disseminated tuberculosis after 38 days, respectively.
Table I.
Effect of consanguinity
| Types of graft | No. of cases | No. of survivals | No. of deaths |
|---|---|---|---|
| Mother-to-son* | 8 | 6 | 2 |
| Mother-to-daughter | 5 | 5 | 0 |
| Father-to-daughter | 2 | 1 | 1 |
| Brother-to-brother†* | 10 | 6 | 4 |
| Brother-to-sister | 1 | 0 | 1 |
| Sister-to-brother* | 6 | 4 | 2 |
| Unrelated | |||
| Same sex* | 8 | 3 | 5 |
| Female-to-male | 4 | 1 | 3 |
| Male-to-female | 1 | 1 | 0 |
| Total | 45 | 27 | 18 |
In each group, 1 additional homograft was donated and removed either immediately or early in the postoperative course. The failures were due either to breeches of ABO incompatibility (2 cases) or to the imposition of excessive ischemia (2 cases). Second homografts were placed, and, in the above tabulation, only the final donors are considered.
Includes 2 sets of fraternal twins. One patient is alive after more than a year. The other died of a fungus brain abscess and gastrointestinal hemorrhage after 208 days.
Two father-to-daughter homografts were performed. The first patient died of an electrolyte imbalance during a massive postoperative diuresis. The second is well after 3 months.
There were 17 sibling donations with 7 deaths; 6 of these cases were female-to-male transfers and 1, male-to-female (Table I). The surviving patients have been followed from 70 to 380 days (average 227.7 days).
Poor results were obtained when the donors were not related to the recipients. Only 5 of 13 such patients are alive; the survivals ranged from 124 to 276 days (average 175.2 days). In the unsuccessful cases, the patients lived from 10 to 113 days postoperatively (average 55.5 days).
Influence of age
Eighteen of 26 patients who were 34 years of age or younger are alive. Of 19 patients who were 35 years of age or older, only 9 are alive. Such statistics are, of course, heavily weighted by the fact that more than half of the latter group received kidneys from donors not related genetically (Table II). Nevertheless, the incidence of nonrenal complications was much higher in the older recipients and contributed to the attrition as has been recently described.42 Postoperative complications included gastrointestinal hemorrhages, pancreatitis, ileus, neurologic disorders, myocardial infarction, peripheral venous thrombosis, and pulmonary embolization.
Table II.
Influence of age on survival
| Age in years | No. of patients | Nonrelated donors | No. of survivals | No. of deaths |
|---|---|---|---|---|
| 0 to 10 | 3 | 0 | 3 | 0 |
| 11 to 20 | 12 | 2 | 9 | 3 |
| 21 to 34 | 11 | 1 | 6 | 5 |
| 33 to 44 | 11 | 6 | 5 | 6 |
| 45 to 55 | 8 | 4 | 4 | 4 |
| Total | 45 | 13 | 27 | 18 |
Considerations of blood type
In previous communications, general suggestions were formulated concerning the combinations of donor and recipient blood group incompatibilities which were and were not safe.41, 43 Briefly, it appeared that the direction of permissible tissue transfer follows the same rules as those which apply to the emergency use of mismatched blood in that type O is the universal tissue donor and type AB the universal recipient. Subsequent experience has tended to confirm these conclusions. When combinations have been selected which do not place renal tissue in contact with preformed host hemagglutinins, immediate good renal function has always been observed and the late results have not been significantly different from those of the groups with donor-recipient ABO identity (Table III). That such dicta were not absolute is evident (Table III) from the fact that an A patient who received a kidney from a B donor and an O recipient who received a kidney from an A donor have good renal function at 12½ and 6½ months, respectively. Nevertheless, violations of this general scheme resulted in the loss of 2 homografts immediately after their revascularization and this was apparently caused by the acute hemagglutination reactions.41
Table III.
Blood groups of donors and recipients. (In the 4 patients receiving 2 homografts, only the second or definitive donor is tabulated)
| Blood type | No. of cases | No. of survivals | No. of deaths |
|---|---|---|---|
| Type O recipient* with: | |||
| Related O donor | 12 | 9 | 3 |
| Unrelated O donor | 5 | 4 | 1 |
| Related A donor | 1 | 1 | 0 |
| Type A recipient with: | |||
| Related A donor | 13 | 7 | 6 |
| Unrelated A donor | 4 | 1 | 3 |
| Related O donor | 3 | 2 | 1 |
| Unrelated O donor | 2 | 0 | 2 |
| Related B donor | 1 | 1 | 0 |
| Type B recipient with: | |||
| Related B donor | 1 | 1 | 0 |
| Unrelated O donor | 1 | 1 | 0 |
| Type AB recipient with: | |||
| Unrelated AB donor | 1 | 0 | 1 |
| Unrelated A donor | 1 | 0 | 1 |
| Total | 45 | 27 | 18 |
In 2 of these O recipients, A and B homografts were placed. The transplants were destroyed immediately after revascularization by an apparent hemagglutinative process, removed, and second homografts from O donors placed 10 and 14 days later.41
Within the larger categories of patients who had identical donor-recipient ABO blood types, there was a subtler difference in late results which warrants description but which is of unknown significance. In 17 homotransplants of kidneys from A donors to A recipients, there were 9 deaths, a failure rate which was exorbitantly high with the use of both related and unrelated donors (Table III). In contrast, there were only 4 deaths in the comparable series of 17 patients with O blood type (Table III). In both groups, excellent early homograft function was obtained but the subsequent rejection episodes in A-type matches tended to be more severe and protracted (Fig. 1), suggesting that a specific sensitization to the red cell antigens, which have been demonstrated within renal cells,16, 45 may have occurred and contributed to the difficulty of maintaining homograft viability. Slight differences in the blood substance of the A donor and recipient, which are commonly present despite the absence of agglutination with direct cross-matching,27 could presumably lead to the delayed production of hemagglutinins and other circulating antibodies. Further observations will be necessary before it can be determined whether the difficulties in transplanting kidneys from A donors to A recipients are renal or whether they are due to coincidence. An interinstitutional cooperative study would be of great value in quickly arriving at a statistically significant conclusion.
Fig. 1.
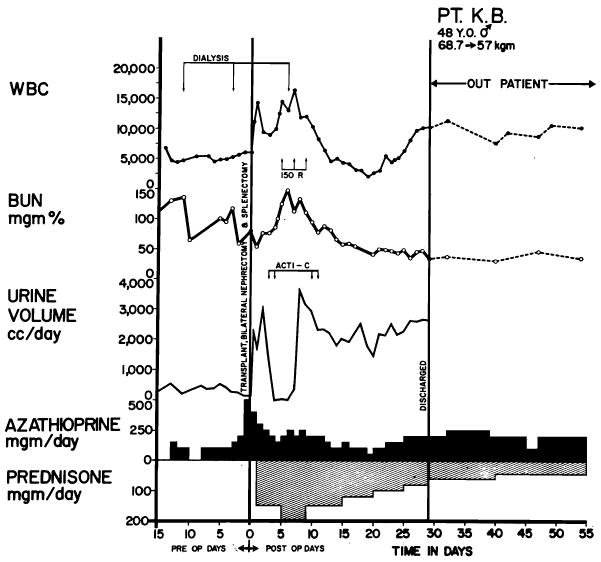
Typical case treated unsuccessfully. The kidney was obtained from the patient’s brother; both of them were of A+ blood type. Good renal function postoperatively was interrupted by a violent rejection crisis after 12 days and anuria developed. After 2 weeks of renal shutdown, secondary diuresis occurred. The patient died from drug toxicity, leukopenia, and septicemia (Table V, Case 32). Exceptionally vigorous rejection episodes seemed more common in recipients of blood type A than in those of other blood types. Note use of hemodialysis during height of rejection crisis. Acti C: each arrow represents the intravenous administration of 200 gamma actinomycin C.
Excision of lymphoid masses
Thymectomy was performed upon eight of the patients but not upon the other thirty-seven. Splenectomy was performed in all 45 cases, usually at the same time as bilateral nephrectomy in the recipient and homotransplantation. Despite strenuous efforts in the laboratory, it has been impossible to demonstrate a potentiated homograft survival in dogs after splenectomy, making the use of this adjuvant procedure entirely empirical. Despite this, continued use of splenectomy may be justified for several reasons. First, it is possible that removal of the spleen makes the use of larger doses of azathioprine feasible, as least early in the postoperative period, because of the consequent leukocytosis. Second, it has been demonstrated in other experimental settings that splenectomy results in definite reduction of antibody response to various intravenous antigens.15, 35 Third, the animal experiments of Wissler and his associates12, 46 suggest that, in the adult stage, the spleen may assume a preceptor role in the organization of total immunologic response (Fig. 2) not dissimilar to that attributed to the thymus in neonatal life by Miller.25, 26 Finally, Hume18 and Hamburger13 and their associates have reported late sequelae of hypersplenism in several which otherwise had been successfully treated; this necessitated a delayed splenectomy.
Fig. 2.
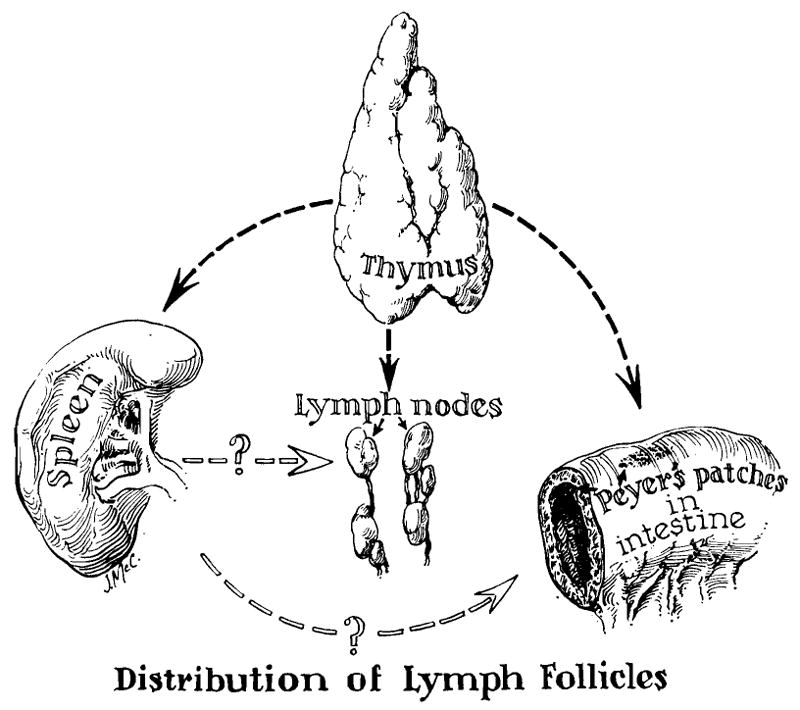
Theoretical considerations in the use of adjuvant thymectomy and splenectomy for the conditioning of recipient patients. The concept of the function of the thymus as an organizer in establishing fetal and neonatal immunologic reactivity is based largely on the work of Miller,25, 26 and may have no relevance in the adult animal. The role of the spleen in controlling response to antigenic stimuli in adult life is suggested by the work of Wissler and his colleagues.12, 46 The contribution of these ancillary procedures to homograft survival is highly speculative at present.
Pretreatment with azathioprine
Recipients are usually treated with 2 to 4 mg per kilogram azathioprine for 7 to 10 days preoperatively. The rationale for this practice is based upon the 60 experiments on dogs summarized in Table IV, in which homotransplantation and bilateral nephrectomy were performed. Untreated animals lived for 10.8 ± .06 (S.E.) days. Animals whose treatment with azathioprine started on the day of operation lived 36 ± 3.0 (S.E.) days. Animals which received treatment with azathioprine for 7 to 30 days preoperatively as well as postoperatively lived for 69 ± 16.1 (S.E.) days. The difference between the 2 groups that received azathioprine was highly significant (p < .001).
Table IV.
Influence of pretreatment with azathioprine upon posttransplant longevity in dogs*
| No. | Mean survival days | S.D. | S.E. | |
|---|---|---|---|---|
| Control animals, untreated | 21 | 10.8 | 2.75 | .06 |
| Animals started on azathioprine on day of operation | 21 | 36 | 13.7 | 3.0 |
| Animals pretreated with azathioprine | 18 | 69.1 | 68.1 | 16.1 |
Comparison of groups 2 and 3 (p < .001).
The stages of recovery
The events which typify the course of a successfully treated case have been previously described in detail39, 40 and will not be exhaustively treated here. Uninterrupted recovery is almost never seen with the therapeutic regimen employed in the present study. Postoperatively, there is a period lasting from 1 to 42 days during which good homograft function is present. At some time during convalescence, a rejection crisis has almost invariably supervened with systemic manifestations of fever, hypertension, and malaise; with local transplant wound tenderness; and with manifold evidence of renal functional deterioration. The addition of large doses of prednisone and actinomycin C to pre-existing therapy with azathioprine results in reversal of these events in most cases (Fig. 3). Later in the course, the drugs added secondarily can be reduced or stopped; this lends support to the belief that a state of host-graft adaptation occurs promptly in those cases which can survive the attempt at graft repudiation.39 Subsequent secondary rejection crises have been observed in only 2 patients.
Fig. 3.
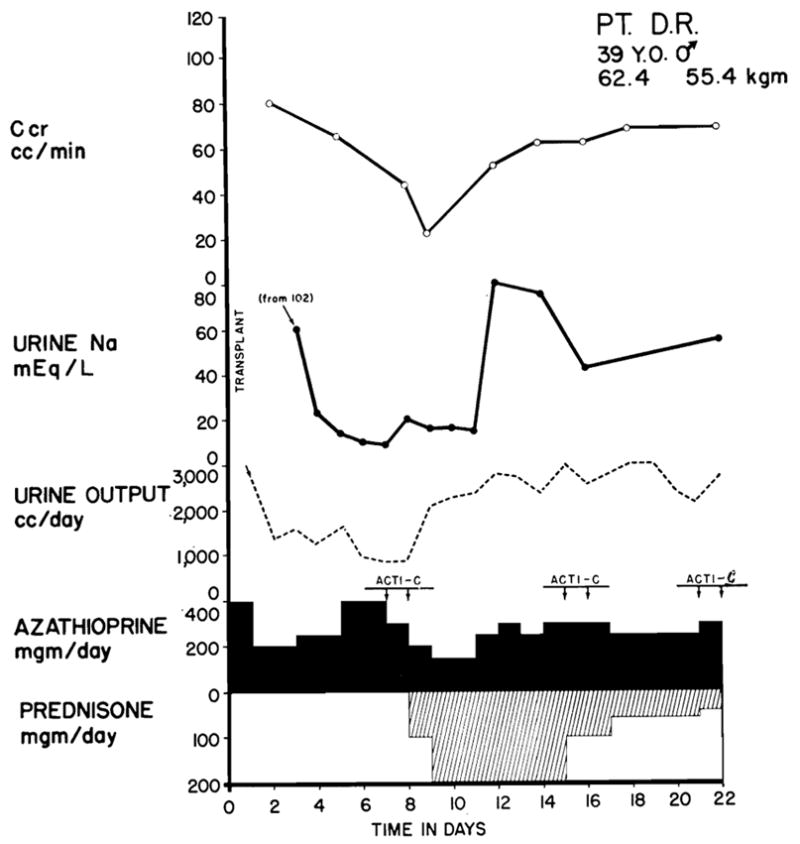
Typical case treated successfully. The patient received a kidney from his sister; both had type O blood. Fever, azotemia, hypertension, transplant wound tenderness, and proteinuria accompanied the relative oliguria and the decline in creatinine clearance which are depicted graphically. The changes were all reversed with the addition of prednisone and actinomycin C to the regimen. Leukopenia did not develop. Note the diminution of urine sodium concentration which is almost always observed during rejection and the paradoxical effect of prednisone in restoration of sodium excretion. Bilateral nephrectomy and splenectomy were performed at the same time as transplantation. Acti C: each arrow represents 200 gamma actinomycin C given intravenously.
Rejection crises not only varied in time of onset as mentioned above, but in vigor. Impairment of homograft function has ranged from the development of asymptomatic azotemia to the onset of complete anuria (Figs. 1 and 4), which has lasted for 1 to 14 days before the beginning of a secondary diuresis. Recently, intermittent local irradiation has been given by the method of Hume17 to 4 patients who had violent rejection episodes and anuria, with the use of 150 r (at depth) every other day for 3 days (Fig. 4). The dose was delivered with a 250 kv. unit at 30 Ma., HVL 2.5 mm. Cu, with the use of a Thoraeus II filter at a skin distance of 70 cm. Output was 31.8 r per minute. Resumption of urine excretion occurred on 3 occasions within a few hours after the first treatment. Equally impressive was the almost immediate relief of exquisite tenderness in the transplant wound.
Fig. 4.
Value of Hume’s method of local irradiation17 in the treatment of severe rejection. The patient (A blood type) received a kidney from his sister (O blood type). After good initial function, gross hematuria developed, followed by anuria and other features of acute rejection. Wound tenderness, which was so extreme that urinary extravasation was feared, was largely relieved within an hour after the first dose of 150 r and resumption of urine excretion followed within 24 hours. The subsequent course was uncomplicated. Despite the rapidity of onset of rejection, it was possible, with 4 hourly analyses of urine composition, to demonstrate the changes that are thought to illustrate an ischemic component of rejection (see text and Fig. 3). Acti C: each arrow represents 200 gamma actinomycin C given intravenously. The conditions of x-ray therapy are described in the text.
Urine composition during rejection
In many patients, urinary constituents were serially measured before, during, and after a rejection episode. Similar changes were observed in almost all these cases. Sharp declines in sodium concentrations were most characteristic (Fig. 3); they sometimes fell as low as 0 to 10 mEq. per liter despite a continuing although reduced urinary output. Chloride alterations tended to be similar. Potassium values were variable. Urea and especially creatinine concentrations usually rose. The effect of prednisone upon urine composition was paradoxical in that this drug, which ordinarily promotes salt retention, reversed these changes and appeared to promote sodium excretion (Fig. 3). In one patient who had 3 distinct rejection episodes, such alterations were noted on all 3 occasions. Even in those patients who had a sudden early rejection with profound oliguria or anuria, similar but more rapidly evolving changes have been documented (by Dr. David Ogden) with the analysis of 4 hour urine aliquots. The marked salt retention is undoubtedly an important factor in the development of the fluid retention and acute hypertension which are often seen at the time of rejection.39
These findings also provide inferential information concerning pathophysiologic mechanisms of rejection inasmuch as the alterations in water, electrolyte, and creatinine excretion are similar to those which have been observed in experimental Goldblatt procedures and which form the basis of the Howard7 and Stamey37 clinical tests for renal ischemia. It has been known for many years that canine kidneys which are rejected have diffuse vascular injury consisting of fibrinoid degeneration and intramural edema collection in and between the layers of vessel walls, endothelial proliferation, and infiltration with pyroninophilic mononuclear cells.8,36 The effect of such lesions upon renal blood flow is speculative, but it seems reasonable to believe that they may add an important element of secondary ischemia to the process of rejection in view of the above findings and because of the common presence of coincident secondary hypertension39 at this time. The indictment of a vascular mechanism is consistent with the observation that some homografts which fail after initial good excretion do not show any evidence of cellular rejection (Fig. 9), and that others resume function after varying periods of delayed anuria in a way not unlike that seen in recovery from acute tubular necrosis (Figs. 1 and 4). Of interest is the recent observation of Porter32 concerning chronic proliferative changes in blood vessels of homografts from which specimens were taken for biopsy late in the postoperative period. It is not unlikely that such lesions are the delayed sequelae of earlier insults upon the vascular system of the transplanted kidney.
Fig. 9.
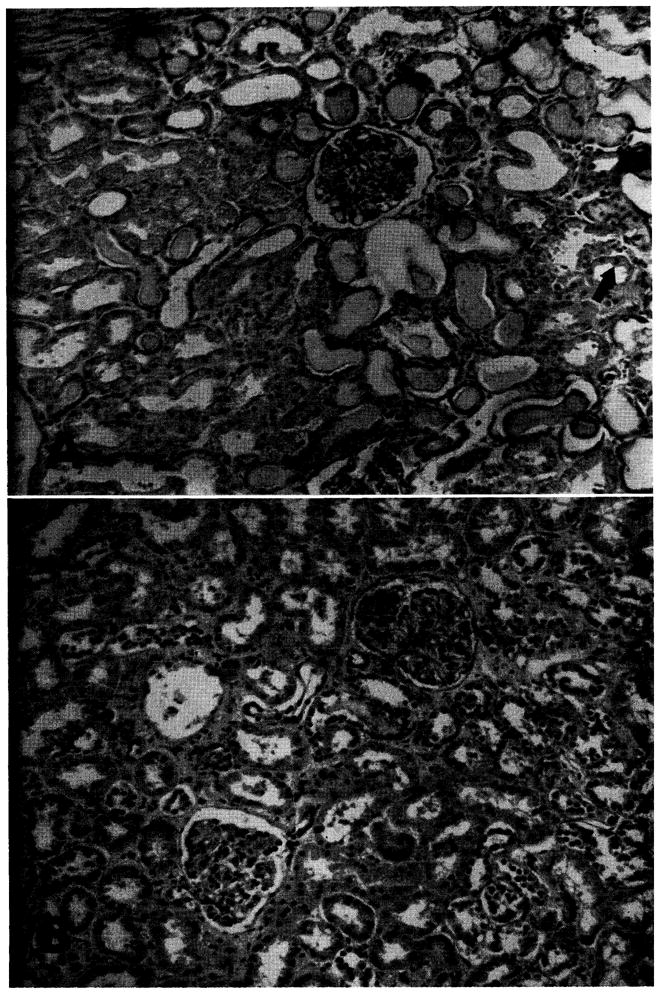
Homografts recovered at early and late autopsy. A, Case No. 29, 8 days after operation. Death was due to drug toxicity and septicemia that occurred after an early vigorous rejection with anuria. Note the evidence of acute tubular necrosis with necrotic and flattened tubular epithelium. Protein casts are evident. Mitosis is indicated by arrow. Cellular infiltrate is minimal. (Hematoxylin and eosin ×80; reduced ⅓.) Ba Case No. 38, 48 days after operation. A rejection episode had been successfully treated. Death was due to pneumonia. The kidney is essentially normal. (Hematoxylin and eosin ×80; reduced ⅓.)
Postoperative eosinophilia
Prednisone was administered during the postoperative period to 43 of the 45 patients, the 2 exceptions being patients who received kidneys from brothers and in whom a recognizable rejection episode did not occur. In both cases, eosinophilia (accounting for as much as 46 percent of the total white count) was observed, beginning 32 and 18 days after operation and lasting for 55 and 38 days, respectively. There was no deterioration of renal function. One patient was completely asymptomatic. The other (Fig. 5) became febrile, and 2 wound re-explorations were performed because infection was suspected. Similar phenomena have recently been noted in a least 2 other centers.4, 13 Inasmuch as the abnormal differential count was not accompanied by impairment of homograft function, the significance of the finding is not evident although it appears to have been an index of immunologic activity. It is probable that eosinophilia might be more commonly observed if it were not for the masking effect of the steroids which are used in most cases.
Fig. 5.
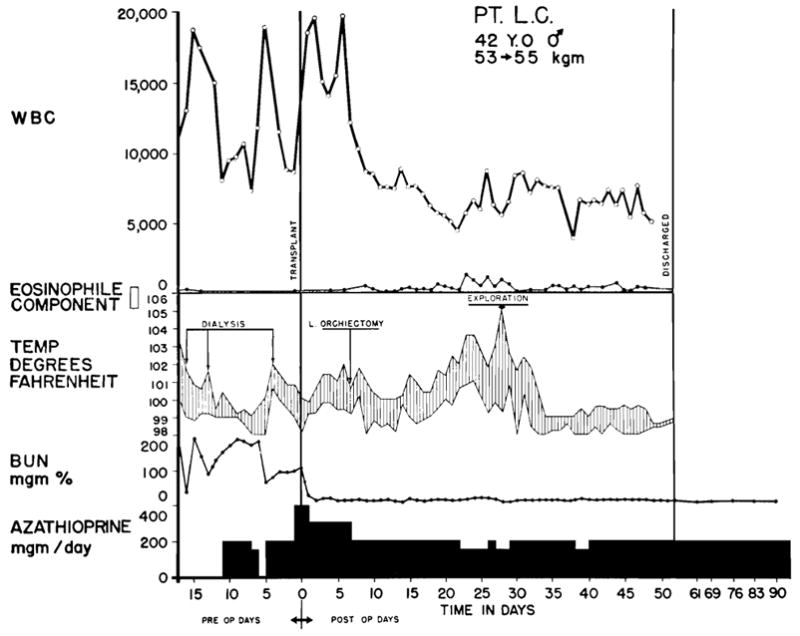
Course of recovery in patient who did not have a classical rejection crisis. Evidence of an immunologic reaction was present after the second postoperative week with eosinophilia and high fever—findings which ultimately resolved spontaneously. The kidney for homograft was provided by his brother, both donor and recipient being A– blood type. The patient who was operated upon on July 5, 1963, continues to have normal renal function.
Causes of death
In the following analysis of failures, the essential information is given in tabular form for each case (Table V). In addition, an attempt has been made to identify recurrent clinicopathologic patterns which defeated efforts at treatment in 18 of the first 45 patients. The principal emphasis in the following remarks will be upon the causes of death which were seen repeatedly.
Table V.
Principal factors in deaths
| Patient no. | Age | Drug toxicity* | Died during rejection crisis | Died after rejection crisis | Infection | Organism | Time survival (days) | Other factors in mortality |
|---|---|---|---|---|---|---|---|---|
| 4 | 35 | X | Subphrenic abscess, septicemia, pneumonia, mediastinitis | Staphylococcus, Pseudomonas | 113 | |||
| 5 | 50 | X | X | Mediastinitis, septicemia | Staphylococcus, Pseudomonas | 11 | Cardiac arrest and thoracotomy during transplantation | |
| 7 | 25 | X | In transplant wound, septicemia | Staphylococcus, Pseudomonas | 79 | |||
| 8 | 29 | None | 62 | Cause of death not found | ||||
| 9 | 30 | X | Brain abscesses, septicemia | Pseudomonas, Candida albicans, Candida krusei, Nocardia | 207 | Gastrointestinal hemorrhage | ||
| 11† | 35 | X | X | In old nephrectomy wound | Staphylococcus, Pseudomonas, Proteus | 24 | ||
| 16 | 45 | X | None | 83 | Acute and chronic pancreatitis | |||
| 19 | 17 | X | Pneumonia | Pneumocystis carinii | 95 | |||
| 21 | 41 | X | Brain abscesses, pneumonia | Candida stellatoidea, Escherichia coli | 76 | |||
| 24 | 42 | X | X | Septicemia | Pseudomonas, Proteus | 37 | ||
| 26 | 16 | None | 1 | Died during diuresis of electrolyte imbalance | ||||
| 28 | 49 | X | X | Septicemia, tuberculosis | E. coli Micrococcus tuberculosis | 25 | Jejunal necrosis, pancreatic abscess, pulmonary emboli | |
| 29† | 40 | X | X | Septicemia | Paracolon, E. coli, Proteus mirabilis | 18 | ||
| 31† | 54 | X | X | Pneumonia | Pseudomonas | 15 | Pulmonary embolus | |
| 32† | 20 | X | X | Myocarditis, pyelonephritis, septicemia | Candida albicans, E. coli | 41 | ||
| 35 | 38 | X | X | Pneumonia, septicemia | Pseudomonas | 48 | ||
| 38 | 21 | X | Pneumonia, pulmonary edema | Unknown | 48 | |||
| 43† | 25 | X | X | Disseminated tuberculosis, septicemia | M. tuberculosis, Enterococcus | 48 |
Bone marrow depression.
Posttransplant dialysis required 1 or more times.
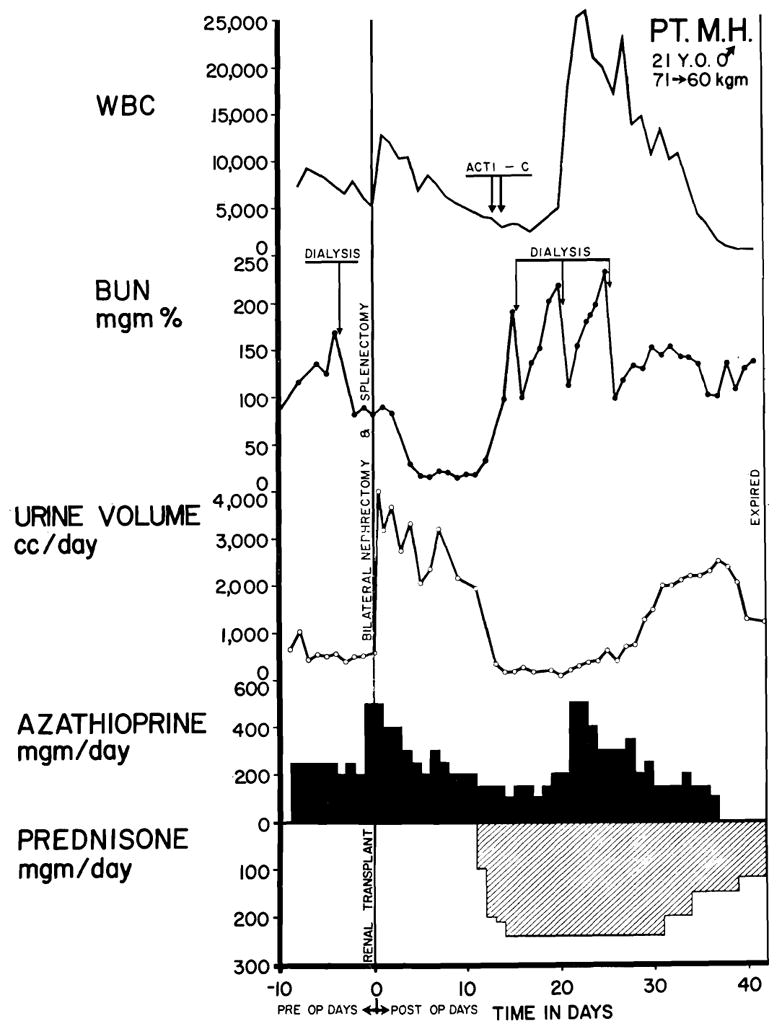
The most frequent cause of death was drug toxicity which occurred during the effort to treat a rejection crisis; 9 patients died of this. The clinical courses of 2 such patients are shown in Figs. 1 and 6. In all but one of the 9 cases, the homografts provided excellent immediate function. The vigor of the subsequent rejection process was frequently greater than usual. In some instances, the disquieting occurrence of decreasing urinary volume, falling creatinine clearance, and mounting azotemia provoked unwise increases in drug dosage to a dangerous level. In others cases however, the therapeutic regimen employed was not thought to be predictably excessive, even upon retrospective analysis.
Fig. 6.
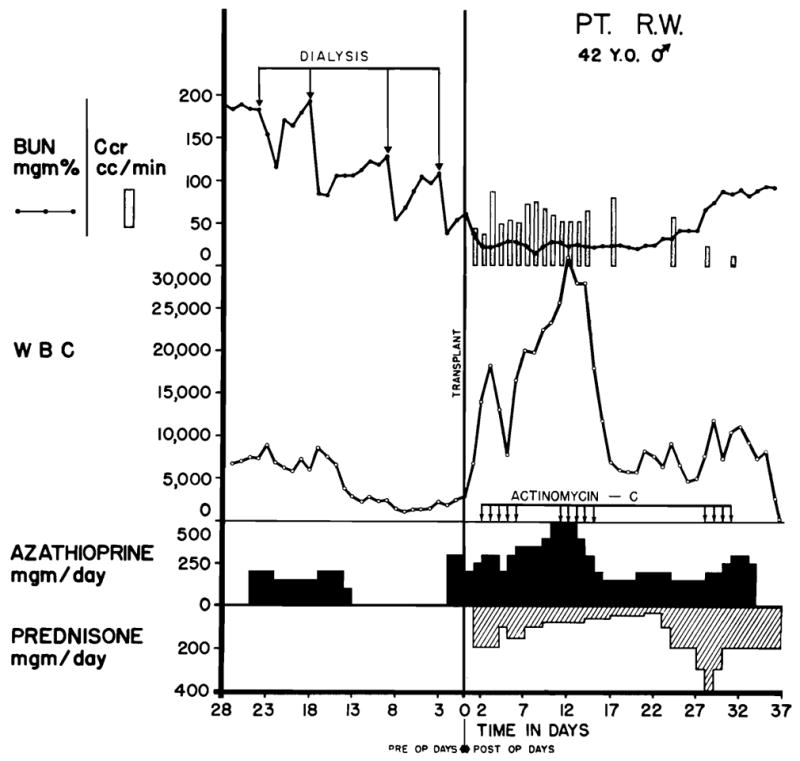
Fatal course of Case No. 24, a blood type A recipient. The donor was a convict volunteer of the same blood type. Note the precipitate drop in the white blood count in the last 2 days of life, a which time thrombocytopenia also developed. The cause of death was septicemia. Each arrow represents 200 gamma actinomycin C.
In such unsuccessfully managed cases, the acute renal failure that occurred during the rejection crisis was accompanied by acute leukopenia (Figs. 1 and 6) and often by thrombocytopenia as if the patient had undergone a sudden change in sensitivity to azathioprine. Within a few hours or a few days, elevation of temperature most always indicated the onset of inexorable deterioration which usually terminated with fatal pneumonia or septicemia or both (Table V). Three patients on whom dialysis was performed when the white blood counts were 3,000 or lower developed septicemia almost immediately after treatment, and all died shortly thereafter. The infecting organisms in such cases are listed in Table V.
There is also evidence that actinomycin C may contribute to bone marrow depression. It has been said that this drug is effective for reversal of rejection in the dose range used without causing leukopenia3; however such a sanguine attitude is not justified. In several cases, a distinct rapid leukopenic and thrombocytopenic response was observed. In one instance, the association of bone marrow depression with administration of actinomycin C was unmistakable because this drug appeared against a background of stable therapy with azathioprine (Fig. 7).
Fig. 7.
Demonstration of leukopenic and thrombocytopenic effect of actinomycin C. Note evidence of sudden bone marrow depression after 5 day course during which a total of 1,200 gamma was administered. After discontinuance of actinomycin C, it was possible to resume the previous dose schedule of azathioprine.
In a second group of 6 patients (Gases 4, 7, 9, 19, 21, and 38), a rejection episode was successfully reversed; however, death occurred 48 to 208 days after operation (Table V). In several of these cases, the ultimately fatal complications were thought to have originated during treatment of the preceding rejection crisis. An example is shown in Fig. 8 of a 25-year-old man (Case 7) who received a homograft from his wife. A ureteral fistula that occurred one week after operation required ureteral reimplantation. After the second operation, the wound apparently healed without infection. However, at the peak of the rejection crisis, fever and toxemia developed as a result of a peritransplant infection. The wound was widely drained. Staphylococcus aureus was found in the tissues, but there was no pus or other evidence of active cellular reaction against the pathogenic organisms. The patient ultimately died of septicemia. Similarly, another patient (Case 4) died almost 4 months after transplantation from a subphrenic abscess which apparently had its inception at the time a laparotomy for intestinal obstruction was performed 3 months previously.
Fig. 8.
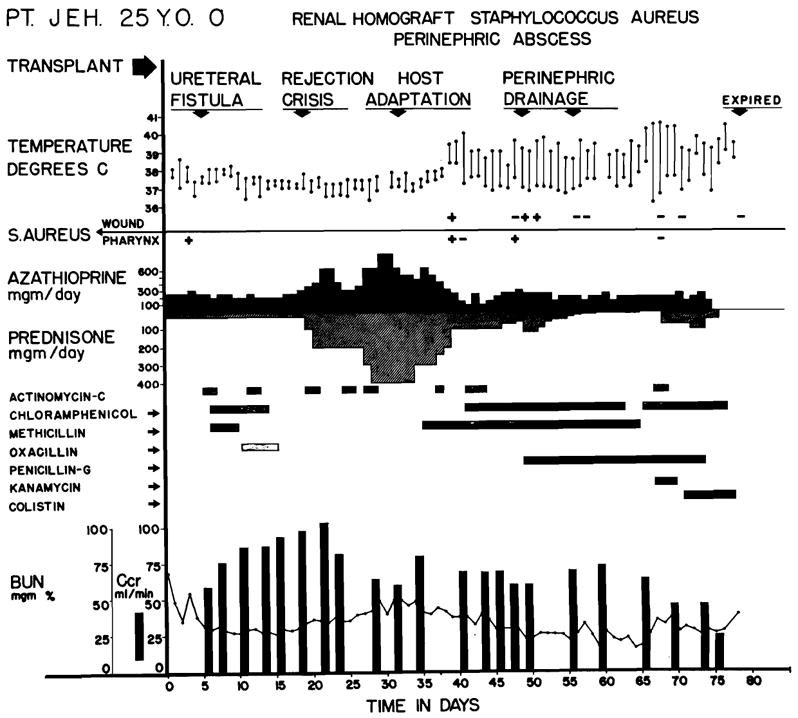
Course of Case No. 7. Fatal wound infection was first noted during late stages of the rejection crisis. Note that fever developed coincidentally with reversal of rejection and was uninfluenced by antibiotics or by surgical drainage. Death was due to septicemia. The simultaneous occurrence of rejection reversal and the development of uncontrollable sepsis suggested that loss of reactivity to bacterial and renal antigens may have occurred simultaneously.
the pathogenic organisms. The patient ultimately died of septicemia. Similarly, another patient (Case 4) died almost 4 months after transplantation from a subphrenic abscess which apparently had its inception at the time a laparotomy for intestinal obstruction was performed 3 months previously.
Of particular interest were 2 cases (Table V) in which death occurred 76 and 208 days after operation. Recovery had seemed to be without serious incident, although both patients had headache, behavioral disturbances, and unexplained neurologic disability. Fungus abscesses of the brain and a mixed fungal and bacterial pneumonia were found at autopsy. These complications probably originated at the time rejection was reversed although they did not become evident until months later.
Two other patients (Cases 19 and 38) died of a peculiar pneumonic process which is described more fully in the following section and which may be of immunologic rather than infectious etiology.
Each of the other 3 deaths was exceptional and cannot be categorized with the two groups described above. One death (Case 26) occurred during a massive postoperative diuresis as the result of electrolyte imbalance. A second patient (Case 8) died with good renal function 62 days after operation. At autopsy, no cause for death was evident. Finally, a 45-year-old woman died 83 days postoperatively of acute and chronic pancreatitis, a complication of steroid therapy that has been described clinically6 and which has been reproduced experimentally in animals.1
The homografts at autopsy
The specimens were reviewed by Dr. K. A. Porter of St. Mary’s Hospital, London, and the following comments are based upon his preliminary analysis as well as that of the University of Colorado Pathology Department. The general architecture of the homografts was comparably intact both in the patients who died at an early time (Fig. 9, A) and those who died later (Fig. 9, B), although evidence of acute tubular damage was more common (Fig. 9, A) in the former group. Some cellular infiltrate was present in all but 3 of the homografts, but this was not extensive in any case and was very minor in most. Blind reading of the sections did not permit ready separation of early from late specimens on the basis of anatomic findings.
Chronic proliferative vascular lesions such as those described by Porter and his associates32 were extremely rare; they were identified, in fact, in only one instance (Case 11). Porter, in reviewing all the pathologic material in this series, has, however, drawn our attention to the possible presence of more acute foci of fibrinoid degeneration in some of the arteries and arterioles which probably represents an early phase of the same process and one which in minimal form may heal without chronic stigmas. Porter has undertaken a detailed study of these more subtle changes in the homografts performed in Colorado employing special histochemical studies, and will report the findings at a later time.
It is worth noting that he did find chronic proliferative arterial lesions in 2 of the 3 cadaveric homografts which were used at this institution, and which are not part of the present study. This observation raised the possibility that the development of an obliterative arteritis may be partially related to the degree of ischemia imposed upon the transferred tissue. Another possibility is that the differences in the prominence of such lesions in different centers may be a measure of the effectiveness of immunosuppressive therapy.
Late noninfectious febrile syndromes
The hyperpyrexia that accompanies a frank rejection crisis and which is almost immediately controlled by the administration of prednisone has already been mentioned. In the absence of sepsis, fever does not subsequently return until the steroid quantities are attenuated to doses of 20 to 60 mg. per day, from 4 to 16 weeks later. In 40 percent of the patients, the return of continuous high fever (103 to 105° F.) is observed at this time; it frequently starts just after the reduction of prednisone from 45 to 30 mg. or 30 to 20 mg. per day. In most cases, there is a protracted concomitant shower of peripheral normoblasts and other immature forms as depicted in Fig. 10. An exhaustive search for microbial complications is always conducted, but an explanation for the febrile illness is not usually found. With maintenance of the steroid dose at the new level, the hyperpyrexia has subsided without treatment in the usual case in 7 days to 4 weeks. Continued withdrawal of steroids has thereafter been possible without further difficulty.
Fig. 10.
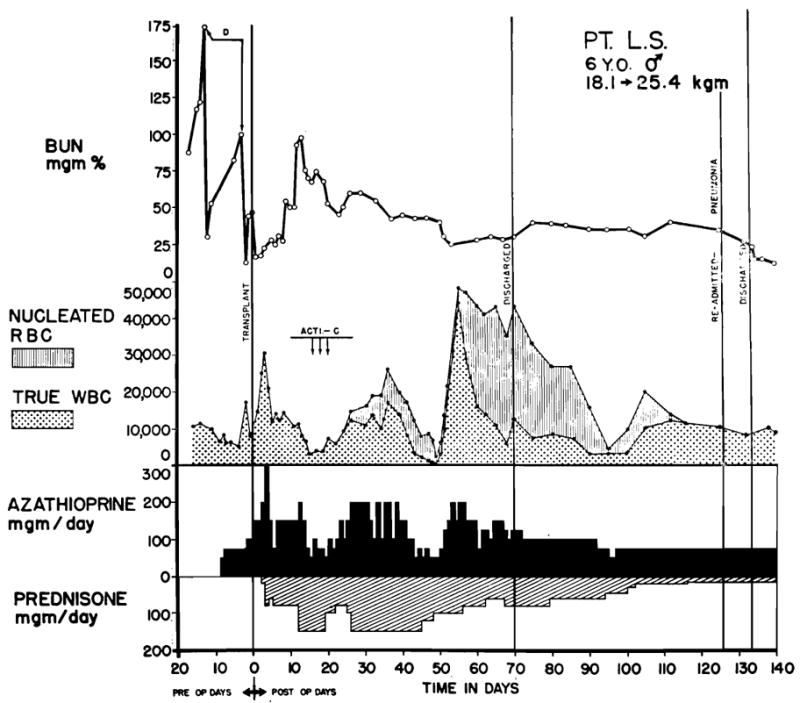
The appearance of nucleated red cells (normoblasts) in the peripheral blood after transplantation to a 6-year-old child. The child was O+ blood type. The maternal donor was A+. Note the high ratio of normoblasts to leukocytes. Drug dosages should be based upon the corrected rather than the falsely high peripheral count. Juvenile white cell forms are also frequently found at such times, although usually in smaller numbers. These changes are most marked in children, and are commonly associated with hyperpyrexia.
In 6 exceptional patients, 3 of whom were children 3 to 8 years of age who were treated with homografts from adults, the late fever was associated with diffuse symmetrical bilateral pulmonary infiltrates which had a parahilar and lower lobar distribution (Fig. 11). Five patients became cyanotic; arterial O2 saturation was as low as 43 percent. Arterial pCO2 was normal or slightly elevated. In each case, administration of nasal oxygen markedly increased arterial oxygen saturation. The condition of an 8-year-old child whose dose of prednisone had just been reduced to 20 mg. per day, was immediately made markedly worse with a further decrease to 10 mg. per day for 2 days. Resumption of the original steroid regimen resulted in equally rapid improvement.
Fig. 11.
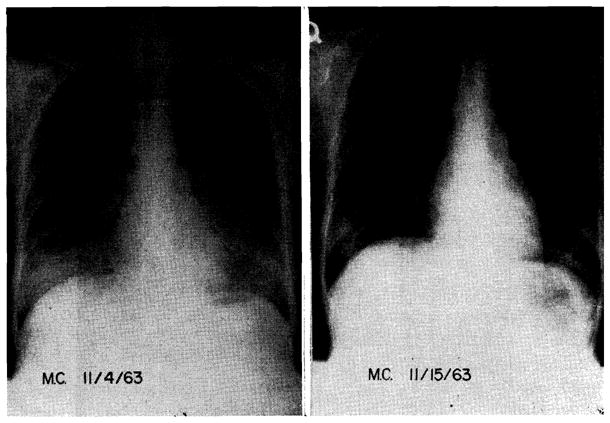
Diffuse bilateral pulmonary infiltrates in a 20-year-old girl of A+ blood type who received a kidney homograft from a convict donor of the same blood group. Note rapid resolution of the extensive infiltrations which first appeared 2 months after operation. Transplantation was performed on Sept. 3, 1963. After the near fatal pulmonary complication, the subsequent convalescence was without complication.
The etiology of the pulmonary lesions is obscure. Elevated heterophil or cold agglutinin titers and suspicious cells on LE preparations were each noted in one or more but not in all cases. The showers of peripheral normoblasts described above were present. One patient died of pulmonary insufficiency. At postmortem, there was extensive consolidation of the lungs. Numerous intra-nuclear inclusion bodies were found in the alveolar lining walls. Other pathologic features of this case were suggestive of Pneumocystis carinii. Nevertheless, the association of the infiltrates with steroid withdrawal, the improvement noted in one case with increased doses of prednisone, and the ultimate sudden disappearance of the abnormalities in those patients who lived all suggest the possibility that the lesions have an immunologic rather than an infectious basis. It is possible that the death described above could have been prevented if the prednisone dose had been increased.
DISCUSSION
As has been made clear, the convalescence of patients who have received a renal homo-transplant is not an easy one. The events of rejection and the therapy necessary to prevent destruction of the homograft impose a period of great risk which lasts for several months after operation. It has been pointed out that patients receiving kidneys from familial donors have a greater chance of surviving this dangerous interval than those whose kidneys have come from genetically unrelated donors.
Although there are differences in the risk to certain classes of recipients as noted in the present study, the steps to recovery are qualitatively similar in almost every case. The variations are only in degree. Thus, in maternal-to-offspring transplants, the crisis is usually temperate and more easily treated, but it is otherwise like that encountered with less favorable genetic combinations. In all groups, moreover, most of the deaths and complications have either occurred in this early postoperative period or the events leading to later morbidity or mortality have apparently had their inception at this time.
In view of the intensity of the immunosuppressive therapy necessary for control and reversal of the rejection crisis, it is not difficult to appreciate the inherent danger of this phase of recovery. The fact that homograft function can be retained at all is perhaps the strongest indication of the extent to which total body immunologic defenses have been weakened. In addition, loss of reactivity to various test antigens,19 the inconstant development of hypogammaglobulinemia,33 and the appearance of steroid diabetes are all indicators of the host’s imperiled state. Rifkind33 has pointed out that isolation procedures are of limited value, since most infections noted during the rejection crisis have originated from endogenous sources.
Hope for long survival with the drug combinations and the dosages used during the rejection crisis would be remote indeed if such stringent measures were permanently required for protection of the homograft. Fortunately, it has become increasingly clear, from various lines of evidence, that a state of homograft tolerance is an integral accompaniment of the successfully treated rejection crisis.14, 18, 23, 24, 21, 47–49 It is probable, however, that the development of tolerance is a mixed blessing. It would be surprising if immunologic memory for antigens other than those of the kidney was not also eliminated during reversal of the rejection crisis. Thus, pockets of pathogenic bacteria could flourish, unchecked by defenses which no longer recognize them as inimical (Fig. 12). This concept has already received strong experimental support from the studies of Forsen and Condie.10 Examples have been cited in the present reports in which such a lethal chain of events has apparently occurred (Fig. 8). It will be of great practical as well as theoretical interest to determine, in future investigations, whether the commonly observed complications caused by infections are due simply to the suppression of greatly weakened but balanced host defenses, or if there is the establishment of relatively specific clonal deficiencies which permit selective overgrowth of certain organisms.
Fig. 12.
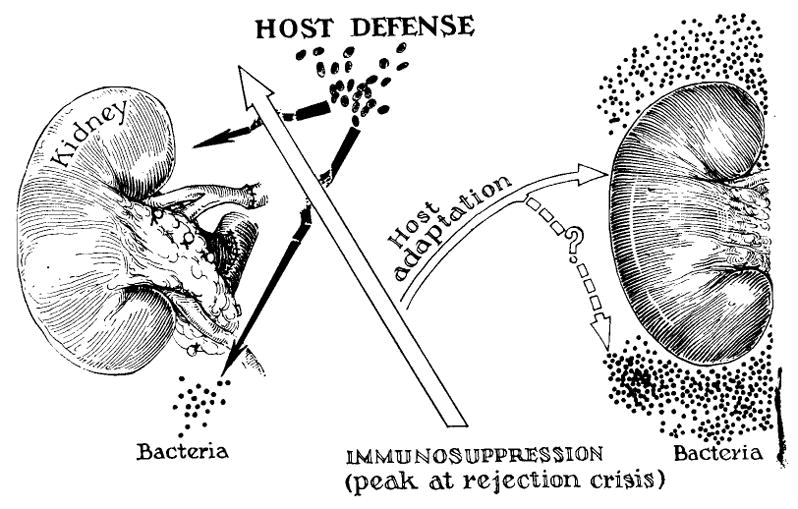
Possible mechanism of simultaneous loss of host reactivity to specific strains of endogenous bacteria, and to the alien renal tissue. See text.
The aforementioned factors may be present to some degree in every case. Nevertheless, it was commonly the additional components of bone marrow depression and leukopenia which appeared to precipitate the beginning of a fatal infection. Although all such cases must be classified as errors in the control of azathioprine dosage, it should be emphasized that the effect of this agent becomes highly unpredictable in the presence of the poor renal function which accompanies a rejection crisis, possibly because azathioprine has a partial renal pathway of detoxification.9 Whether this is the entire explanation is not certain, although it seems clear that the leukopenic effect of this drug is intensified at the time of acute renal failure. Thus, a patient with an unusually severe rejection crisis is most vulnerable to toxicity, despite the fact that this is the kind of case in which exact therapy with azathioprine is most needed. Consequently, the rejection crisis has often seemed to be the initiating influence in the genesis of fatal drug toxicity. It might be reasoned that aggressive dialysis therapy under such circumstances would be remediable. In practice, however, the institution of hemodialysis at this time has, on at least 3 occasions, seemed to be the direct cause of septicemia.
Inasmuch as the greatest risk is during the period of rejection crisis, future attempts must be directed to improved management during this interval. It is probable that more specific pharmacologic agents will eventually become available. At present, attention will have to be focused upon more effective manipulation of the existing agents.
In most of the 45 reported cases, it was attempted to use only one drug, azathioprine, in the early postoperative period, reserving emergency administration of prednisone and actinomycin C for the specific indications of rejection. There were several reasons for this practice. First, it was desirable to obtain information concerning the efficacy of administering azathioprine without the superimposition of other pharmacologic factors. Second, the benefit of the secondary drugs could be assessed only in the situation in which primary therapy with azathioprine had already proved inadequate. Third, only by the graded use of drugs was it possible to demonstrate clearly the presence and timing, first of rejection and then of adaptation. Fourth, it was not at first evident that adaptation would occur if efforts to prevent the rejection crisis were completely successful. Finally, the use of potentially dangerous doses of steroids for periods of time in the early convalescent phase when they did not appear to be needed, did not seem to be warranted. The last argument seemed especially pertinent inasmuch as the time of onset of the rejection crisis was so variable, and because occasionally a patient did not require any treatment with steroids.
It is evident that many of the most forceful reasons for withholding steroid therapy until graft repudiation had clearly begun were for the purpose of delineating the features of rejection and adaptation, and defining the influence of drug therapy upon these processes. From a clinical point of view, this information is now nearly complete, as has been described in this and other publications.39, 40
It has now become desirable to test systematically an alternative use of steroids, employing prednisone prophylactically in the attempt either to prevent altogether or to attenuate the rejection crisis. The potential advantages of this approach are clear. If profound renal failure could be prevented, the loss of predictability of the effect of azathioprine could be avoided, and it would be possible to use this agent more effectively. If mitigation of the rejection process can be demonstrated with this approach, it is conceivable that the total steroid dose would also be lessened since reduction of the prednisone dose could be started at an earlier time.
Based upon these considerations, the protocol of management was changed at the University of Colorado Medical Center beginning Dec. 15, 1963. In addition to azathioprine, all recipients are now pre-treated for 2 days with 100 to 200 mg. prednisone, a dose which is continued into the immediate postoperative period. That the change may have merit has already been suggested by the studies of Hume,18 in which both the frequency and vigor of rejection episodes have seemed to be reduced with the employment of a combination of immunosuppressive agents. It must be emphasized that the value of this alteration in management is highly speculative at present, and that the demonstration of any benefit will have to wait further study.
SUMMARY
Forty-five patients were treated with renal homotransplantation 15 to 2½ months ago. Twenty-seven are alive with adequate renal function after an average postoperative interval of 190.0 days. The other 18 died after an average period of 58 days.
Several factors appeared to have influenced the outcome; the most striking was the presence or absence of donor-recipient relationship. Best results were obtained in parent-to-offspring donations, next with sibling transfers, and poorest with transfers from genetically unrelated donors. Patients in the older age groups had a higher risk but this may have been partially due to an increased use of transfers from unrelated donors in such cases. Inexplicably, there appeared to be poorer results in recipients who had type A blood even when kidneys were from donors with the same blood type.
The immunosuppressive regimen used for this series included adjuvant splenectomy and preoperative treatment with azathioprine for 8 to 10 days. Direct evidence for the value of splenectomy is lacking and the rationale for its continued use is indirect. In contrast, the usefulness of pretreatment with azathioprine is supported by evidence from canine transplantation experiments.
Most of the patients who were treated successfully passed through a rejection crisis at which time the immunosuppressive therapy was intensified. After the patients recovered from such episodes, the drug doses required for continued preservation of homograft function became less stringent, as if a state of homograft tolerance had been established. The preponderance of deaths either occurred during this period of maximum immunosuppressive risk or at a later time as a result of complications which had originated during a rejection. Inferential evidence has been presented that some of the delayed lethal complications caused by infections may have been due to the development of clonal deficiencies to certain bacterial antigens which resulted in later unrestrained growth of specific organisms.
Since the events of rejection crises are the most important factors in causing mortality, alternative methods of immunosuppression have been investigated which are designed to temper the vigor of the immunologic assault at this time. For this purpose, the most significant alteration to be instituted in our study will be the use of prophylactic prednisone which will be started in the preoperative period. The value of this change in protocol is still highly speculative.
Several new observations are recorded concerning delayed host reactions which occur after the immediate threat of rejection is past. These include the development of a delayed febrile syndrome during steroid withdrawal, which is often associated with diffuse pulmonary lesions and the appearance of abnormal peripheral blood smears.
In addition, physiologic data are presented which support the view that the rejection crisis has an ischemic factor as an important component. A pattern of deteriorating renal function can be identified in most patients during rejection which simulates that of a severe Goldblatt lesion. The effect is presumably due to generalized hemodynamic alterations of the homograft vascular tree. That such alterations may be functional and reversible is suggested by the paucity, in homografts recovered in the postoperative period, of chronic proliferative vascular lesions such as those described by Porter.
Acknowledgments
Aided by United States Public Health Service Grants A-6283, A-6344, HE-07735, AM-07772, AI-04152 and OG-27.
Footnotes
Presented at the Twenty-fifth Annual Meeting of the Society of University Surgeons, Los Angeles, Calif., Feb. 13–15, 1964.
References
- 1.Bencosme SA, Lazarus SS. Pancreas of cortisone treated rabbits. A M A Arch Path. 1956;62:285. [PubMed] [Google Scholar]
- 2.Calne RY. Renal transplantation. Baltimore: Williams & Wilkins Company; 1963. [Google Scholar]
- 3.Calne RY, Alexandre GPJ, Murray JE. A study of the effects of drugs in prolonging survival of homologous renal transplants in dogs. Ann New York Acad Sc. 1962;99:743. doi: 10.1111/j.1749-6632.1962.tb45358.x. [DOI] [PubMed] [Google Scholar]
- 4.Calne RY, MacGillivray JB, Loughridge LW, Zilva JF, Levi AJ. Renal transplantation in man. A report of five cases, using cadaveric donors. Brit M J. 1963;2:467. [PMC free article] [PubMed] [Google Scholar]
- 5.Calne RY, Murray JE. Inhibition of the rejection of renal homografts in dogs by Burroughs Wellcome 57-322, S. Forum. 1961;12:118. [PubMed] [Google Scholar]
- 6.Carone FA, Liebow AA. Acute pancreatic lesions in patients treated with ACTH and adrenal corticoids. New England J Med. 1957;257:690. doi: 10.1056/NEJM195710102571502. [DOI] [PubMed] [Google Scholar]
- 7.Connor TB, Berthrong M, Thomas WC, Jr, Howard JE. Hypertension due to unilateral renal disease: With a report on a functional test useful in diagnosis. Bull Johns Hopkins Hosp. 1957;100:241. [PubMed] [Google Scholar]
- 8.Dempster WJ. Kidney homotransplantation. Brit J Surg. 1953;40:447. doi: 10.1002/bjs.18004016309. [DOI] [PubMed] [Google Scholar]
- 9.Elion GB, Callahan S, Bieber S, Hitchings GH, Rundles RW. A summary of investigations with 6-[(1-methyl-4-nitro-5-imidazolyl) thio] purine (B. W. 57-322) Cancer Chemotherapy Reports. 1961 Oct;(14):93. [Google Scholar]
- 10.Forsen NR, Condie RM. Abolition of immunologic memory with 6-mercaptopurine (6-MP), Fed. Proc. 1963;22:500. [Google Scholar]
- 11.Goodwin WE, Kaufman JJ, Mims NM, Turner RD, Glassock R, Goldman R, Maxwell MM. Human renal transplantation. I Clinical experience with 6 cases of renal homotransplantation. J Urol. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 12.Gunderson CH, Juras D, LaVia MF, Wissler RW. Tissue and cellular change associated with antibody formation in the rat spleen. J A M A. 1962;180:1038. [PubMed] [Google Scholar]
- 13.Hamburger J, Crosnier J, Dormont J. A new secondary disease observed in patients with a well tolerated homotransplanted kidney. Presented at the Sixth International Transplantation Conference; New York. Feb 8, 1964. [DOI] [PubMed] [Google Scholar]
- 14.Hamburger J, Vaysse J, Crosnier J, Auvert J, Lalanne CM, Hopper J., Jr Renal homotransplantation in man after radiation of the recipient: Experience with 6 cases since 1959. Am J Med. 1962;32:854. doi: 10.1016/0002-9343(62)90032-3. [DOI] [PubMed] [Google Scholar]
- 15.Hektoen L. Further observations on effects of roentgenization and splenectomy on antibody formation. J Infect Dis. 1920;27:23. [Google Scholar]
- 16.Hogman CF. Blood group antigens A and B determined by means of mixed agglutination on cultured cells of human fetal kidney, liver, spleen, lung, heart and skin. Vox Sang. 1959;4:12. doi: 10.1111/j.1423-0410.1959.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 17.Hume DH, Magee JH, Kauffman HM, Jr, Rittenbury MS, Prout GR., Jr Renal homotransplantation in man in modified recipients. Ann Surg. 1963;158:608. doi: 10.1097/00000658-196310000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hume DM, Magee JH, Prout GR, Jr, Kauffman HM, Jr, Cleveland RH, Bower JD, Lee HM, Kramer N. Studies of renal transplantation in man. Presented at the Sixth International Transplantation Conference; New York. Feb 8, 1964. [Google Scholar]
- 19.Kirkpatrick CH, Wilson WEC, Talmage DW. Immunologic studies in human organ transplantation. I. Observation and characterization of suppressed cutaneous reactivity in uremia. J Exper Med. doi: 10.1084/jem.119.5.727. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Küss R, Teinturier J, Milliez P. Some notes on renal homotransplantation in man. Mém Acad chir. 1951;77:755. [PubMed] [Google Scholar]
- 21.Marchioro TL, Axtell HK, LaVia MF, Waddell WR, Starzl TE. The role of adrenocortical steroids in reversing established homograft rejection. Surgery. 1964;55:412. [PMC free article] [PubMed] [Google Scholar]
- 22.Marchioro TL, Brittain RS, Holmes JH, Hermann G, Waddell WR, Starzl TE. The use of living donors for renal homotransplantation. A M A Arch Surg. 1964;88:711. doi: 10.1001/archsurg.1964.01310220201031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGavick JD, Knight PR, Tomkiewicz ZM, Alexandre GPJ, Murray JE. Analysis of mechanisms of drug induced tolerance in canine renal homotransplants. S Forum. 1963;14:210. [PubMed] [Google Scholar]
- 24.Merrill JP, Murray JE, Takacs FJ, Hager EB, Wilson RE, Dammin GJ. Successful transplantation of kidney from a human cadaver. J A M A. 1963;185:347. doi: 10.1001/jama.1963.03060050025015. [DOI] [PubMed] [Google Scholar]
- 25.Miller JFAP. Immunological significance of the thymus of the adult mouse. Nature. 1962;195:1318. [Google Scholar]
- 26.Miller JFAP. Immunity and the thymus. Lancet. 1963;2:43. [Google Scholar]
- 27.Mollison PL. Blood transfusion in clinical medicine. Oxford: Blackwell Scientific Publications; 1951. [Google Scholar]
- 28.Murray JE, Harrison JH. Management of 50 patients with kidney transplants including 18 pairs of twins. Am J Surg. 1963;105:205. doi: 10.1016/0002-9610(63)90292-7. [DOI] [PubMed] [Google Scholar]
- 29.Murray JE, Merrill JP, Dammin GJ, Dealy JB, Alexandre GW, Harrison JH. Kidney transplantation in modified recipients. Ann Surg. 1962;156:337. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts with immunosuppressive drug therapy. New England J Med. 1963;268:1315. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 31.Pierce JC, Varco RL. Effects of long-term 6-mercaptopurine treatment upon kidney homotransplants in dogs. Surgery. 1963;54:124. [PubMed] [Google Scholar]
- 32.Porter KA, Owen J, Mowbray JF, Thomson WB, Kenyon JR, Peart WS. Obliterative vascular changes in four human kidney homotransplants. Brit M J. 1963;2:468. doi: 10.1136/bmj.2.5358.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rifkind D, Marchioro TL, Waddell WR, Starzl TE. Infectious diseases associated with renal homotransplantation. I. Incidence, types, and predisposing factors. J A M A. doi: 10.1001/jama.1964.03070060007001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rifkind D, Marchioro TL, Waddell WR, Starzl TE. Infectious diseases associated with renal homotransplantation. II. Differential diagnosis and management. J A M A. doi: 10.1001/jama.1964.03070060007001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowley DA. The formation of circulating antibodies in the splenectomized human being following intravenous injection of heterologous erythrocytes. J Immunol. 1950;65:515. [PubMed] [Google Scholar]
- 36.Simonsen M, Buemann J, Gammeltoft A, Jensen F, Jorgenson K. Biological incompatibility in kidney transplantation in dogs. I Experimental and morphologic investigations. Acta path, et microbiol scandinav. 1953;32:1. doi: 10.1111/j.1699-0463.1953.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 37.Stamey TA. The diagnosis of curable unilateral renal hypertension by ureteral catheterization. Postgrad M J. 1961 May; [Google Scholar]
- 38.Starzl TE, Marchioro TL, Talmage DW, Waddell WR. Splenectomy and thymectomy in human renal homotransplantation. Proc Soc Exper Biol & Med. 1963;113:929. doi: 10.3181/00379727-113-28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynec & Obst. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 40.Starzl TE, Marchioro TL, Holmes JH, Brittain RS, Waddell WR. Clinical problems in renal homotransplantation. J A M A. 1964;187:734. doi: 10.1001/jama.1964.03060230062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE, Marchioro TL, Holmes JH, Waddell WR. The incidence, cause, and significance of immediate and delayed oliguria or anuria after human renal transplantation. Surg Gynec & Obst. 1964;188:819. [PMC free article] [PubMed] [Google Scholar]
- 42.Starzl TE, Marchioro TL, Rifkind D, Dickinson TC, Waddell WR. The importance of technical factors in renal homotransplantation: Experiences with 40 cases. A M A Arch Surg. doi: 10.1001/archsurg.1964.01320010089009. In press. [DOI] [PubMed] [Google Scholar]
- 43.Starzl TE, Marchioro TL, Hermann G, Brittain RS, Waddell WR. Renal homografts in patients with major donor-recipient blood group incompatibilities. Surgery. 1964;55:195. [PMC free article] [PubMed] [Google Scholar]
- 44.Starzl TE, Marchioro TL, Morgan WW, Waddell WR. A technique for use of adult renal homografts in children. Surg Gynec & Obst. In press. [PMC free article] [PubMed] [Google Scholar]
- 45.Szulman AE. The histological distribution of the blood group substances A and B in man. J Exper Med. 1960;111:785. doi: 10.1084/jem.111.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wissler RW, Fitch FW, LaVia MF, Gunderson CH. The cellular basis for antibody formation. Symposium on antibodies: their production and mechanism of action. J Cell & Comp Physiol. 1957;50:260. doi: 10.1002/jcp.1030500417. [DOI] [PubMed] [Google Scholar]
- 47.Woodruff MFA. The “critical period” of homografts. Transplantation Bull. 1954;1:221. [Google Scholar]
- 48.Woodruff MFA, Robson JS, Nolan B, Lambie A, Wilson TI, Clark JG. Homotransplantation of kidney. Lancet. 1963 Sept 28;2:675. doi: 10.1016/s0140-6736(63)90465-3. [DOI] [PubMed] [Google Scholar]
- 49.Zukoski CF, Callaway JM. Adult tolerance induced by 6-methylmercaptopurine to a canine renal homograft. Nature. 1963;198:706. doi: 10.1038/198706a0. [DOI] [PubMed] [Google Scholar]