SUMMARY
Purpose
Attempts to overcome multi-drug resistance (MDR) have been limited by toxicities. To investigate the effect of reducing peak drug levels, we performed sequential studies using continuous infusion daunorubicin and cytarabine without (AD) and then with cyclosporine (ADC) in older patients with acute myeloid leukemia (AML).
Patients and Methods
Untreated AML patients (age 56+) participated in phase II studies using daunorubicin (45 mg/m2/day for 3 days) and cytarabine (200 mg/m2/day for 7 days), both by continuous infusion, without (study S0112, 60 patients) and then with (study S0301, 50 patients) the addition of cyclosporine (6 mg/kg bolus, then continuous infusion 16 mg/kg/day for 3 days).
Results
Complete response (CR) rates were 38% on S0112 and 44% on S0301. Toxicities were generally similar, though after enrollment of the first 14 patients, S0301 excluded elderly patients with poor performance status. Fatal induction toxicities occurred in 17% and 12%, respectively, arising primarily from infection and hemorrhage. Median overall and relapse-free survival was 7 and 8 months for AD, respectively, and 6 and 14 months for ADC. Patients with phenotypic or functional P-glycoprotein (Pgp) had somewhat higher CR rates with ADC than AD, although confidence intervals overlapped.
Conclusion
In sequential phase II cooperative group trials, continuous infusion AD produced CR rates comparable to those with bolus daunorubicin. Addition of cyclosporine at doses known to modulate Pgp-mediated drug efflux did not cause undue toxicities, produced a similar CR rate, and possibly improved relapse-free survival. Further correlate analyses did not identify a subpopulation specifically benefitting from the addition of cyclosporine.
INTRODUCTION
The median age of patients with acute myeloid leukemia (AML) exceeds 60 years, and despite advances in therapy for younger patients, older patients continue to have poor outcomes with little improvement over time. Induction outcomes in older patients is often complicated by more frequent co-morbidities, and by fundamental differences in disease biology [van den Heuvel-Eibrink et al, 2007; van der Holt et al, 2007; Appelbaum et al, 2006; Farag et al, 2006; Wilson et al, 2006; Anderson et al, 2002; Goldstone et al, 2001; Godwin et al, 1998]. These biological differences include, among others, an increased frequency of expression of P-glycoprotein (Pgp) and other drug efflux proteins that share homology with the ATP-Binding Cassette (ABC) transporters [Schilthuizen et al, 2007; Mahadevan & List, 2004; van den Heuvel-Eibrink et al, 2002; van den Heuvel-Eibrink et al, 2001; Ross et al, 1994; Ross et al, 1993]. Compounds such as cyclosporine, its derivative PSC-833, as well as unrelated compounds such as zosuquidar have been investigated in younger and older patients with AML in attempts to overcome resistance and improve treatment outcomes [Lancet et al, 2009; Becton et al, 2006; van der Holt et al, 2005; Greenberg et al, 2004; Solary et al, 2003; Baer et al, 2002; List et al, 2002; List et al, 2001; Liu Yin et al, 2001; Chauncey et al, 2000; Advani et al, 1999; Lee et al, 1999; Leith et al, 1999; Tallman et al, 1999; Leith et al, 1997; Solary et al, 1996]. To date, however, none of these strategies have been consistently successful in improving outcome in younger or older patients, and many trials have been complicated by increased chemotherapy-related toxicities owing to higher blood levels of antineoplastics such as anthracyclines and etoposide when adminstered with these Pgp-modulating agents.
Based on the hypothesis that anthracycline-related toxicity in these regimens may be ascribed to higher peak drug concentrations rather than total drug exposure, we previously conducted a trial in patients with recurrent or untreated secondary AML testing the effect of cyclosporine with continuous infusion daunorubicin and high-dose cytarabine. In that prospective randomized trial (S9126), the addition of cyclosporine did not significantly improve the complete response rate, but the incidence of induction failure due to resistant disease was significantly reduced, and both relapse-free and overall survivals were significantly prolonged. The benefit of cyclosporine was greatest in patients whose blasts expressed a multi-drug resistant (MDR) phenotype.
Considering the high frequency of Pgp expression in older AML patients, we performed sequential phase II feasibility studies to assess the tolerance of infusional daunorubicin and cytarabine administration in older patients with AML, either alone (study S0112) or with the addition of cyclosporine (study S0301). The second study would proceed only if continuous infusion daunorubicin had no untoward impact on toxicity or efficacy. The results of S0112 were, within the limits of study size, similar to those in previous SWOG trials in similar patients using bolus daunorubicin, allowing S0301 to proceed. We found that the addition of cyclosporine to the infusional induction regimen was poorly tolerated in the aged and those patients with very poor performance status. After exclusion of those patients from study eligibility, the addition of cyclosporine did not appear to increase toxicities.
PATIENTS, MATERIALS, AND METHODS
Eligibility
Patients 56 years of age or older with a morphologically confirmed diagnosis of AML, except for acute promyelocytic leukemia (M3), were eligible [Cheson et al, 2003]. Diagnosis of AML was by French-American-British (FAB) criteria, i.e., required ≥30% marrow blasts (≥20% if the peripheral blast percentage was ≥30%). Patients with blast crisis of chronic myelogeneous leukemia were excluded. Prior systemic chemotherapy for acute leukemia was not permitted, although treatment for hyperleukocytosis with hydroxyurea, or intrathecal therapy for suspected or proven central nervous system involvement was allowed. Patients with secondary AML, defined as AML arising after a morphological diagnosis as myelodysplasia (MDS) [Cheson et al, 2003] or AML arising after prior chemotherapy or radiotherapy, were not excluded. Prior treatment for MDS with cytarabine (≤100 mg/m2) or azacitidine administered more than 30 days before administration was allowed. Patients were initially required to have performance status of 0-3 by SWOG criteria. However, in S0301, the permissible range of performance status was modified after the initial 14 patients as described below. The modified criteria permitted performance status 0-3 for patients of age ≤60, 0-2 for age 61-70, and 0-1 for age ≥71. Adequate solid organ function was required, as defined by (1) serum bilirubin ≤2 times institutional upper limits of normal (IULN); (2) SGOT and/or SGPT ≤4xIULN; (3) serum creatinine ≤1.5xIULN and/or creatinine clearance ≥40 ml/min; (4) ejection fraction ≥50% as measured by either multigated cardiac blood pool (MUGA) scan or echocardiography; and (5) absence of unstable cardiac arrhythmia or unstable angina. Informed consent in accordance with local institutional guidelines and federal regulations was required of all patients.
Treatment Plans
For S0112, patients received cytarabine 200 mg/m2 daily by 24-hour continuous infusion on days 1-7 and daunorubicin 45 mg/m2, also as 24-hour continuous infusion on days 1-3. For S0301, cyclosporine A was added as a loading dose of 6 mg/kg over 2 hours followed by 16 mg/kg/day by continuous infusion for 3 days. A bone marrow aspirate and biopsy was obtained on day 14 and if the marrow blast percentage was more than or equal to 5%, a second cycle of induction of identical chemotherapy was administered upon the earliest recovery of marrow cellularity to more than 20%. Once the marrow blast percentage of less than 5% was documented, patients received either G-CSF or GM-CSF, administered intravenously once daily until the absolute neutrophil count (ANC) reached 1500/mcL [Godwin et al, 1998]. The dose and drugs administered for cycle two were the same as cycle one except daunorubicin was reduced by 50% for serum bilirubin between 2-4 x IULN, or discontinued for higher serum bilirubin concentration .
Supportive Care
All patients received antimicrobial prophylaxis as follows: (1) ciprofloxacin 500 mg orally or 200 mg intravenously twice daily until administration of broad spectrum antibiotics for febrile neutropenia; (2) fluconazole, to begin 48 hours after completion of chemotherapy at 200 mg orally or intravenously daily or until administration of broad spectrum antifungal therapy, and (3) among patients who were seropositive for herpes simplex virus, acyclovir 800 mg orally or 250 mg/m2 intravenously twice daily, to begin 48 hours after completion of chemotherapy. If patients developed febrile neutropenia, ciprofloxacin was discontinued and parental broad-spectrum antipseudomonal antibiotic coverage was instituted. Antibiotics were continued until the ANC was over 500/mcL, and clinical signs of infection resolved. Guidelines for transfusion support included prophylactic platelet transfusion at a threshold of 10,000/mcL in asymptomatic patients and red cell transfusion at a hemoglobin threshold of 8 g/dL. Early institution of hyperalimentation was recommended for patients with poor oral intake.
Post-remission Therapy
Patients on either study who achieved a CR after one or two cycles of induction chemotherapy were registered to receive two cycles of post-remission therapy. For S0112, this consisted of daunorubicin (45 mg/m2) intravenously as 24-hour continuous intravenous infusion on days 1 and 2, and cytarabine (200 mg/m2) daily as 24-hour continuous infusion on days 1-5. On S0301, an identical regimen was used with the addition of cyclosporine with a loading dose of 6 mg/kg over 2 hours, followed by 16 mg/kg/day by continuous intravenous infusion on days 1-2. Performance status 0-2 by SWOG criteria and serum bilirubin less than or equal to twice the IULN were required for post-remission therapy, as was an MUGA scan demonstrating an ejection fraction more than or equal to 45%, for patients with cardiac toxicity after induction therapy. Use of G-CSF or GM-CSF with post-remission therapy was left to the discretion of the treating physician.
DEFINITIONS OF OUTCOMES
Response criteria were adapted from National Cancer Institute-sponsored workshop guidelines [Cheson et al, 1990]. Complete response (CR) required the following for ≥28 days: marrow with >20% cellularity, <5% blasts and no Auer rods; peripheral blood with neutrophils >1500/mcL, platelets >100,000/mcL, and no blasts; and absence of extra-medullary disease. Responses meeting CR criteria but without evidence of ≥28-day duration were designated as “unconfirmed CR.” Patients who failed to achieve CR after induction therapy were classified according to type of failure: resistant disease (RD); death during aplasia; or indeterminate, defined as death <7 days after completion of induction therapy or with no persistent leukemia in the peripheral blood, but no post-induction marrow examination [Cheson et al, 2003]. Overall survival (OS) was measured from the date of randomization until death from any cause, with observation censored at the date of last contact for patients last known to be alive. Relapse-free survival (RFS) was measured from the date of CR until relapse or death from any cause, with observation censored at the date of last contact for patients last known to be alive without report of relapse. Toxicities were defined and graded according to the National Cancer Institute (NCI) Common Toxicity Criteria version 2.0 for S0112 and Common Terminology Criteria for Adverse Events version 3.0 for S0301.
LABORATORY AND CYTOGENETIC DATA
MDR-1 expression by the leukemic blasts was measured using MRK-16 and three color flow cytometric assays for blasts co-staining with CD34 (hematopoietic stem/progenitor cell antigen) and CD33 (panmyeloid antigen) as previously described [Leith et al, 1999]. To assess cyclosporine-inhibited functional drug efflux of the leukemia blasts, two fluorescent dyes, Di(OC)2 and rhodamine 123 (Rh123), were measured with and without exposure to cyclosporine (CSA) in a single-color flow cytometric assay as described in previous reports [Leith et al, 1999]. MRK-16 expression and CSA-inhibited Di(OC)2 and Rh123 efflux were measured as continuous values using the Kolmogorov-Smirnov statistic, denoted D [Young, 1977]. Cytogenetic studies were performed at previously approved laboratories and centrally reviewed by the SWOG Cytogenetics Committee. Cytogenetic abnormalities were grouped according to published criteria adopted by SWOG as favorable, intermediate, unfavorable, or unknown [Slovak et al, 2000].
STATISTICAL CONSIDERATIONS
Each trial was conducted in two steps to permit termination if early results proved unfavorable. In S0112, if ≥9 of the first 30 patients achieved CR, 25 more would be enrolled; if ≥23 of the 55 achieved CR, results would be considered favorable. This design had critical (α) level 4% if the true CR rate with AD is 30%, and power 91% if the true CR rate is 50%. S0301 tested both response and the following indicator toxicity: any Grade 4+ non-hematologic toxicity except (1) hyperbilirubiunemia or (2) mucositis assigned Grade 4 due solely to ulceration (not requiring enteral/parenteral nutrition or prophylactic intubation). Initially S0301 would accrue 25 patients; if <7 achieved CR or >10 experienced indicator toxicity, the trial would end, otherwise another 25 would be enrolled. Results would be favorable for ADC if ≥16 of 50 achieved CR and <18 experienced indicator toxicity. S0301 had α level <5% if the true CR rate is ≤25% or the true risk of indicator toxicity is ≥45%, and power ≥89% if the true CR rate is ≥45% and the true risk of indicator toxicity is ≤25% Neither trial was stopped early, although the S0301 eligibility criteria were modified as described. Logistic regression analysis and Fisher’s exact test (for CR and RD) and proportional hazards regression (for OS and RFS) were used to test the effects on treatment outcomes of patient and disease characteristics including MDR. Distributions of OS and RFS were estimated by the method of Kaplan and Meier [Kaplan & Meier, 1958]. Confidence intervals (CIs) were calculated at the 95% confidence level. Results are based on data available as of March 23, 2009.
RESULTS
Patient Accrual and Characteristics
Study S0112
A total of 71 patients from 24 institutions were enrolled on study S0112 between August 2001 and April 2003. Ten were ineligible because of non-AML diagnosis (N=8), PS=4 (N=1), or prestudy MUGA <50% (N=1). An additional patient died before treatment and is excluded from the analysis. Demographic and disease characteristics for the 60 included patients are summarized in Table 1. Sixteen patients (27%) had AML secondary to prior MDS (N=14) or prior cytotoxic chemotherapy or chemoradiotherapy (N=2). Pre-treatment cytogenetic results were not available for 9 (15%) eligible patients, either because specimens were not submitted to approved laboratories (N=2) or evaluable studies were not obtained (N=7). Among 51 patients with cytogenetic data, 37% had unfavorable karyotypes. MRK16 expression was detected in 64% of patients, and CSA-inhibited efflux of Di(OC)2 and Rh123 occurred in 73% and 72%, respectively, of patients tested for MDR-1 expression or function (Table 2).
Table 1.
Demographic and Disease Characteristics at Baseline, by Study
| S0112 (N=60) |
S0301: original eligibility criteria (N=14) |
S0301: revised eligibility criteria (N=50) |
||
|---|---|---|---|---|
| Age (yrs) | 68 (56, 85) | 69 (57, 80) | 65 (56, 81) | |
| Sex (Males) | 29 (48%) | 11 (79%) | 30 (60%) | |
|
| ||||
| Perfor- mance Status |
0 | 20 (33%) | 2 (14%) | 18 (36%) |
| 1 | 25 (42%) | 7 (50%) | 28 (56%) | |
| 2 | 11 (18%) | 2 (14%) | 4 (8%) | |
| 3 | 4 (7%) | 3 (21%) | 0 | |
|
| ||||
| Race | White | 57 (95%) | 14 (100%) | 43 (86%) |
| Black | 2 (3%) | 0 | 3 (6%) | |
| Asian | 1 (2%) | 0 | 0 | |
| Unknown | 0 | 0 | 4 (8%) | |
|
| ||||
| AML Onset |
De novo | 44 (73%) | 10 (71%) | 42 (84%) |
| MDS-related | 14 (23%) | 4 (29%) | 7 (14%) | |
| Treatment related | 2 (3%) | 0 | 0 | |
| Unknown | 0 | 0 | 1 (2%) | |
|
| ||||
| Laboratory Values a | ||||
| WBC (1000/mcL) | 8.5 (0.6, 152.4) | 8.4 (0.7, 91.6) | 10.4 (0.6, 172.6) | |
| Platelets (1000/mcL) | 57 (5, 438) | 47 (12, 159) | 57 (13, 643) | |
| Neutrophils (%) | 16 (0, 75) | 7 (0, 31) | 12 (0, 67) | |
| Hemoglobin (g/dL) | 9.4 (5.6, 14.6) b | 9.0 (7.5, 10.6) | 9.2 (5.7, 12.4) | |
| Blasts(%)–blood | 16 (0, 91) b | 35 (0, 88) | 23 (0, 98) | |
| Blasts(%)–marrow | 51 (13, 97) c | 54 (23, 90) | 62 (17,98) | |
|
| ||||
| FAB Classif- ication |
M0 | 4 (7%) | 0 | 4 (8%) |
| M1 | 15 (25%) | 5 (36%) | 10 (20%) | |
| M2 | 15 (25%) | 4 (29%) | 10 (20%) | |
| M4 | 14 (23%) | 3 (21%) | 15 (30%) | |
| M4eos | 1 (2%) | 0 | 2 (4%) | |
| M5 | 8 (13%) | 2 (14%) | 6 (12%) | |
| M6 | 2 (3%) | 0 | 2 (4%) | |
| M7 | 1 (2%) | 0 | 1 (2%) | |
|
| ||||
| Cyto- genetic Risk Classif- ication |
Evaluable | 51 | 11 | 31 |
| Unfavorable d | 19 (37%) | 2 (18%) | 12 (39%) | |
| Intermediate d | 29 (57%) | 7 (64%) | 16 (52%) | |
| -Normal d | 25 (49%) | 4 (36%) | 11 (35%) | |
| Favorable d | 3 (6%) | 2 (18%) | 3 (10%) | |
Median (min, max)
Not reported for one patient
Not reported for two patients
Percentages based on number with evaluable cytogenetics
Table 2.
MDR1 Expression and Functional Efflux at Baseline, by Study
| S0112 (N=60) |
S0301: original eligibility criteria (N=14) |
S0301: revised eligibility criteria (N=50 ) |
||
|---|---|---|---|---|
| MRK16 a | Negative | 13 (26%) | 1 (7%) | 1 (42%) |
| Weak Positive | 5 (10%) | 4 (29%) | 4 (8%) | |
| Positive | 32 (64%) | 9 (64%) | 25 (50%) | |
|
| ||||
| Di(OC)2 Efflux a |
Negative | 9 (24%) | 1 (10%) | 17 (47%) |
| Weak Positive | 1 (3%) | 1 (10%) | 3 (8%) | |
| Positive | 27 (73%) | 8 (80%) | 16 (44%) | |
| Not Done | 13 | 4 | 14 | |
|
| ||||
| Rh123 Efflux a |
Negative | 8 (25%) | 2 (22%) | 20 (56%) |
| Weak Positive | 1 (3%) | 0 (0%) | 3 (8%) | |
| Positive | 23 (72%) | 7 (78%) | 13 (36%) | |
| Not Done | 18 | 5 | 14 | |
Negative: Kolmogorov-Smirnov D<0.15; Weak Positive: 0.15≤D<0.20; Positive: D≥0.2.
Study S0301
Study S0301 was activated in October 2003, but was temporarily closed in March 2004 after accruing 14 patients at 6 institutions due to concern about possible excessive toxicity and early mortality, particularly among older patients with poor performance status. At the time, six patients had died within 30 days of study entry, an additional patient had grade 4 non-hematologic toxicities, and median survival was only 2 months, though for the 6 (43%) who achieved CR, the median RFS was 8 months. Because of these results, study S0301 was amended to exclude patients of age 61-70 with performance status 3, and patients of age 70+ with performance status 2-3. The two-stage design was reinitiated after this modification, so that 25-30 patients meeting the amended eligibility criteria would be enrolled. The results for study S0301 presented below focus primarily on patients registered after the eligibility criteria modification, although results for the first 14 patients are included in some summaries for completeness. After the modification, 55 patients were accrued between August 2004 and December 2006, at 16 institutions. Three patients were ineligible because of non-AML diagnosis (N=2), or presence of concomitant malignancy (N=1) at the time of registration. Two additional patients who did not receive study treatment are excluded from the analysis. Among the 50 eligible patients, 42 (84%) had de novo disease, 7 (14%) had prior MDS, and 1 (2%) had unknown prior disease status. Pre-treatment cytogenetic results were not available for 19 (40%) eligible patients because specimens were not submitted to approved laboratories (N=7) or evaluable studies were not obtained (N=12). Among 31 patients with cytogenetic data, 39% had unfavorable karyotypes. MRK16 expression was found in 50% of patients, and CSA-inhibited efflux of Di(OC)2 and Rh123 was seen in 44% and 36%, respectively (Table 2).
Response to Induction Therapy
Study S0112
As shown in Table 3, 23 (38%) of the 60 patients on S0112 achieved CR (CI 26%-52%). Nineteen patients (32%) achieved CR after a single course of induction therapy. An additional 20 patients received a second course of identical induction therapy, and four (20%) of these achieved CR. Nineteen patients (32%, CI 20-45%) had resistant disease (RD) following one (N=7) or two (N=12) courses of induction therapy. Responses of nine patients (15%) were indeterminate due to death within 7 days of completing induction therapy or during subsequent aplasia, and the response of nine others was not adequately assessed. Twenty of the first 55 patients achieved CR, fewer than the 23 required for the primary test of effectiveness. Fifty-six (93%) patients experienced non-hematologic toxicities of grade 3 or higher. These included 10 (17%) with fatal induction toxicities, most commonly infection (N=6), hemorrhage (N=4) and/or cardiac toxicity (N=2), and 12 others (20%) with grade 4 non-hematologic toxicities.
Table 3.
Response to Induction Chemotherapy, by Study
| S0112 (N=60) |
S0301: original eligibility criteria (N=14) |
S0301: revised eligibility criteria (N=50) |
||
|---|---|---|---|---|
| Complete | Confirmed | 6 (10%) | 4 (29%) | 10 (20%) |
| Response | Unconfirmed | 17 (28%) | 2 (14%) | 12 (24%) |
| Total | 23 (38%) | 6 (43%) | 22 (44%) | |
| Resistant Disease | 19 (32%) | 2 (14%) | 13 (26%) | |
| Died During Aplasia | 5 (8%) | 2 (14%) | 2 (4%) | |
| Died <7 Days after 1st Course | 4 (7%) | 3 (21%) | 4 (8%) | |
| Died without Bone marrow exam | 2 (3%) | 1 (7%) | 4 (8%) | |
| Assessment Inadequate | 7 (12%) | 0 (0%) | 5 (10%) | |
Study S0301
Twenty-two (44%) of the 50 patients on S0301 achieved CR (CI 30%-59%), all after a single cycle of induction chemotherapy (Table 3). Seven patients received a second course of induction, but none achieved CR. Thirteen patients (23%) had RD (CI 15%-40%) following one (N=10) or two (N=3) courses of induction therapy. Responses of six patients (12%) were indeterminate due to death within 7 days of completing induction therapy or during subsequent aplasia, and responses of nine others were not adequately assessed. Forty patients (80%) had non-hematologic toxicities of grade 3 or higher. Six patients (12%) suffered fatal induction toxicities including infection (N=3), pulmonary hemorrhage (N=2) and asystole (N=1) and eight others (16%) experienced grade 4 non-hematologic toxicities. Consequently, the ADC regimen met both the response and toxicity criteria defined for study S0301.
Post-remission Therapy
Study S0112
On S0112, 31 patients were registered for consolidation, though 10 were ineligible and excluded from the analysis. Three patients who achieved a CR were not registered for consolidation. Of the 21 evaluable patients on S0112 who received protocol post-remission therapy, 19 completed the two cycles of consolidation therapy specified in the protocol. There were no fatal toxicities, and one patient experienced grade 4 non-hematologic toxicities including lung infection, ARDS, CNS hemorrhage, confusion, anxiety, and hepatotoxicity.
Study S0301
On S0301, 19 patients were registered for consolidation. One patient was excluded due to incomplete blood count recovery following induction therapy. Of the 18 patients, there was one fatality from sepsis, and another patient experienced grade 4 non-hematologic toxicity (high glucose). Three patients did not receive the second cycle of protocol-specified consolidation therapy.
Overall and Relapse-Free Survival
Study S0112
Of the 60 evaluable patients on S0112, 57 have died. The remaining patients were last known to be alive 73, 75, and 81 months after entry into the study. The median OS was 7 months (CI 3-10 months), and the estimated probability of survival was 33% (CI 21%-45%) at one year, and 15% (CI 6%-24%) at two years (Figure 1).
Figure 1.
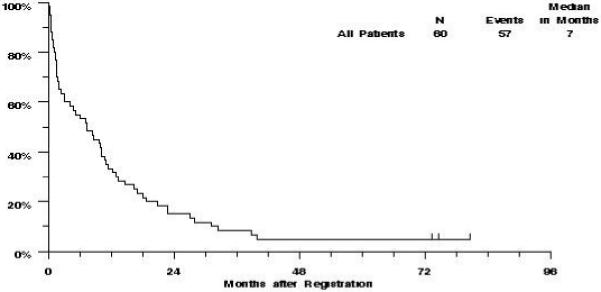
S0112 Overall Survival
Of the 23 patients who achieved complete remission on S0112, 19 have relapsed and one other died without report of disease status. The estimated median RFS was 8 months (CI 5-11 months), and the estimated probability of RFS was 35% (CI 15%-54%) at one year, and 22% (CI 5%-39%) at two years (Figure 2).
Figure 2.
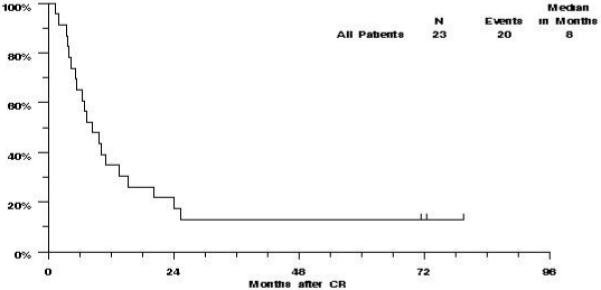
S0112: Relapse Free Survival
Study S0301
Of the 50 evaluable patients on S0301, 40 have died, and the remaining 10 patients were last known to be alive between 22 and 34 months (median 27 months). The estimated median OS was 6 months (CI 2-11 months), and the estimated probability of survival was 38% (CI 24%-51%) at one year, and 28% (CI 16%-40%) at two years (Figure 3).
Figure 3.
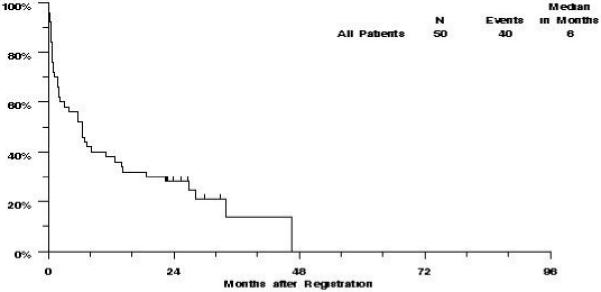
S0301 revised eligibility: Overall Survival
Of the 22 patients who achieved CR on S0301, eleven have relapsed and four others have died without report of relapse. The estimated median RFS was 14 months (CI 7-19 months) and the estimated probability of RFS was 55% (CI 34%-75%) at one year and 36% (CI 16%-56%) at two years (Figure 4).
Figure 4.
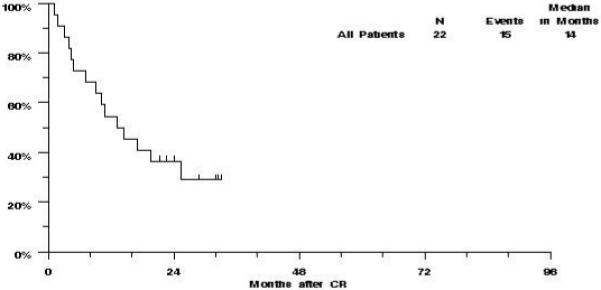
S0301 revised eligibility: Relapse Free Survival
Effect of MDR and other demographics on Treatment Outcomes
Analyses of cytogenetics, phenotypic drug efflux, CD34 expression, white count, performance status and history of prior antecedent myelodysplastic syndrome did not identify a population of patients specifically benefitting from the addition of cyclosporine (Supplementary Tables).
The impact of MDR phenotype on treatment outcome was analyzed separately for S0112 (without cyclosporine) and S0301 (with cyclosporine). In S0112, MDR as measured by Rh123 efflux was associated with a lower CR rate (p=0.036), but neither MRK16 expression nor Di(OC)2 efflux was statistically significantly associated with remission rates (Table 4). Resistant disease as a cause of induction failure tended to increase with CSA-inhibited efflux of both Di(OC)2 (p=0.48) and Rh123 (p=0.54). None of the measures of MDR were significantly associated with OS or RFS, a finding that is not unexpected given the small number of remitting patients. In S0301, the CR rate did not vary significantly according to the three measures of MDR, although the RD rate tended to increase with increasing Di(OC)2 efflux (p=0.049). None of the other associations of MDR phenotype and treatment outcome were significant.
Table 4.
Effects of MDR1 Expression and Functional Efflux on Response to Induction Chemotherapy, by Study
| Study S0112 |
Study S0301: revised eligibility criteria |
||||||
|---|---|---|---|---|---|---|---|
| Patients | Complete Response |
Resistant Disease |
Patients | Complete Response |
Resistant Disease |
||
| MRK16 | Negative | 13 | 5 (38%) | 4 (31%) | 21 | 10 (48%) | 3 (14%) |
| Weak Positive | 5 | 4 (80%) | 1 (20%) | 4 | 1 (25%) | 3 (75%) | |
| positive | 32 | 10 (31%) | 11 (34%) | 25 | 11 (44%) | 7 (28%) | |
| Trend a | P = 0.15 | P = 0.098 | P = 0.30 | P = 0.94 | |||
|
| |||||||
| Di(OC)2 Efflux |
Negative | 9 | 6 (67%) | 1 (11%) | 17 | 9 (53%) | 2 (12%) |
| Weak Positive | 1 | 1 (100%) | 0 (0%) | 3 | 1 (33%) | 0 (0%) | |
| Positive | 27 | 8 (30%) | 11 (41%) | 16 | 6 (38%) | 7 (44%) | |
| Not Done | 13 | 4 (31%) | 4 (31%) | 14 | 6 (43%) | 4 (29%) | |
| Trend a | P = 0.34 | P = 0.48 | P = 0.52 | P = 0.049 | |||
|
| |||||||
| Rh123 Efflux |
Negative | 8 | 5 (63%) | 2 (25%) | 20 | 12 (60%) | 2 (10%) |
| Weak Positive | 1 | 1 (100%) | 0 (0%) | 3 | 0 (0%) | 1 (33%) | |
| Positive | 23 | 6 (26%) | 10 (43%) | 13 | 4 (31%) | 6 (46%) | |
| Not Done | 18 | 7 (39%) | 4 (22%) | 14 | 6 (43%) | 4 (29%) | |
| Trend a | P = 0.036 | P = 0.54 | P = 0.49 | P = 0.12 | |||
Two-sided p-value based on logistic regression, treating MRK16 expression or efflux D-value as a continuous variable, excluding patients with assay not done.
DISCUSSION
In these sequential phase II studies, we found that administrating daunorubicin by continuous infusion along with cytarabine did not significantly alter the toxicity profile or the efficacy of the standard “7+3” regimen in older adults with newly diagnosed AML. In contrast, the addition of cyclosporine to the continuous infusion regimen increased toxicity in very old patients with poor performance status. This finding is consistent with earlier SWOG studies that have shown the interaction of age and performance status in determining outcome for this older AML population [Appelbaum et al, 2006]. Once this subgroup of patients was excluded from enrollment, the toxicities in S0301 were not markedly different from those seen in S0112. Although study S0112 did not meet its planned test criterion for efficacy, the CR rate for patients on both studies were consistent with past experience for older AML patients; 38% (CI 26-52%) with AD on S0112, and 44% (CI 30-59%) with ADC on S0301.
These phase II studies were not designed to formally compare the effectiveness of these two regimens. Nevertheless, it is noteworthy that the addition of cyclosporine in S0301 did not produce a markedly higher CR rate or lower RD rate than seen in S0112, even though the S0301 patients had a better overall performance status. Still, RFS appeared to be somewhat longer in S0301 (with cyclosporine, 14 months) compared to S0112 (without cyclosporine, 8 months). Nevertheless, this was not a prospective randomized study and the confidence intervals for RFS and OS overlap between studies. Such comparisons must be interpreted cautiously in two sequential trials. Indeed, after the eligibility criteria were revised for S0301, restricting enrollment for older patients with poor performance status, baseline patient characteristics became slightly more favorable on S0301 than S0112. Nonetheless, the possibility of improved remission maintenance with the addition of cyclosporine cannot be excluded. Such an effect is consistent with the outcome of the phase III randomized trial, S9126, where the addition of cyclosporine to a regimen of continuous infusion daunorubicin plus high-dose cytarabine did not improve complete response rates, but did lead to a significantly lower rate of resistant disease and prolonged relapse-free and overall survivals in younger patients with recurrent or secondary AML. The current phase II studies suggest that cyclosporine as a modulator of MDR in older patients may impact remission duration rather than remission induction.
Various measures of the multi-drug resistant phenotype were studied in S0112 and S0301 with the hypothesis that their association with outcome would be seen most clearly in S0112, but attenuated in S0301 with the addition of cyclosporine. The baseline assessment of phenotypic and functional Pgp appeared to differ substantially between the two studies (Table 3). Whether this difference was a chance occurrence or the result of an unidentified change in methodology is unknown. Although there were trends in association for several measures of MDR with lack of CR and persistent disease in S0112, these patterns were not strikingly altered by the addition of cyclosporine in S0301 (Table 4). However, because of the differences in baseline measures, the proportion of patients not evaluable for MDR function in S0301 (28%), and the limited number of complete responders, comparisons of MDR expression and treatment responses between studies are difficult.
Other studies have investigated Pgp-modulation in patients with relapsed and refractory AML. In the MRC’s AML-R study, patients with relapsed or refractory AML were randomized to ADE with and without cyclosporine at 5 mg/kg/day [Liu Yin et al, 2001]. There was no apparent benefit, though cyclosporine dosing was arguably inadequate for reversing Pgp-mediated efflux [Mahadevan & List, 2004; List et al, 2001; Qadir et al, 2005].
A salient feature of many of the Pgp-efflux inhibitors is their pharmacokinetic effects on other cytotoxic drugs, such as anthracyclines and etoposide. This has typically required dose-attenuation of cytotoxic drugs with the goal of achieving equitoxic regimens with and without the modifying agent. This strategy, in turn, creates a complex interaction of cytotoxic drugs with a spectrum of toxicities and risks underdosing of patients without pharmacokinetic changes. Other Pgp-inhibitors, such as zosuquidar, show very little pharmacodynamic effects on cytotoxic agents, although a recent ECOG study in older patients with newly diagnosed AML using zosuquidar by prolonged infusion with conventionally administered daunorubicin and cytarabine (E3999) was also ineffective at improving outcomes [Cripe et al, 2006].
Other investigators have found similar results using the cyclosporine analog, PSC-833. In the CALGB 9720 study of patients over 60 with untreated AML, daunorubicin was given by conventional bolus infusion along with etoposide and cytarabine in parallel prospective comparative studies with and without PSC-833 [Baer et al, 2002)]. Prior to this study, in separate phase I studies, daunorubicin was dose adjusted to achieve equivalent toxicity in those patients receiving PSC-833 to those not receiving PSC-833. The use of PSC-833 required a 30% dose reduction of daunorubicin and 40% reduction in etoposide dose. Despite this dose adjustment, the arm receiving PSC-833 was closed early due to excessive toxicity. Though there were trends toward improved response and DFS for patients receiving PSC-833, they did not reach statistical significance. Likewise, results were also found in the similarly designed CALGB 9621, evaluating AML in patients younger than 60 years-old [Kolitz et al, 2004]. In this set of phase II trials, PSC-833 appeared to improve DFS and first round induction efficacy, as well as improve OS in patients younger than 45, however, a confirmatory phase III study (CALGB 19808) was stopped early when PSC-833 became unavailable [Kolitz et al, 2005].
HOVON and MRC performed a parallel prospective comparative study with cytarabine and daunorubicin by bolus infusion at attenuated doses with and without PSC-833, in patients 60 and over. Patients received two induction cycles followed by a single cycle without PSC-833 as consolidation [van der Holt et al, 2005]. Again, there was a trend toward improved disease-free survival for patients who received PSC-833, though other outcomes were not improved. Similarly, the ECOG performed a prospective comparative study utilizing PSC-833 along with conventional bolus infusion of mitoxantrone, etoposide, and cytarabine in patients with relapsed and refractory disease in E2995 [Greenberg et al, 2004], again without improvement in any outcome parameters assessed, though PSC-833 decreased clearance of mitoxantrone and etoposide in predictable fashion.
SWOG also performed a phase I dose-escalation study (S9617) using mitoxantrone and etoposide by conventional bolus administration along with PSC-833 by continuous infusion, and determined a maximum tolerated dose (MTD) consistent with other studies in patients with AML over 55 years-old [Chauncey et al, 2000]. However, after completion of the study, the regimen was not further pursued based on results of S9333 showing mitoxantrone and etoposide regimen given as first-line therapy for older patients with AML, was not superior to conventional daunorubicin and cytarabine [Anderson et al, 2002]. Of note, in the AML11 prospective comparative trial of three different induction regimens, the MRC showed that their etoposide-containing regimen were less effective at achieving remission than the combination of anthracyclines and cytarabine in older AML patients [Goldstone et al, 2001].
In contrast, the GOELAMS study in patients with de novo AML between 15 and 60 years old, used quinine by continuous infusion along with cytarabine by continuous infusion and idarubicin by conventional bolus administration [Solary et al, 2003]. Patients with persistent disease after this regimen received high-dose cytarabine and mitoxantrone, and patients in CR received consolidation with an amsacrine and cytarabine regimen. After that, patients underwent ablative allogeneic transplant from a sibling donor or chemotherapy consolidation with high-dose cytarabine and mitoxantrone followed by amsacrine and etoposide with and without quinine. In this study, quinine improved response, EFS, and OS for those patients with demonstrated Rh123 efflux, though this subgroup analysis did not reach statistical significance for EFS and OS.
MDR-1 expression may serve as a marker for other mechanisms of resistance that parallel the induction and/or selection of MDR-1 expression. In addition to Pgp, other ATP-binding cassette (ABC)-efflux mediators such as MRP and BCRP, as well as LRP, correlate with resistance in various clinical settings, and PSC-833 and cyclosporine may be clinically less effective at modulating drug-efflux by these other transporters. Because the presence of other transporters was not assessed in the current study, their significance in this dataset is unknown.
It is likely that many mutations exist in leukemia cells in older patients with AML, as well as redundant mechanisms of resistance, complicating any therapeutic approach. While the increased expression of an MDR phenotype in the blasts of older patients continues to represent a theoretically attractive target, attempts to reverse that phenotype as a single approach have not had the anticipated success in this patient population.
Supplementary Material
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA04919, CA11083, CA12644, CA13612, CA20319, CA27057, CA35090, CA35119, CA35128, CA35176, CA35178, CA35261, CA35431, CA37981, CA45807, CA45808, CA46113, CA46282, CA46441, CA58416, CA58658, CA58861, CA63845, CA67575, CA74647 and CA76462
REFERENCES
- Advani R, Visani G, Milligan D, Saba H, Tallman M, Rowe JM, Wiernik PH, Ramek J, Dugan K, Lum B, Villena J, Davis E, Paietta E, Litchman M, Covelli A, Sikic B, Greenberg P. Treatment of poor prognosis AML patients using PSC833 (valspodar) plus mitoxantrone, etoposide, and cytarabine (PSC-MEC) Adv Exp Med Biol. 1999;457:47–56. doi: 10.1007/978-1-4615-4811-9_6. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Kopecky KJ, Willman CL, Head D, O’Donnell MR, Luthardt FW, Norwood TH, Chen IM, Balcerzak SP, Johnson DB, Appelbaum FR. Outcome after induction chemotherapy for older patients with acute myeloid leukemia is not improved with mitoxantrone and etoposide compared to cytarabine and daunorubicin: a Southwest Oncology Group study. Blood. 2002;100:3869–76. doi: 10.1182/blood-2001-12-0354. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer MR, George SL, Dodge RK, O’Loughlin KL, Minderman H, Caligiuri MA, Anastasi J, Powell BL, Kolitz JE, Schiffer CA, Bloomfield CD, Larson RA. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B Study 9720. Blood. 2002;100:1224–32. [PubMed] [Google Scholar]
- Becton D, Dahl GV, Ravindranath Y, Chang MN, Behm FG, Raimondi SC, Head DR, Stine KC, Lacayo NJ, Sikic BI, Arceci RJ, Weinstein H, Pediatric Oncology Group Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood. 2006;107:1315–24. doi: 10.1182/blood-2004-08-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauncey TR, Rankin C, Anderson JE, Chen I, Kopecky KJ, Godwin JE, Kalaycio ME, Moore DF, Shurafa MS, Petersdorf SH, Kraut EH, Leith CP, Head DR, Luthardt FW, Willman CL, Appelbaum FR. A phase I study of induction chemotherapy for older patients with newly diagnosed acute myeloid leukemia (AML) using mitoxantrone, etoposide, and the MDR modulator PSC 833: a southwest oncology group study 9617. Leuk Res. 2000;24:567–74. doi: 10.1016/s0145-2126(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, International Working Group for Diagnosis Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD, Brunning R, Gale RP, Grever MR, Keating MJ, Sawitsky A, Stass S, Weinstein H, Woods WG. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- Cripe LD, Li X, Litzow M, et al. A randomized placebo-controlled, double blind trial of the MDR modulator, zosuquidar, during conventional induction and post-remission therapy for pts > 60 years of age with newly diagnosed acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome (HR-MDS): ECOG 3999. 2006 ASH #423. [Google Scholar]
- Farag SS, Archer KJ, Mrózek K, Ruppert AS, Carroll AJ, Vardiman JW, Pettenati MJ, Baer MR, Qumsiyeh MB, Koduru PR, Ning Y, Mayer RJ, Stone RM, Larson RA, Bloomfield CD, Cancer and Leukemia Group B Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. doi: 10.1182/blood-2005-11-4354. 8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin JE, Kopecky KJ, Head DR, Willman CL, Leith CP, Hynes HE, Balcerzak SP, Appelbaum FR, A Southwest Oncology Group study A double blind placebo controlled trial of G-CSF in elderly patients with previously untreated acute myeloid leukemia. Blood. 1998;91:3607–3615. 9031. [PubMed] [Google Scholar]
- Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE, Medical Research Council Adult Leukemia Working Party Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–11. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- Greenberg PL, Lee SJ, Advani R, Tallman MS, Sikic BI, Letendre L, Dugan K, Lum B, Chin DL, Dewald G, Paietta E, Bennett JM, Rowe JM. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995) J Clin Oncol. 2004;22:1078–86. doi: 10.1200/JCO.2004.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kolitz JE, George SL, Dodge RK, Hurd DD, Powell BL, Allen SL, Velez-Garcia E, Moore JO, Shea TC, Hoke E, Caligiuri MA, Vardiman JW, Bloomfield CD, Larson RA, Cancer and Leukemia Group B Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol. 2004;22:4290–301. doi: 10.1200/JCO.2004.11.106. [DOI] [PubMed] [Google Scholar]
- Kolitz JE, George SL, Marcucci G, Ravi Vij, Bayard L, Powell BL, Allen SL, De Angelo DJ, Shea T, Stock W, Hars V, Hoke E, Vardiman JW, Bloomfield CD, Larson RA. A Randomized Comparison of Induction Therapy for Untreated Acute Myeloid Leukemia (AML) in Patients < 60 Years Using P-Glycoprotein (Pgp) Modulation with Valspodar (PSC833): Preliminary Results of Cancer and Leukemia Group B Study 19808. 2005 ASH #157. [Google Scholar]
- Lancet JE, Baer MR, Duran GE, List AF, Fielding R, Marcelletti JF, Multani PS, Sikic BI. A phase I trial of continuous infusion of the multidrug resistance inhibitor zosuquidar with daunorubicin and cytarabine in acute myeloid leukemia. Leuk Res. 2009;33:1055–61. doi: 10.1016/j.leukres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Lee EJ, George SL, Caligiuri M, Szatrowski TP, Powell BL, Lemke S, Dodge RK, Smith R, Baer M, Schiffer CA. Parallel phase I studies of daunorubicin given with cytarabine and etoposide with or without the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age or older with acute myeloid leukemia: results of Cancer and leukemia group B study 9420. J Clin Oncol. 1999;17:2831–9. doi: 10.1200/JCO.1999.17.9.2831. [DOI] [PubMed] [Google Scholar]
- Leith CP, Kopecky KJ, Chen IM, Eijdems L, Slovak ML, McConnell TS, Head DR, Weick J, Grever MR, Appelbaum FR, Willman CL. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood. 1999;94:1086–99. [PubMed] [Google Scholar]
- Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, Head DR, Appelbaum FR, Willman CL, A Southwest Oncology Group study Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. Blood. 1997;89:3323–9. [PubMed] [Google Scholar]
- List AF, Kopecky KJ, Willman CL, Head DR, Slovak ML, Douer D, Dakhil SR, Appelbaum FR. Cyclosporine inhibition of P-glycoprotein in chronic myeloid leukemia blast phase. Blood. 2002;100:1910–2. [PubMed] [Google Scholar]
- List AF, Kopecky KJ, Willman CL, Head DR, Persons DL, Slovak ML, Dorr R, Karanes C, Hynes HE, Doroshow JH, Shurafa M, Appelbaum FR. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood. 2001;98:3212–20. doi: 10.1182/blood.v98.12.3212. [DOI] [PubMed] [Google Scholar]
- Liu Yin JA, Wheatley K, Rees JK, Burnett AK, UK MRC Adult Leukemia Working Party Comparison of ‘sequential’ versus ‘standard’ chemotherapy as re-induction treatment, with or without cyclosporine, in refractory/relapsed acute myeloid leukaemia (AML): results of the UK Medical Research Council AML-R trial. Br J Haematol. 2001;113:713–26. doi: 10.1046/j.1365-2141.2001.02785.x. [DOI] [PubMed] [Google Scholar]
- Mahadevan D, List AF. Targeting the multidrug resistance-1 transporter in AML: molecular regulation and therapeutic strategies. Blood. 2004;104:1940–51. doi: 10.1182/blood-2003-07-2490. 2004. [DOI] [PubMed] [Google Scholar]
- Qadir M, O’Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, Baer MR. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–6. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- Ross DD, Wooten PJ, Sridhara R, Ordóñez JV, Lee EJ, Schiffer CA. Enhancement of daunorubicin accumulation, retention, and cytotoxicity by verapamil or cyclosporin A in blast cells from patients with previously untreated acute myeloid leukemia. Blood. 1993;82:1288–99. [PubMed] [Google Scholar]
- Ross DD, Wooten PJ, Tong Y, Cornblatt B, Levy C, Sridhara R, Lee EJ, Schiffer CA. Synergistic reversal of multidrug-resistance phenotype in acute myeloid leukemia cells by cyclosporin A and cremophor EL. Blood. 1994;83:1337–47. [PubMed] [Google Scholar]
- Schilthuizen C, Broyl A, van der Holt B, de Knegt Y, Lokhorst H, Sonneveld P. Influence of genetic polymorphisms in CYP3A4, CYP3A5, GSTP1, GSTM1, GSTT1 and MDR1 genes on survival and therapy-related toxicity in multiple myeloma. Haematologica. 2007;92:277–8. doi: 10.3324/haematol.10618. [DOI] [PubMed] [Google Scholar]
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83. [PubMed] [Google Scholar]
- Solary E, Drenou B, Campos L, de Crémoux P, Mugneret F, Moreau P, Lioure B, Falkenrodt A, Witz B, Bernard M, Hunault-Berger M, Delain M, Fernandes J, Mounier C, Guilhot F, Garnache F, Berthou C, Kara-Slimane F, Harousseau JL, Groupe Ouest Est Leucémies Aiguës Myéloblastiques Quinine as a multidrug resistance inhibitor: a phase 3 multicentric randomized study in adult de novo acute myelogenous leukemia. Blood. 2003;102:1202–10. doi: 10.1182/blood-2002-11-3419. [DOI] [PubMed] [Google Scholar]
- Solary E, Witz B, Caillot D, Moreau P, Desablens B, Cahn JY, Sadoun A, Pignon B, Berthou C, Maloisel F, Guyotat D, Casassus P, Ifrah N, Lamy Y, Audhuy B, Colombat P, Harousseau JL. Combination of quinine as a potential reversing agent with mitoxantrone and cytarabine for the treatment of acute leukemias: a randomized multicenter study. Blood. 1996;88:1198–205. [PubMed] [Google Scholar]
- Tallman MS, Lee S, Sikic BI, Paietta E, Wiernik PH, Bennett JM, Rowe JM. Mitoxantrone, etoposide, and cytarabine plus cyclosporine for patients with relapsed or refractory acute myeloid leukemia: an Eastern Cooperative Oncology Group pilot study. Cancer. 1999;85:358–67. [PubMed] [Google Scholar]
- van den Heuvel-Eibrink MM, van der Holt B, Burnett AK, Knauf WU, Fey MF, Verhoef GE, Vellenga E, Ossenkoppele GJ, Löwenberg B, Sonneveld P. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann Hematol. 2007;86:329–37. doi: 10.1007/s00277-007-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel-Eibrink MM, Wiemer EA, de Boevere MJ, van der Holt B, Vossebeld PJ, Pieters R, Sonneveld P. MDR1 gene-related clonal selection and P-glycoprotein function and expression in relapsed or refractory acute myeloid leukemia. Blood. 2001;97:3605–11. doi: 10.1182/blood.v97.11.3605. [DOI] [PubMed] [Google Scholar]
- van den Heuvel-Eibrink MM, Wiemer EA, Prins A, Meijerink JP, Vossebeld PJ, van der Holt B, Pieters R, Sonneveld P. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML) Leukemia. 2002;16:833–9. doi: 10.1038/sj.leu.2402496. [DOI] [PubMed] [Google Scholar]
- van der Holt B, Breems DA, Berna Beverloo H, van den Berg E, Burnett AK, Sonneveld P, Löwenberg B. Various distinctive cytogenetic abnormalities in patients with acute myeloid leukaemia aged 60 years and older express adverse prognostic value: results from a prospective clinical trial. Br J Haematol. 2007;136:96–105. doi: 10.1111/j.1365-2141.2006.06403.x. [DOI] [PubMed] [Google Scholar]
- van der Holt B, Löwenberg B, Burnett AK, Knauf WU, Shepherd J, Piccaluga PP, Ossenkoppele GJ, Verhoef GE, Ferrant A, Crump M, Selleslag D, Theobald M, Fey MF, Vellenga E, Dugan M, Sonneveld P. The value of the MDR1 reversal agent PSC-833 in addition to daunorubicin and cytarabine in the treatment of elderly patients with previously untreated acute myeloid leukemia (AML), in relation to MDR1 status at diagnosis. Blood. 2005;106:2646–54. doi: 10.1182/blood-2005-04-1395. [DOI] [PubMed] [Google Scholar]
- Wilson CS, Davidson GS, Martin SB, Andries E, Potter J, Harvey R, Ar K, Xu Y, Kopecky KJ, Ankerst DP, Gundacker H, Slovak ML, Mosquera-Caro M, Chen IM, Stirewalt DL, Murphy M, Schultz FA, Kang H, Wang X, Radich JP, Appelbaum FR, Atlas SR, Godwin J, Willman CL. Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood. 2006;108:685–96. doi: 10.1182/blood-2004-12-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young IT. Proof without prejudice: use of the Kolmogorov-Smirnov test for the analysis of histograms from flow systems and other sources. J Histochem Cytochem. 1977;25:935. doi: 10.1177/25.7.894009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


