In this paper, “hyperacute rejection” of renal homografts will be discussed on the basis of information obtained in hypersensitized dogs. Attention will be focused upon three specific topics: (1) the variety of humoral antibodies that may be produced in the course of sensitizing a recipient to donor tissue; (2) the coagulation changes that can be produced within a homograft or systemically by transplantation to a pre-sensitized recipient; (3) the mitigation of hyperacute rejection that may be obtained by transplantation of consecutive organs from the same donor.
METHODS
Pairs of adult mongrel dogs were sensitized to common canine donors by the repeated orthotopic transplantation (5 to 8 times, mean 6) of 2 circular pieces of full-thickness skin, 3 to 4 cm. in diameter. The successive skin grafts were placed within a few days after the preceding grafts were rejected. The mean times before necrosis was evident of the first and last skin transplants, respectively, were 8.5 and 2 days. The mean time for completion of sensitization was 48 days.
Organs were transplanted from the same donor an average of 7 days after the last exposure to skin, under two general circumstances. In control experiments, a donor kidney was inserted by the standard intra-abdominal technique. In conditioning experiments, the kidney was similarly transplanted to specifically sensitized animals but only after the donor’s liver, spleen or other kidney was first revascularized in the recipient for 51 to 240 (mean, 120) minutes. The conditioning organs were anastomosed to vessels of the neck or abdomen. When the liver was used, only the hepatic arterial supply was restored.
All but 3 recipients had bilateral nephrectomy. The homograft ureter was drained by a cutaneous ureterostomy and urine flow was observed 2 or 3 times daily until the development of anuria which was taken as the time of rejection. All dogs were given 500 to 1,500 ml. intravenous fluids intraoperatively and 500 ml. daily thereafter.
Systemic blood samples were obtained before and during sensitization with skin and before and after whole organ transplantation. From these bloods were determined the hematocrit, total white blood cell and differential counts, and platelet counts.2 Sera were analyzed for: (1) isohemagglutinins against donor red cells; (2) heterohemagglutinins against sheep red cells; (3) anti-donor leukoagglutinins23; (4) antidonor lymphocytotoxins determined in the presence of pooled male dog complement.21
In 15 special experiments, arteriovenous differences of the aforementioned formed blood elements and antibodies were repetitively measured across kidneys, spleens, or livers that had been transplanted to pre-sensitized recipients or alternatively, to normal dogs. In addition, the following clotting tests and assays were performed in citrated, platelet-poor plasma: euglobulin lysis time3; thrombin time with 3 units per milliliter thrombin; prothrombin time with rabbit brain thromboplastin; partial thromboplastin time with kaolin15; fibrinogen16; prothrombin (factor II) 13; Ac-globulin (factor V)6; antihemophilic globulin (factor VIII)14; and plasma thromboplastin component (factor IX).8 Fibrin split products were quantitatively measured in thrombinized Trasylol containing serum according to the method of Claman and Merrill4 except that rabbit antidog–fibrinogen antisera were employed instead of anti–immunoglobulin sera.
In the experiments for which special pathologic studies were planned, fresh tissues were fixed with formalin or snap frozen on dry ice. The snap frozen tissue was studied for the presence of dog IgG, B1C globulin and fibrinogen by a direct immunofluorescent method which will be fully described elsewhere.26
RESULTS
Humoral antibodies with sensitization
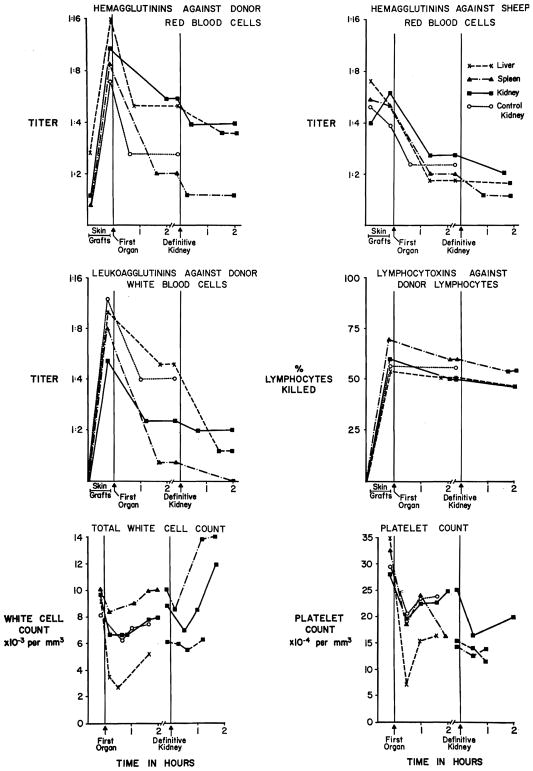
Before skin grafting, 13 of 40 dogs (32.5 percent) had isoagglutinins against donor red cells with titers of 1:2 to 1:16 (mean 1:5). After sensitization, isoagglutinins became detectable (titers 1:2 to 1:64, mean, 1:9) in 32 dogs (78 percent) (Fig. 1). Another kind of hemagglutinin directed against sheep red cells was initially present in 39 (97.5 percent) of the animals at titers of 1:2 to 1:16 (mean, 1:6). The incidence and titers of the heterohemagglutinins were not changed by the sensitization (Fig. 1).
Fig. 1.
Changes in humoral antibodies, peripheral white cell counts, and platelet counts during the period of multiple skin grafting and at the time of a first and second whole organ transplantation from the same donor. Codes for the different kinds of first organs (kidney, spleen, and liver) are identified in the upper right graph. All second organs were kidneys. See text for details.
Before skin grafting, lymphocytotoxins were never detectable and leukoagglutinins were found in only one of 40 dogs. After sensitization, 22 of 40 animals (54 percent) developed leukoagglutinins in titers of 1:2 to 1:64 (mean, 1:8), and 28 of 34 tested (82 percent) had lymphocytotoxins (Fig. 1).
Depletion of antibodies by homografts
When the sensitized recipients were exposed to 24 kidneys, 10 livers, and 7 spleens of their skin donors, there was a fall of all the measured antibodies (Fig. 1), with the lymphocytotoxins least reduced. None of the three organs appeared to be a superior antibody remover in comparison with the others.
In 21 of the foregoing recipients, kidneys were placed after the first organs had been removed. The residual leukoagglutinins were almost totally eliminated, the two kinds of hemagglutinins were further reduced, and as with the first organ homotransplantation the lymphocytotoxins were only minimally reduced (Fig. 1).
Absorption of the antibodies by the whole organ homografts was proved in 12 special experiments in which arteriovenous gradients were obtained across the transplants. Extraction was evident of isoagglutinins, heterohemagglutinins, and leukoagglutinins. However, the lymphocytotoxins had much less significant arteriovenous differences.
Depletion of the heterohemagglutinins by the homografts was unexpected. Consequently absorption of this antibody by canine red cells and canine renal cells was tested. Only the renal cells absorbed these agglutinins.
Hematologic changes
In all 40 sensitized recipients, the numbers of peripheral platelets and white blood cells fell within a few minutes after whole organ transplantation and then tended to return toward but not to pre-existing levels (Fig. 1). Liver transplants caused more severe and lasting depressions than spleens and kidneys (Fig. 1). When kidneys were transplanted secondarily after removal of a first organ, there were again declines in the platelet and white cell counts (Fig. 1). Comparable fluctuations in the hematocrit did not occur.
In the 12 experiments in which homograft arteriovenous gradients were obtained, platelets and white cells in the venous effluent fell 66 and 57 percent, respectively, from the arterial values within one minute following revascularization (Table I). The extensiveness of the clearance was indicated by arterial leukopenia and thrombocytopenia (Table I), 20 to 40 minutes later. In three other experiments in which a kidney, spleen, or liver was transplanted to nonsensitized dogs, venous platelets and white cells fell 32 and 41 percent, respectively, from the arterial values. However, the sequestration was so transient that the platelets and white cells in the systemic arterial blood fell detectably in only one of the three experiments.
Table I.
Maximum arterial changes in formed blood elements and in coagulation tests after the transplantation to sensitized recipients of 12 primary homografts (3 livers, 2 spleens, and 7 kidneys) and after the subsequent transplantation of 7 kidney homografts. By also repetitively analysing the venous effluent, the maximum arteriovenous (A–V) gradients were established across the various organs
| Platelet count (per mm.3) | Leucocyte count (per mm.3) | Fibrinogen (mg. %) | Factor* II (% normal) | Factor* V (% normal) | Factor* VIII (% normal) | Factor* IX (% normal) | Thrombin time (sec.) | Prothrombin time (sec.) | Partial thromboplastin time (sec.) | Euglobulin lysis time (min.) | Fibrin split products | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average maximum change in venous effluent compared to arterial blood† | ||||||||||||
| Liver | −420,000 | −8,577 | −194 | −47 | −44 | −70 | −48 | +40 | +5.4 | +9.3 | ||
| Spleen | −190,000 | −5,233 | −110 | −15 | −8.5 | −76 | −31 | +3.8 | +4.1 | +3.0 | ||
| First kidney | −116,000 | −4,536 | −71 | −10.5 | −15 | −19 | −44 | +3.3 | +0.3 | +1.7 | ||
| Second kidney | −89,000 | −5,325 | −46 | −9.5 | −9.0 | −15 | −19 | +4.5 | +1.7 | +2.2 | ||
| Maximum changes in arterial blood† | ||||||||||||
| Liver | −358,000 | −9,011 | −257 | −61 | −82 | −76 | −38 | +7.5 | +3.2 | +14.2 | −49 | ++ |
| Spleen | −105,000 | −2,996 | −83 | −20.5 | −25.5 | −31 | −55 | +1.4 | +2.2 | +3.5 | −50 | + |
| First kidney | −49,000 | −1,735 | −59 | −12 | −18.7 | −27.5 | −29.2 | +2.3 | +0.3 | +2.8 | −22 | + |
| Second kidney | −76,000 | −4,348 | −34 | −8.5 | −10.1 | −7 | −16 | +1.8 | +1.4 | +4.3 | −16 | + |
Normal (100 percent) activity was defined as that present in canine plasma pooled from 8 healthy donors. The figures given are the mean changes in percentage activity.
The maximum arteriovenous gradients almost invariably developed within 1 to 5 minutes and then became progressively smaller with the passage of time. However, there were continuing changes in the systemic blood so that the maximum alterations in the aortic blood did not evolve until 20 to 40 minutes after the most extreme gradients.
Coagulation changes
In the 12 sensitized dogs in which arterial and venous samples were obtained across primary homografts (7 renal, 2 splenic, and 3 hepatic) there was always a major consumption of all the measured clotting factors within a few minutes, to the greatest degree with the livers (Table I). In addition, prolongation of the thrombin time (10 of 12 experiments), shortening of the euglobulin lysis time (6/6), and the appearance of fibrin split products (4/6) indicated fibrinolysis. The arteriovenous gradients tended to be eliminated with time but when new kidneys were then transplanted, consumption was seen again in these second organs (Table I). The changes in the systemic aortic blood reflected those measured across the homografts but occurred later, usually at about 30 minutes. In 9 of the 12 animals the coagulation tests of systemic blood returned essentially to normal. However, the other 3 dogs continued to have abnormalities that were too great to be explained solely by consumption within the homograft and in fact these alterations persisted and became worse even after the organs were removed. In 2 of the latter 3 animals the titers of leukoagglutinins or lymphocytotoxins or both were unusually high.
When 3 nonsensitized dogs were given a renal, splenic, or hepatic transplant, consumption of clotting factors and fibrinolysis occurred within the grafts. However, the gradient changes were relatively minor and of short duration. Moreover, abnormalities in the aortic blood became detectable only in the liver experiment.
Effect of repetitive grafting
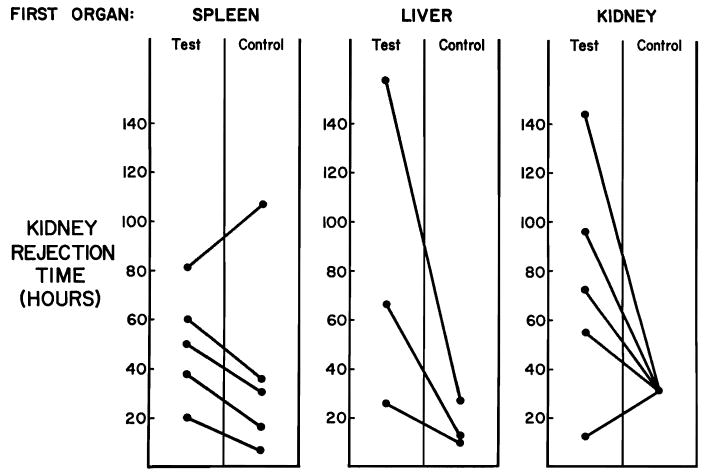
There were 8 experiments in which a renal homograft was placed after the removal of a conditioning spleen (5 examples) or liver (3 examples). All but one of these kidneys functioned for longer than the other paired donor kidneys which were transplanted to sensitized but unmodified control recipients (Fig. 2).
Fig. 2.
Functional times of kidney homografts after transplantation to sensitized unmodified recipients (control) compared to the functional intervals when kidneys were inserted into comparably sensitized recipients but as second organs following spleens, livers or kidneys. A description of the control and test animals is in the text.
In 5 other experiments, the conditioning organ was the first donor kidney which was revascularized in the recipient for 51 to 240 minutes and removed. The other kidney was then placed. Urine excretion occurred from the second kidney for a mean of 75.8 hours. In 4 of the 5 individual animals the duration of function was longer than the average in the 8 control experiments of the preceding paragraph.
The fate of kidney homografts either primarily placed or transplanted as second organs could not be significantly correlated to titers of any special kind of antibody measured at the time of revascularization.
Immunofluorescent and histologic studies
Immmunofluorescent and histologic studies were made on ten conditioning organs (4 kidneys, 3 spleens, and 3 livers) at the time of their removal. Minimal amounts of IgG and B1C were present in all organs in a diffuse interstitial distribution without any significant anatomic concentrations. Fibrin was present in focal deposits randomly distributed in the spleens. Lesser amounts were seen concentrated about the hepatic arteries in the liver and in the peritubular capillary areas of the kidneys. Light fibrin deposits were seen along glomerular capillary walls in three of four conditioning kidneys. Routine histologic examination revealed moderate numbers of polymorphonuclear (PMN) leukocytes in glomeruli of the kidney examined earliest following transplantation. A few PMN leukocytes were seen in the glomeruli of the remaining kidneys and in the parenchyma of the livers and spleens. There was little evidence of thrombosis.
Serial biopsies on one dog with disseminated intravascular coagulation indicated a changing pattern of fibrin deposition with time. Seventy minutes after anastomosis of the second kidney fibrin was deposited largely in glomeruli and about peritubular capillaries. At 2 hours the glomerular deposits had largely disappeared. At 35 hours, one day after cessation of function, fibrin was found primarily in small arteries and about peritubular capillaries of the transplant. At this time, the dog’s own kidney contained only small amounts of fibrin about peritubular capillaries.
DISCUSSION
The relationship of two specific factors to accelerated or hyperacute rejection were examined in this study. The first was the pre-existence of a family of circulating anti–red cell and anti–white cell antibodies which appeared coincident with sensitization to donor skin and which could be absorbed by the cells of the donor. The second factor was the coagulation process coincident with the rapid destruction of the grafts.
The fact that humoral antibodies seemed to be an integral part of the sensitized state was not unexpected. Since the publications of Gorer and O’Gorman7 and Stetson19 the concept has been frequently advanced that homograft rejection is due not only to cell mediated immunity but to an immunoglobulin response as well. In human recipients of renal transplants, antigraft antibodies have been found in an incidence that varied with the sensitivity of the detection method used. Moreover, recent publications have underscored the bad prognostic implications of antibodies particularly if these antedated operation.11, 18, 22, 25
It remains to be settled if certain tests such as the mixed agglutination method of Klassen and Milgrom11 detect unusually harmful antibodies in an especially discriminating way. The mixed agglutination test was not employed in this present study. However, the different antibodies analyzed tended to rise or fall in parallel with sensitization or depletion procedures, although the lymphocytotoxins proved to be the most resistant to absorption. The heterogeneity of the response to the skin grafts was consistent with the fact that well studied histocompatibility loci determine not only the nature of cell mediated reactions but also a wide range of humoral antibody responses, including those with anti–red cell and anti–white cell activity.7
The controversial aspect of the presensitized state has not been whether humoral antibodies play a role in rejection but rather how their injurious effects are mediated. Clinical observations from our own institution18 were interpreted as indicating that an immediate antigen antibody reaction following revascularization of renal homografts caused alterations in coagulation, leading to deposition of fibrin in the homograft vasculature and an ultimate pathologic appearance which resembled the Shwartzman reaction. This view of the pathogenetic role of clotting was not confirmed by Colman and Merrill5 but indirectly supported by the observations of MacDonald and associates12 who showed that hyperacute renal rejection could be regularly prevented in sensitized dogs by prophylactic heparin therapy.
The present study provided strong evidence that acute coagulation changes are in fact precipitated by an immeditae immunologic reaction when homografts are transplanted to sensitized recipients. Invariably, there was consumption within the transplants of platelets and all measured clotting factors. Furthermore, in a fourth of the experiments there developed a systemic clotting disorder indistinguishable from that of disseminated intravascular coagulation (DIC). These observations were consistent with the immunofluorescent detection of fibrin deposits in all conditioning grafts. The single definitive graft studied by serial biopsies showed early deposition and rapid removal of fibrin suggesting active fibrinolysis accompanying this coagulation process. With either the localized or general coagulopathy the transplanted organs were at specific risk since the native kidneys or other host organs were not perceptibly harmed as “innocent bystanders” at the same time as the homograft was undergoing variable damage, and as fibrin became deposited in its vasculature.
The means of inducing this local and/or generalized coagulopathy have not been identified but our experimental observations suggest a likely mechanism. Since the induced antibodies appear to react with antigens in the transplants, clotting could be induced directly by the antibody-antigen reaction. 1, 17 In addition, antibody-antigen reactions attract PMN leukocytes via C′ activation24 and these white cells appear capable of inducing clotting9 and are essential in the causation of the thrombosis seen in the local20 and generalized9,10 Shwartzman reactions. Our failure to find large numbers of PMN leukocytes in these grafts may have been because our observations were too late, i.e., after cessation of leukocyte sequestration in the graft. Heavy PMN accumulations and thrombosis have been seen in man where preformed antibodies induced immediate hyperacute rejections of renal transplants.18, 25
One objective of the present study was to evaluate means of mitigating hyperacute or accelerated rejection. This was achieved by transplanting successive organs from the same donor. The reason for protection cannot be precisely defined from our observations although the absorption of humoral antibodies by the first or conditioning organ is the most obvious explanation. However, it is conceivable that the coincident depletion of the clotting factors could have been responsible in part for the extended survival of the second organ.
SUMMARY
Dogs were sensitized with repeated skin homotransplantation and then given a spleen, kidney, or liver from their skin donor. Coincident with sensitization, there developed a family of anti–donor antibodies which apparently participated in the accelerated or hyperacute rejection of the whole organs. An important component of the destructive process was a coagulopathy which always occurred within the grafts and which sometimes led to systemic alterations resembling a disseminated intravascular coagulation. Kidneys were usually temporarily protected from hyperacute rejection by the prior transplantation of another organ (kidney, spleen, or liver) from the same donor.
Acknowledgments
This work was supported by United States Public Health Service Grants AI-04152, AI-07007, AI-AM-08898, AM-12148, AM-06344, AM-07772, FR-00051, and FR-00069; by United States Public Health Service Contract PH-43-68-621; and by Atomic Energy Commission contract AT (04-3)-410.
Footnotes
Presented at the Thirty-first Annual Meeting of the Society of University Surgeons, Pittsburgh, Pa., Feb. 12 to 14, 1970.
References
- 1.Bettex-Galland M, Luescher EF, Simon G, Vasalli P. Induction of viscous metamorphosis in human blood platelets by means other than by thrombin. Nature. 1963;200:1109. doi: 10.1038/2001109a0. [DOI] [PubMed] [Google Scholar]
- 2.Brecher G, Cronkite EP. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950;3:365. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- 3.Buckell M. The effect of citrate on euglobulin methods of estimating fibrinolytic activities. J Clin Path. 1958;11:403. doi: 10.1136/jcp.11.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claman HN, Merrill D. Quantitative measurement of human gamma-2, beta-2A, and beta-2M serum immunoglobulins. J Lab Clin Med. 1969;69:685. [PubMed] [Google Scholar]
- 5.Colman RW, Braun WE, Busch GJ, Dammin GJ, Merrill JF. Coagulation studies in the hyperacute and other forms of allograft rejection. New Eng J Med. 1969;280:685. doi: 10.1056/NEJM196909252811301. [DOI] [PubMed] [Google Scholar]
- 6.Deutsch E, Schaden W. Zur Reinigung und Charakterisierung des VII. Blutgerinnungs-faktors. Biochem Z. 1953;324:266. [PubMed] [Google Scholar]
- 7.Gorer PA, O’Gorman P. Cytotoxic activity of isoantibodies in mice. Transplantation Bull. 1956;3:142. [Google Scholar]
- 8.Henderson ES, Rapaport SI. The thrombotic activity of activation product. J Clin Invest. 1962;41:235. doi: 10.1172/JCI104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horn RG, Collins RD. Studies on the pathogenesis of the generalized Shwartzman reaction. The role of granulocytes. Lab Invest. 1968;18:101. [PubMed] [Google Scholar]
- 10.Johnstone DE, Michaelson SM, Tuttle L, Howland JW. Attempts to produce localized Shwartzman reaction in seven species of animals. Proc Soc Exp Biol Med. 1958;99:15. doi: 10.3181/00379727-99-24230. [DOI] [PubMed] [Google Scholar]
- 11.Klassen J, Milgrom F. The role of humoral antibodies in the rejection of renal homografts by rabbits. Transplantation. 1969;8:566. doi: 10.1097/00007890-196911000-00003. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald A, Busch GJ, Alexander JL, Pheteplace EA, Menzoian J, Murray JE. Heparin and aspirin in the treatment of hyperacute rejection of renal allografts in presensitized dogs. Transplantation. 1970;9:1. doi: 10.1097/00007890-197001000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Owren PA, Aas K. The control of dicumarol therapy and quantitative determination of prothrombin and proconvertin. Scand J Clin Lab Invest. 1951;3:201. doi: 10.3109/00365515109060600. [DOI] [PubMed] [Google Scholar]
- 14.Pool JG. Preparation and testing of anti-hemophilia globulin (factor VIII) sources for transfusion therapy in hemophilia. Scand J Clin Lab Invest. 1965;17(Suppl 84):70. [PubMed] [Google Scholar]
- 15.Proctor RR, Rapaport SI. The partial thromboplastin time with kaolin. Amer J Clin Path. 1961;36:212. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- 16.Ratnoff OD, Menzie C. A new method for the determination of fibrinogen in small samples of plasma. J Int Clin Med. 1951;31:316. [PubMed] [Google Scholar]
- 17.Robbins J, Stetson CA., Jr An effect of antigen antibody interaction on blood coagulation. J Exp Med. 1959;109:1. doi: 10.1084/jem.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Lerner RA, Dixon FJ, Groth CG, Brettschneider L, Terasaki PI. Shwartzman reaction after human renal homotransplantation. New Eng J Med. 1968;278:642. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stetson CA. The role of humoral antibody in the homograft reaction. In: Dixon FJ Jr, Humphrey JH, editors. Advances in immunology. III. New York: Academic Press, Inc; 1963. p. 97. [Google Scholar]
- 20.Stetson CA, Good RA. Studies on the mechanism of the Shwartzman phenomenon. Evidence for the participation of polymorphonuclear leukocytes in the phenomenon. J Exp Med. 1951;93:49. doi: 10.1084/jem.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terasaki PI, McClelland JD. Micro-droplet assay of human serum cytotoxins. Nature. 1964;204:998. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 22.Terasaki PI, Trasher DL, Hauber TH. Advance in transplantation. Copenhagen: Ejnar Munksgaard Forlag; 1968. Serotyping for homotransplantation. XIII. Immediate kidney transplant rejection and associated preformed antibodies; p. 225. [Google Scholar]
- 23.Van Rood JJ, van Leeuwen A. Leukocyte grouping. A method and its application. J Clin Invest. 1963;42:1382. doi: 10.1172/JCI104822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward PA, Cochrane CG. Bound complement and immunologic injury of blood vessels. J Exp Med. 1965;121:215. doi: 10.1084/jem.121.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams GM, Hume DM, Hudson RP, Jr, Morris PJ, Kano K, Milgrom F. “Hyperacute” renal homograft rejection in man. New Eng J Med. 1968;279:611. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 26.Wilson CB, Dixon FJ. Antigen quantitation in experimental immune complex glomerulonephritis. Acute serum sickness. I In preparation. [PubMed] [Google Scholar]