Cytomegalovirus (CMV*), the first reported transplant-associated opportunistic virus (1), has remained the most frequent single cause or co-cause of infections throughout the ensuing three decades (2). Because prophylactic drug and hyperimmune globulin therapy has not been effective in these CMV-infected patients (2–4), Rubin (5) suggested withholding treatment until the phase of rapid viral replication (preemptive therapy). Early diagnosis of CMV infection, upon which this strategy depends, can be accomplished in the presymptomatic phase (6, 7) with a rapid quantitative direct demonstration of CMV antigen pp65 in cytospin preparations of peripheral blood leukocytes (PBL) (8, 9). This method has aided in the management of 20 CMV seronegative recipients of livers from seropositive donors, a circumstance that carries an 85–90% risk of virus transmission of CMV disease, presumably from migratory monocytes from the graft (10) that are known to harbor the latent virus in healthy seropositive individuals (11).
The 20 recipients under tacrolimus/prednisone immunosuppression were followed for 308 ± 100.5 days (range, 63–520 days) after transplantation; a death at 63 days unrelated to CMV accounted for the only follow-up of <3 months. Heparinized blood samples were obtained by schedule during the first 3 months or when clinically indicated during this period and later. The pp65 antigen was detected in duplicate leukocyte spots (2 × 105 cells in 200 μl/glass slide) with the technique of Van der Bij et al. (8), using immunofluorescence (12) instead of immunoperoxidase staining (9). The anti-pp65 mouse monoclonal antibody was clone 1C3 (Biosoft, Argene, France). Fluorescein-isothiocyanate-labeled goat anti-mouse Fab2 fragment (Cappel, Durham, NC) was applied as the conjugate. Slides were counterstained with Evans blue dye and observed under 400 x magnification for the typical green nuclear fluorescence.
A conventional shell vial culture assay was done on the same blood specimens. Anti-CMV antibodies (IgG and IgM) were measured before and after transplantation using a semiautomated immunofluorescence test (FIAX test system, Whittaker Bioproducts, Inc., Walkersville, MD). IgG titers >20 were considered positive. CMV IgG was also the routine test used for donor screening.
Intravenous ganciclovir therapy (Cytovene; Syntex Pharmaceutical Ltd., Palo Alto, CA) was started only with the first detection of pp65 antigenemia, and continued until pp65 clearance. The ganciclovir dose was governed by creatinine clearance: >50 ml/min → 5 mg/kg of ganciclovir twice daily, 10–50 ml/min → 5 mg/kg every 24–48 hr, and <10 ml/min → 5 mg/kg every 48–96 hr.
As expected, 17 (85%) of the 20 patients had positive pp65 tests (CMV antigenemia) after a median posttransplant interval of 34 days (range, 9–83 days). Positive shell vial CMV cultures (viremia) were obtained at the same time as the first antigenemia diagnosis in only 5 of these 17 cases.
Twelve of the 17 patients were asymptomatic when the first positive pp65 test was obtained, including a recipient who was diagnosed only 9 days after transplantation (Fig. 1). The pp65 levels were generally low: 20±28 (SD) per 2 × 105 leukocytes. However, 6 of the 12 had further rises in antigenemia for 1 to 12 days after therapy was started, to a median peak of73 stained PBLs (range, 29–446 PBLs), before antigenemia began to clear. An example is shown in Figure 1. Ganciclovir instituted at the time of the first antigenemia prevented clinical disease in 11 of the 12 infected patients; the exceptional patient had a transient fever. The five other patients had CMV hepatitis at the time antigenemia was detected 12–83 days after surgery (median, 34 days). The median number of pp65-positive cells was 475 (range, 177–872).
Figure 1.
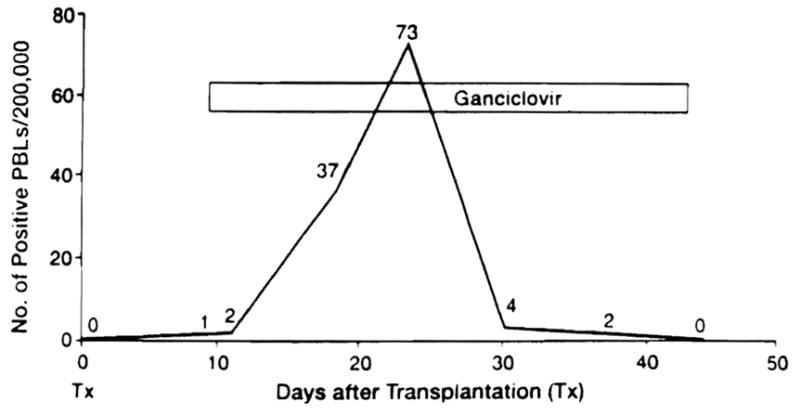
Preemptive ganciclovir treatment guided with CMV antigenemia in an asymptomatic, previously seronegative liver transplantation recipient whose donor was CMV positive.
Antigenemia was cleared with intravenous ganciclovir therapy in all 17 patients but recurred in 10 (59%) patients 5–174 days (median, 57 days) after the drug had been discontinued. Recurrence was independent of IgG conversion (P=0.26). The only patient who was symptomatic with recurrence had 430 pp65-stained cells, which cleared after resumption of ganciclovir. The nine asymptomatic patients had 3–101 pp65-stained cells. Ganciclovir retreatment promptly cleared the antigenemia in the recipient with 101 stained cells. Retreatment was withheld in the other eight patients whose samples had <100 stained cells, and the antigenemia resolved spontaneously in all.
In these cases, the antigen assay was useful as a signal to begin preemptive antiviral treatment, as envisioned by Rubin (2), as a means to decide whether to reinstitute ganciclovir in the event of a recurrence (previously emphasized by The et al. [6]) and as a guide to individualize the duration of a ganciclovir course in all of these circumstances. The assay also can be an important differential diagnostic tool. The persistence of symptoms despite clearance of the CMV pp65 antigen is an unambiguous warning of co-infection by another pathogen. Conversely, failure of antigen clearance should raise suspicion of ganciclovir-resistant CMV mutants. The pp65 findings are specific. With a novel method for quantitation of CMV DNA in leukocytes, a highly significant correlation was demonstrated between leukoDNAemia and the presence and quantity of pp65 antigenemia (13).
The sensitivity and quantitation nature of the pp65 test allowed new observations about response to therapy, such as the reason for increases in pp65 levels seen during the first few days after beginning ganciclovir in half of the preemptively treated patients. This may have reflected a delay in suppression of viral replication or, alternatively, phagocytosis by PBLs of degrading CMV matrix protein from lysed, infected leukocytes (14) or from the ballooned and infected endothelial cells that shed into the circulation (15).
Acknowledgments
We thank Elena Percivalle for assistance in developing the antigenemia assay, Drs. Giuseppe Gerna (Pavia) and Robert Rubin (Boston) for helpful consultations, and the clinical virology staff for technical support.
Footnotes
This study was aided by project grant DK 29661 from the National Institutes of Health, Bethesda, MD. Dr. Paolo Grossi was supported in part by a grant from Ministero della Sanita’. Ricerca Finalizzata 1989 e 1992 IRCCS San Matteo, Pavia, Italy. This work was also supported in part by the Pathology Education and Research Foundation of the University of Pittsburgh.
Abbreviations: CMV, cytomegalovirus; PBL, peripheral blood leukocyte.
References
- 1.Rifkind D, Starzl TE, Marchioro TL, Waddell WR, Rolands DT, Jr, Hill RB., Jr Transplantation pneumonia. JAMA. 1964;189:808. doi: 10.1001/jama.1964.03070110010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin RH. Infection in the organ transplant recipient. In: Rubin RH, Young LS, editors. Clinical approach to infection in the compromised host. New York: Plenum; 1994. p. 629. [Google Scholar]
- 3.Martin M, Manez R, Linden P, et al. A prospective randomized trial comparing ganciclovir-high dose acyclovir to high dose acyclovir for prevention of cytomegalovirus disease in adult liver transplant recipients. Transplantation. 1994;58:779. doi: 10.1097/00007890-199410150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snydman DR, Werner BG, Dougherty NN, et al. Cytomegalovirus immunoglobulin prophylaxis in liver transplantation. Ann Intern Med. 1993;119:984. doi: 10.7326/0003-4819-119-10-199311150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Rubin RH. Preemptive therapy in immunocompromised hosts. N Engl J Med. 1991;324:1057. doi: 10.1056/NEJM199104113241509. [DOI] [PubMed] [Google Scholar]
- 6.The TH, Van Der Ploeg M, Van Der Berg AP, Vlieger AM, Van Der Giessen M, Van Son WJ. Direct detection of cytomegalovirus in peripheral blood leukocytes: a review of the antigenemia assay and polymerase chain reaction. Transplantation. 1992;54:193. doi: 10.1097/00007890-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Grossi P, Minoli L, Percivalle E, Irish W, Vigano M, Gerna G. Clinical and virological monitoring of human cytomegalovirus infection in 294 heart transplant recipients. Transplantation. 1995;59:847. [PubMed] [Google Scholar]
- 8.Van der Bij W, Schirm J, Torensma R, Van Son WJ, Tegzess AM. Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J Clin Microbiol. 1988;26:2531. doi: 10.1128/jcm.26.12.2531-2535.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revello MG, Percivalle E, Zavattoni M, Parea M, Grossi P, Gerna G. Detection of human cytomegalovirus immediate-early antigen in leukocyte as a marker of viremia in immunocompromised patients. J Med Virol. 1989;29:88. doi: 10.1002/jmv.1890290204. [DOI] [PubMed] [Google Scholar]
- 10.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor-Wiedeman J, Sissons JGP, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 12.Gerna G, Revello MG, Percivalle E, Morini F. Comparison of different immunostaining techniques and monoclonal antibodies to the lower matrix phosphoprotein (pp65) for optimal quantitation of human cytomegalovirus antigenemia. J Clin Microbiol. 1992;30:1232. doi: 10.1128/jcm.30.5.1232-1237.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerna G, Furione M, Baldanti F, Sarasini A. Comparative quantitation of human cytomegalovirus DNA in blood leukocytes and plasma of transplant and AIDS patients. J Clin Microbiol. 1994;32:2709. doi: 10.1128/jcm.32.11.2709-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerna G, Zipeto D, Percivalle E, et al. Human cytomegalovirus infection of the major leukocyte subpopulations and evidence for initial viral replication in polymorphonuclear leukocytes from viremic patients. J Infect Dis. 1992;166:1236. doi: 10.1093/infdis/166.6.1236. [DOI] [PubMed] [Google Scholar]
- 15.Percivalle E, Revello MG, Vago L, Morini F, Gerna G. Circulating endothelial giant cells permissive for human cytomegalovirus (HCMV) are detected in disseminated HCMV infections with organ involvement. J Clin Invest. 1993;92:663. doi: 10.1172/JCI116635. [DOI] [PMC free article] [PubMed] [Google Scholar]


