Abstract
In biological systems, carbon dioxide exists in equilibrium with bicarbonate and protons. The individual components of this equilibrium (i.e., CO2, HCO3−, and H+), which must be sensed to be able to maintain cellular and organismal pH, also function as signals to modulate multiple physiological functions. Yet, the molecular sensors for CO2/HCO3−/pH remained unknown until recently. Here, we review recent progress in delineating molecular and cellular mechanisms for sensing CO2, HCO3−, and pH.
Keywords: Adenylyl cyclase, Bicarbonate, Carbon dioxide, Carbonic anhydrase, Channels, pH, Protons, Sensory transduction
Carbon dioxide (CO2), bicarbonate ions (HCO3−), and pH/ protons (H+) are inextricably linked in biological systems. Due to ubiquitous carbonic anhydrases, CO2 is in nearly instantaneous equilibrium with its hydrated form H2CO3, which in turn rapidly dissociates into H+ and HCO3−. Consequently, changes to any one of these molecules are reflected by variations in the other two, and all three (CO2, HCO3−, and pH) play central roles in biology. Cellular enzymes and chemical reactions are sensitive to pH, and cells actively transport H+ and HCO3− across their cell membrane to maintain intracellular pH (pHi) (reviewed in [1, 2]). Plus, CO2/HCO3− buffers intracellular and extracellular physiological fluids and is the essential substrates or end products of biological calcification, photosynthesis, and respiration. Thus, cellular homeostasis and organismal homeostasis depend on sensing and tightly regulating levels of CO2, HCO3−, and H+.
While all cells must possess mechanisms to sense, and respond to, the levels of CO2, HCO3−, and H+, multicellular organisms have specialized cells which measure intracellular HCO3− ([HCO3−]i) and pHi as surrogates for the levels of CO2, pH (pHe), and [HCO3−] ([HCO3−]e) in their immediate environment. In these sensory cells, variations in pHi or [HCO3−]i elicit such diverse responses as secretion/absorption of H+, HCO3−, and other ions, as adjustments in lung ventilation rate, as changes in metabolism, and as regulation of gene expression.
Implicit in the definition of a biological sensor, in addition to “sensing” at least one of the relevant variables, a CO2, HCO3−, or pH sensor must trigger appropriate downstream responses. This signaling function can be achieved by altering membrane potential, by producing a second messenger molecule, or by modulating other proteins and enzymes via allosteric conformational changes or posttranslational modifications such as phosphorylation/ dephosphorylation. In this review, we describe recent advances about how molecular pH, HCO3−, and/or CO2 sensors function.
pH sensors
Extracellular pH (pHe) sensors
Organisms sense pHe to maintain and restore systemic acid/ base balance, to adjust cellular metabolism to environmental conditions, and to detect and transduce sensory stimuli.
At least three G protein-coupled receptors (GPCRs) are activated by physiological increases in extracellular [H+] (decreases in pHe): OGR1, GPR4, and TDAG8. In general, these pHe-sensing GPCRs are inactive at pHe>7.5 and are fully activated at pHe<6.8. The mechanism of activation by H+ probably involves the protonation of histidines exposed to the extracellular medium [3, 4], inducing conformational changes that promote activation of the particular downstream heterotrimeric G proteins. The end results are increased production of the intracellular second messengers cAMP (via Gs stimulation of transmembrane adenylyl cyclase activity) or IP3 and diacylglycerol (via Gq family stimulation of phospholipase C activity).
Ovarian cancer G protein-coupled receptor 1(OGR1, GPR68) stimulation of IP3 production has been reported in osteoblasts [3] and osteoclasts [5], where it is thought to play roles in differentiation, metabolism, and systemic pH homeostasis [3, 5–8], as well as in aortic smooth muscle cells [6], where it is thought to mediate vasorelaxation via production of prostaglandin I2 [7]. OGR1-dependent IP3 formation is inactive at pHe 7.8 and maximally active at pHe 6.8 [3]. Activation of OGR1 also leads to the formation of cAMP in aortic smooth muscle cells [6]; however, this cAMP increase may be secondary to the IP3-elicited increase in intracellular calcium, rather than through direct Gs stimulation of a transmembrane adenylyl cyclase. Additionally, OGR1 is detectable in virtually every tissue tested and has been specifically identified in lung, kidney, and nervous system [3, 6, 8], suggesting there are additional roles of pHe sensing.
GPR4 (aka GPRC6.1 [9]) is predominantly found in lung, although it is also present in kidney, heart, liver, skeletal muscle, ovary, and placenta [8–10]. In an overexpression system, GPR4 was linked to the activation of Gs and production of cAMP with pH responsiveness similar to OGR1 [3]; however, its signaling in more physiologically relevant contexts (i.e., aortic smooth muscle cells) remains unclear [7]. GPR4 is important for regulating angiogenesis [10], and it may also be important for the inflammatory response in endothelial cells [8].
The third H+-sensing GPCR is T cell death-associated gene (8TDAG8, GPR65), which seems to be exclusively present in immune cells and lymphoid tissues [6, 11]. Activation of TDAG8 by acidic pHe leads the production of cAMP in a variety of transfected cultured cell lines [4, 12, 13] as well as in thymocytes and splenocytes [13]. TDAG8 overexpression in thymocytes promoted glucocorticoid-induced apoptosis [14]. TDAG8 genetic knockdown did not affect immune development or glucocorticoid-induced apoptosis [15]; however, the lack of phenotype may be due to compensation by other pH-sensing GPCRs or redundant pH-sensing mechanisms.
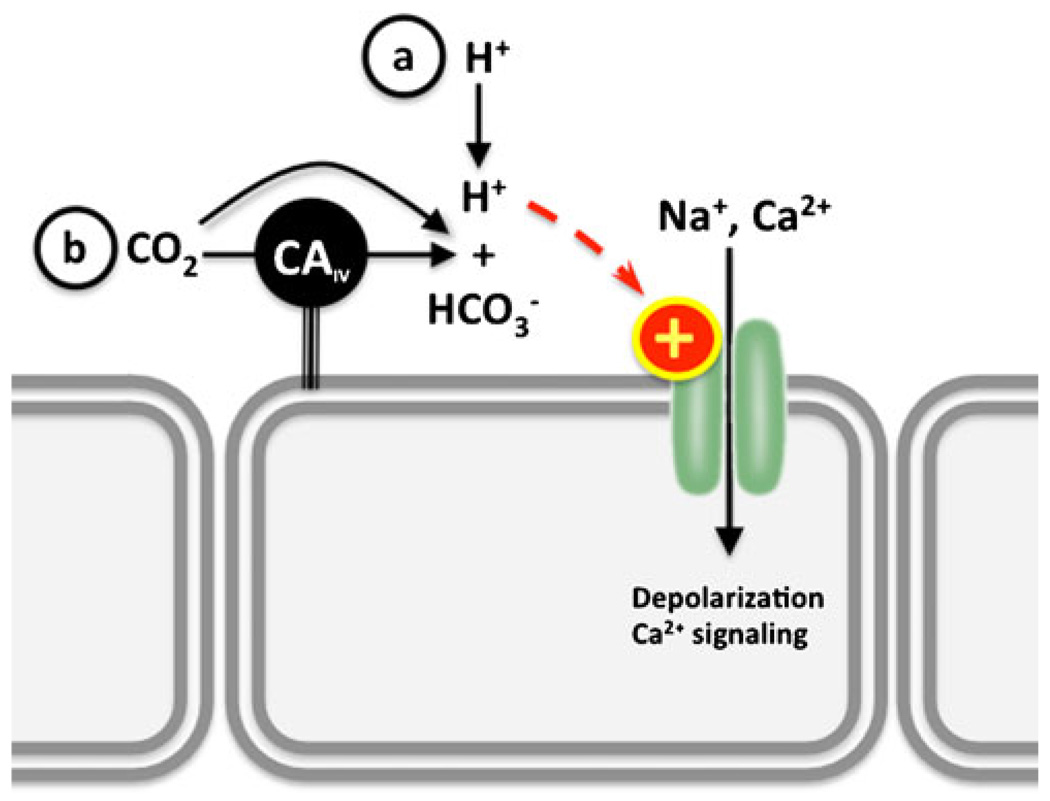
In addition to the H+-sensing GPCRs, pHe is also sensed via H+-sensitive ion channels, for example, some inwardly rectifying K+ channels are regulated by intra- and/or extracellular pH. In general, acidic pH inhibits channel current while alkaline pH enhances it (reviewed in [16]). The better-characterized pH-sensitive K+ channels are the rat outer medullary K+ channel (ROMK) [17] and the TWIK-related acid-sensitive K+ channel (TASK) [18]. ROMK, TASK, and related inwardly rectifying K+ channels have been proposed to function as pH sensors throughout the body (especially in the kidney and in the brain stem) by modulating cell membrane potential (recently reviewed in [16, 19, 20]). Other H+-sensitive ion channels are predominantly found in sensory neurons and are involved in the signal transduction of pain or taste. These channels are gated by increases in [H+]e, and their activation stimulates conductance of K+, Na+, and/or Ca2+, which triggers action potentials (Fig. 1). There are two major groups of H+-stimulated sensory channels: transient receptor potential (TRP) channels and acid-sensing ion channels (ASICs).
Fig. 1.
Extracellular pH/CO2 sensing by H+-gated channels in sensory neurons. a Extracellular H+, which could derive from elevated CO2 (b), stimulate cation currents. Examples include TRP channels, such as PKD2L1 and TRPV1, and ASICs. If coexpressed with extracellular carbonic anhydrase IV (CAIV), these channels can indirectly sense extracellular CO2
Polycystic kidney disease 2-like 1(PKD2L1) is a TRP channel stimulated by low pHe. PKD2L1 is present in taste receptor cells in the tongue, where it transduces sour stimuli (i.e., pHe<5) [21]. PKD2L1 is also present in a discrete population of neurons around the central canal of the spinal cord, which respond to minute pH variations around pH 7.4 [21]. This differential sensitivity to pHe, as well as its functional expression on cellular membranes, is presumed to be due to tissue-specific association with modifier proteins [21, 22]. Additional information can be found in recent reviews on taste receptors [23] and PKD channels [24]. PKD2L1 is also involved in CO2 sensing; in tongue taste buds, PKD2L1 senses extracellular acidification subsequent to carbonic anhydrase IV-dependent hydration of CO2 (see below).
A second TRP channel activated by acidic pHe is the vanilloid receptor-1 (VR1 or TRPV1). At pHe<5.9, TRPV1 channels display increased open probability [25]. The stimulatory mechanism depends on the interaction of protons with two glutamic acid residues in the extracellular loop (reviewed in [26]. Because TRPV1 is also stimulated by capsaicin (the main ingredient of hot chili peppers) and heat [27], this channel is thought to sense burning pain throughout the body [28, 29].
Acid-sensing ion channels (ASICs), which are predominantly expressed in neuronal and neuroepithelial tissues [30–33], are activated by acidic pHe<7 [32]. ASICs, like all members of the degenerin family of channels, are more permeable to Na+, but they also transport Ca2+ and K+ [31, 32]. There are several ASIC isoforms and splice variants, and their association into hetero- or homo-multimers may confer specific regulatory and kinetic properties in different tissues [33, 34].
In ASICs, gating by protons involves multiple residues in the extracellular domain, most notably carboxyl–carboxylate interactions between amino acids in the acidic pocket. These interactions result in the movement of a distal domain that blocks or unblocks the channel pore (ball and socket joint) [35]. Proton gating may also involve displacing Ca2+ ions which stabilize the closed state (reviewed in [36]).
ASICs are known to be important for nociception and taste [30–33], and they are also implicated in touch sensation in hairy skin [37], control of circulation by arterial baroreceptors [38], and pHe sensing by carotid body glomus cells [39]. In addition, certain ASIC variants are present in the pituitary gland [40], bone [41], and smooth muscle [42] (among others [43]), with proposed roles in endocrine function, mineralization, and cell migration and control of circulation, respectively. However, it is not known if these functions are related to pHe sensing. Finally, similar to PKD2L1, ASICs can also mediate CO2 sensing via the associated variation in pHe [44] (see below).
Intracellular pH (pHi) sensors
Originally, it was posited that the H+ and HCO3− transporting proteins which restore pHi would be directly responsible for pHi sensing. While it is true that a subset of these transporters are allosterically modulated by pHi (e.g., sodium/hydrogen exchanger (NHE) [45]), their responsiveness is not sufficiently sensitive: pHi regulation demands finer control than the observed kinetic responsiveness of NHEs and other pHi regulatory ion transporting proteins (reviewed in [46]).
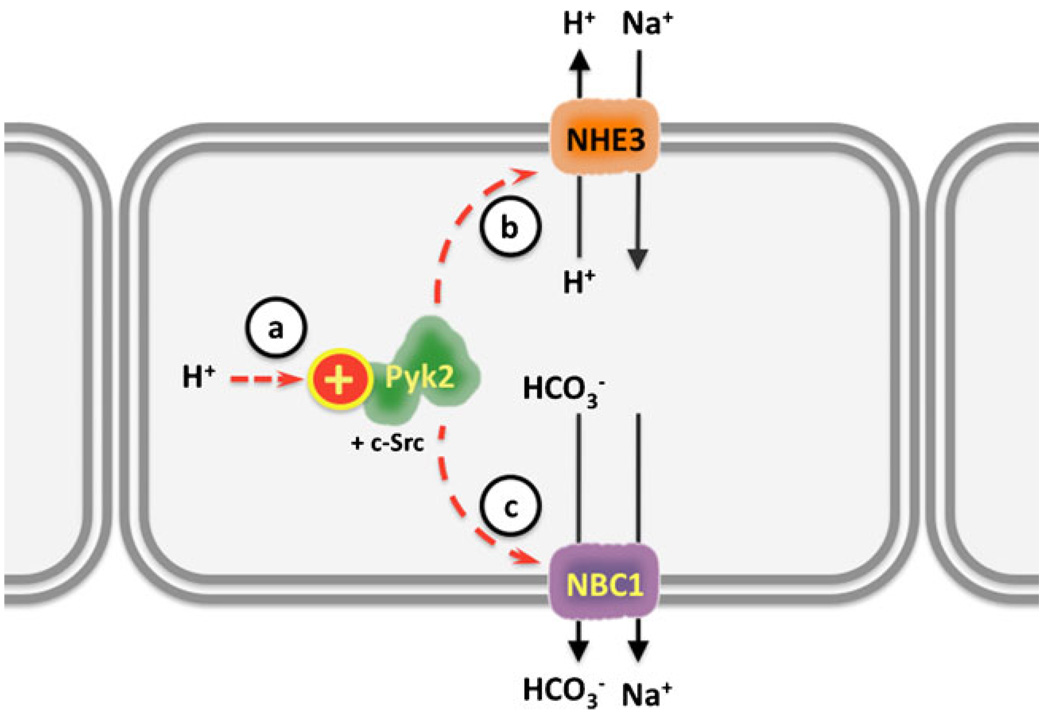
One “signaling” pHi sensor is Pyk2, a member of the focal adhesion kinase family of tyrosine kinases [47]. Pyk2 is directly activated by acidic pH, both in cultured cells and in cell-free systems [48]. Although the exact molecular mechanism for acid activation is not known, it results in increased affinity for Pyk2’s substrate ATP (decreased Km) without changing its Vmax and is dependent on Ca2+ ions in the medium [48]. The initial steps of Pyk2 activation by acidic pH are autophosphorylation of tyrosine 402 [49] followed by a physical association with [48], and phosphorylation of, the tyrosine kinase c-Src [49]. While both c-Src and Pyk2 are activated by acidic pH [50], Pyk2 is considered the pHi sensor because its activation precedes the other events, and c-Src activation is prevented by dominant-negative Pyk2 or siRNA-mediated inhibition of Pyk2 [48].
One of the consequences of acid stimulation of Pyk2 is NHE3 activation [48], which may be mediated by enhanced NHE3 transcription [51, 52] and/or by increasing NHE3 insertion into apical membranes [52]. NHE3 activation enhances H+ secretion to restore pHi (reviewed in [49]). The Pyk2/c-Src/NHE3 pathway is thought to be important for the compensation of systemic metabolic acidosis by the renal proximal tubule (reviewed in [46, 49]) (Fig. 2). Pyk2 and c-Src also mediate the activation of the Na+/HCO3− cotransporter 1 (NBC1, Slc4a4) in response to intracellular acidification by CO2 [53]. Thus, Pyk2-mediated responses to acidosis can involve increases in both apical H+ secretion via NHE3 and basolateral HCO3− absorption via NBC1 (Fig. 2). Finally, Pyk2 and c-Src are also important for the normal functioning of osteoclasts [54] and endothelial cells [55, 56], suggesting their pHi sensory role extends beyond the kidney (reviewed in [46]).
Fig. 2.
Intracellular pH sensing by the tyrosine kinase Pyk2 in the renal proximal tubule. a Intracellular H+ activate PyK2 and c-Src, which promote apical H+ secretion and Na+ absorption by b sodium hydrogen exchanger 3 (NHE3) and c Na+ and HCO3− absorption by sodium bicarbonate cotransporter (NBC)
The renal proximal tubule possesses a pHi-sensing pathway independent of Pyk2 which leads to ERK activation via an unknown mechanism [49, 57]. One candidate for this Pyk2-independent pHi sensing would be HCO3−-sensitive soluble adenylyl cyclase (sAC) (described below). The sAC has been shown to be upstream of ERK activation in other cells [58], and it is present in the renal proximal tubule [59]. However, the involvement of sAC in renal ERK activation has not yet been examined.
The pH-sensing protein kinases are also implicated in bacteria. Salmonella sense the lower pH inside the mammalian phagosome to stimulate transcription of the genes essential for virulence. In this system, the sensor for lowered pH is kinase PhoQ, which is directly activated by exposure of the sensor domain to pH 5.5 [60]. Also in Salmonella, pHi sensing is important for activation of the machinery necessary for delivering toxin to their host’s cells, but in this case, the sensor is not yet known [61].
Bacteria also sense pHi via second messenger generation. The adenylyl cyclase from Mycobacterium tuberculosis (Rv1264) [62] is activated by low pH. The molecular mechanism that confers Rv1264 pH sensitivity depends on the separation of an inhibitory subunit from the catalytic subunit [62]. Although Rv1264 was hypothesized to be important for sensing and counteracting acidification of phagolysosomes during host invasion [62], mutants lacking this cyclase demonstrated normal virulence [63]; no other in vivo role of Rv1264 is known.
The adenylyl cyclases from enteric bacteria (CyaA) such as Escherichia coli are also pH sensitive, but they are activated by high pH [64]. Purified CyaA demonstrates a very steep pH-dependency in the physiological range (between pH 7 and 9) in vitro, and while CyaA mediates the well-studied process of catabolite repression (reviewed in [65]), there is no defined role for pHi sensing in this process. Conversely, it has been postulated that cAMP mediates physiological responses to pHi changes [66, 67], but a definitive role for CyaA has not been established. Even though orthologs of Rv1264 and CyaA have not been found in eukaryotes, these studies reveal an evolutionarily ancient connection between second messenger signaling and pHi sensing, and establish the paradigm of nucleotidyl cyclases acting as molecular sensors of pHi.
Recent reviews about other molecular pHi-sensing mechanisms can be found in [68] and [69].
Bicarbonate (HCO3−) sensors
Inside cells, HCO3− is used as a cofactor for carboxylation reactions, particularly via the vitamin K-dependent carboxylases, whose substrates play roles in blood coagulation, apoptosis, bone mineralization, calcium homeostasis, growth control, and signal transduction in the brain [70, 71]. However, vitamin K-dependent carboxylase is maximally stimulated at a [HCO3−]<2 mM [72], which is way below its intracellular level, indicating that their activity would not fluctuate with physiologically relevant changes in bicarbonate. Thus, although they are HCO3−-utilizing enzymes involved in signaling cascades, they do not satisfy the requirements for functioning as HCO3− sensors.
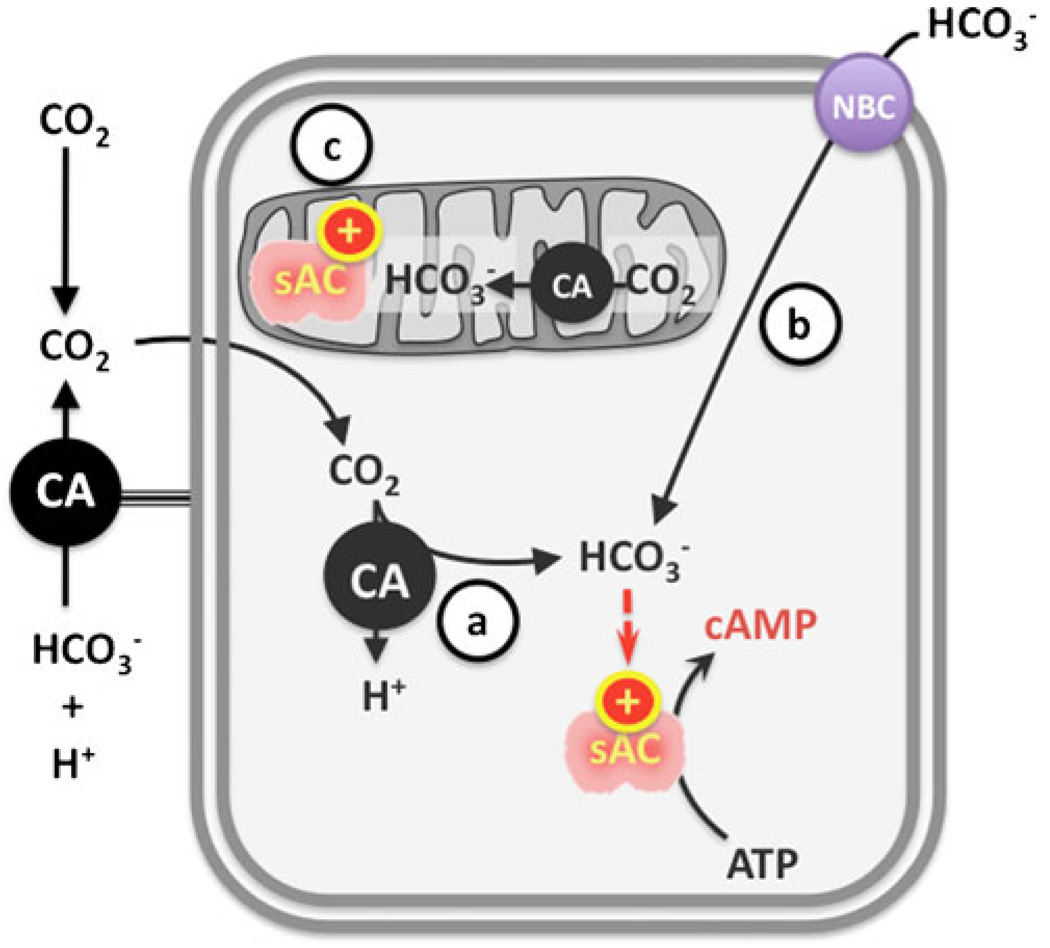
In contrast, two vertebrate signaling enzymes are responsive to physiologically relevant changes in intracellular [HCO3−]—soluble adenylyl cyclase (sAC, also known as ADCY10 and Sacy) and guanylyl cyclase-D (GC-D) [73–75]—for example, sAC has been demonstrated to be modulated in vivo by [HCO3−] which (a) originates via carbonic anhydrase-dependent hydration of exogenous CO2 [59, 75–77], (b) enters the cell via DIDS-sensitive NBCs [Choi et al., unpublished], and (c) is generated from metabolic CO2 production inside mitochondria [78, 79] (Fig. 3).
Fig. 3.
Activation of sAC inside the cells. Production of cAMP by sAC can be modulated by HCO3− which a originates via carbonic anhydrase (CA) hydration of exogenous CO2, b enters the cell via sodium bicarbonate cotransporter (NBC), or c is generated from metabolic CO2 production inside mitochondria
sAC is directly activated by HCO3− to produce the second messenger cAMP [73]. sAC is widely expressed [80–82], and it is distributed throughout the cell’s cytoplasm and in organelles such as the nucleus, centriole, mitotic spindle, mid-body, mitochondria [83], and in cilia [84]. sAC orthologs are present from cyanobacteria to mammals, suggesting that HCO3− sensing via sAC-like cyclases is evolutionarily conserved [73, 85].
A mechanistic model for cAMP formation from ATP by sAC-like enzymes has been deduced from the HCO3−-regulated cyanobacterial sAC homolog CyaC [86], which is both structurally and kinetically similar to mammalian sAC [86, 87]. Like other class III adenylyl cyclases, sAC requires two divalent cations for activity [88, 89]. Though active with Mg2+ as the only available divalent ion, addition of Ca2+ increases the affinity (decreases Km) for its substrate ATP to values consistent with the concentration of ATP found inside the cells [90]. These data suggest that in vivo mammalian sAC utilizes both Mg2+ and Ca2+, and that its activity will be sensitive to ATP fluctuations inside the cells. The structure- and kinetics-based model predicts that Ca2+ bound to the γ-phosphate of ATP enters the catalytic site and coordinates with specific residues in the sAC catalytic center, resulting in an “open sAC state”. The second divalent metal, in this case a Mg2+ ion, then binds to the α-phosphate of ATP, leading to a “closed state” by interaction with a different set of catalytic residues. The change from “open” to “closed” states induces the release of the β- and γ-phosphates and esterification of the remaining alpha phosphate with C3 of the ribose in adenosine (“cyclizing”). HCO3− stimulates substrate turnover (Vmax) by inducing an allosteric change which leads to active site closure, recruitment of the catalytic Mg2+, and rearrangement of the phosphates in the bound ATP, thus facilitating cAMP formation and release [87].
There is a single functional sAC gene in the human genome; however, it utilizes multiple promoters, and sAC mRNA undergoes extensive alternative splicing [80, 81, 84, 91]. Full-length mammalian sAC (sACfl) is comprised of two heterologous catalytic domains (C1 and C2) which constitute the amino terminal 50 KDa of protein. The additional ~140-kDa C terminus of sACfl includes several putative regulatory domains such as an autoinhibitory region [92] and canonical P-loop and leucine zipper sequences [85] of as yet unknown functions. The minimal functional sAC variant, termed sACt, is a truncated form almost exclusively comprised of C1 and C2 [85, 91]. This C1C2 protein has robust intrinsic basal and HCO3−-stimulated activities, and it is sensitive to all known sAC inhibitors. Other sAC variants have not yet been characterized in detail, but molecular studies predict the existence of C2-only isoforms resulting from alternative splicing and promoter utilization [80, 81, 84]. Existence of these C2 isoforms provide a rationale to explain why male infertility is the only reported phenotype of the existing sAC-knockout mouse model (which deleted exons encoding the C1 domain) [93–95]; this sAC-C1 knockout mouse seems to retain the alternative promoter generating the putative C2-only proteins [80]. Confirmation of this hypothesis awaits generation of KO mice which specifically disrupt the C2 domain.
For mammalian sAC, HCO3− half-maximal effect (EC50) ranges between 10 and 25 mM [73, 90], which is roughly equivalent to the [HCO3−] levels found inside the cells and in plasma. In contrast, the HCO3− EC50 for sAC from the dogfish shark is ~5 mM [76], reflecting the lower [HCO3−] of the internal fluids of aquatic animals compared to air breathers. It is thus possible that the kinetics of HCO3− activation will be specific for each sAC ortholog so they reflect the characteristics of that species physiology.
sAC serves as a sensor of extracellular pH/HCO3− in the acid/base regulatory epithelia of the mammalian epididymis [59, 96] and kidney collecting duct [97, 98], and in shark gills [76]. Despite the evolutionary and physiological diversity of these epithelia, the sAC-dependent mechanisms for sensing and regulating pH and [HCO3−] in extracellular fluids are astonishingly similar (Fig. 4). In each case, the extracellular stimulus (i.e., elevated luminal pH or [HCO3−]) is transmitted inside of the cell either by HCO3− movement through anion transporters [99] or by entrance of CO2 via diffusion (or by facilitated diffusion through aquaporins [76, 99, 100]). When CO2 diffusion into the cell is the initiating signal, extracellular carbonic anhydrase IV presumably functions to rapidly equilibrate increases in [HCO3−]e into readily available CO2 [77, 99], which can diffuse into the cell. A second carbonic anhydrase (type II) inside the cell would be essential for hydrating CO2 back into H+ and HCO3− [59, 77, 99].
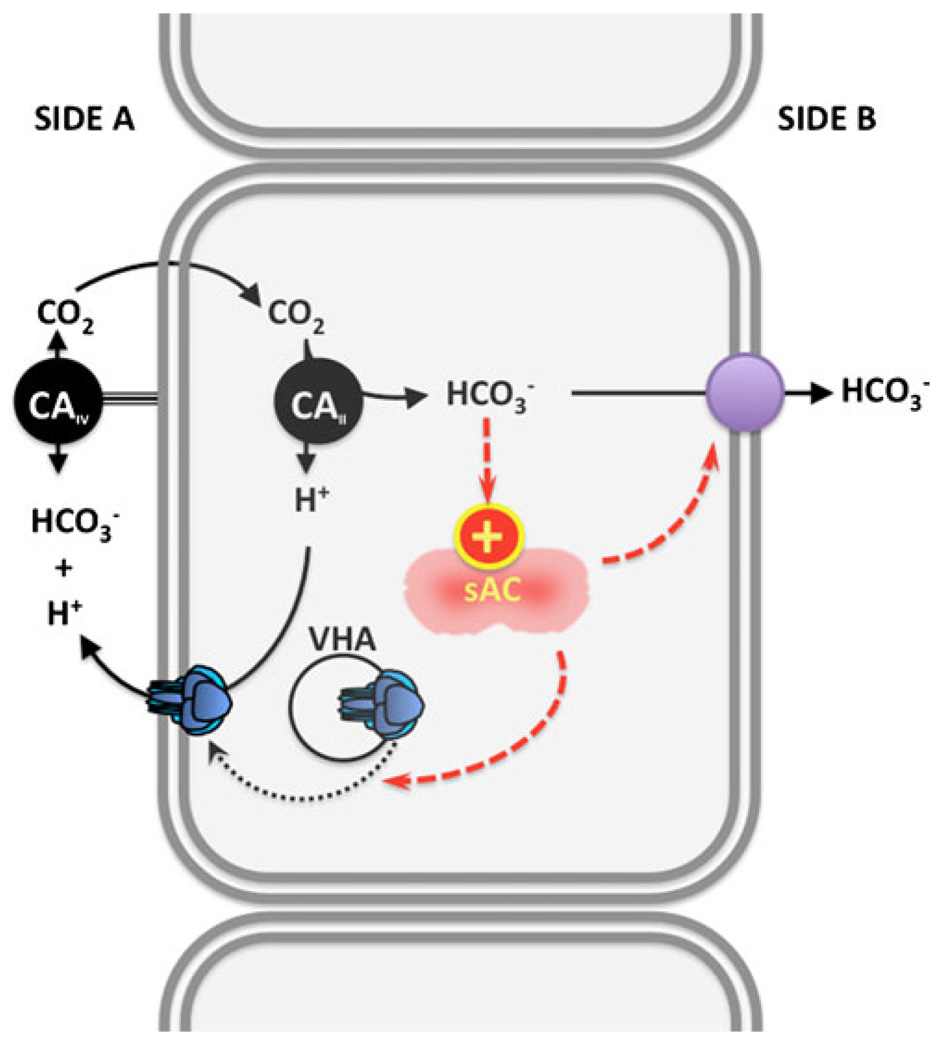
Fig. 4.
Sensing and regulation of systemic pH and acid/base status by carbonic anhydrase (CA), sAC, and vacuolar proton pump (VHA). Alkalosis due to elevated extracellular HCO3− and/or pH results in elevated extracellular CO2, a reaction catalyzed by extracellular CAIV. CO2 diffuses inside the cell, where it is hydrated into H+ and HCO3−. sAC is activated by intracellular HCO3− to produce cAMP, which promotes (via PKA) the insertion of VHA-containing vesicles into the cell membrane facing the alkalosis. Membrane-inserted VHAs secrete H+, which counteract the alkalosis. sAC may also modulate the activity of HCO3− transporters in the opposite membrane. In the clear cells of the epididymis and in A-type renal intercalated cells, Side A is the apical (mucosal) side and Side B is the basolateral (serosal) side. The polarity is reversed in base-secreting cells of the shark gill and in B-type renal intercalated cells
Regardless of how the bicarbonate enters the cells, in these pH/HCO3− sensing epithelia, the elevated [HCO3]i stimulates sAC to produce cAMP, which activates protein kinase A (PKA) [96, 98]. The end effect is translocation of vacuolar proton pump (V-ATPase)-containing vesicles into the cell membrane facing the increase in pHe/[HCO3−]e. In the clear cells of the epididymis and in renal A-type cells, the sAC-dependent V-ATPase translocation is into the apical membrane, producing the elongation of apical microvilli [59, 98, 101], while in shark gill base-secreting cells, the sAC-dependent V-ATPase translocation is into the basolateral membrane [76, 77, 102, 103]. The transport of H+ to the side with the original alkalotic stimulus not only counteracts the original alkalosis but it also supplies the H+ which can combine with the elevated HCO3− to generate the CO2 which fuels the cycle. In the whole animal experiments in the shark, this cellular mechanism was demonstrated to be relevant for systemic pH regulation. The components of this self-regulating loop, sAC, carbonic anhydrase, and V-ATPase are evolutionarily ancient enzymes, suggesting that sensing and regulation of pH/ [HCO3−] via this signaling module is likely to be conserved. Variations of this mechanism due to differential CO2 permeability, cell polarization, and association with other sensors and/or downstream effector proteins could result in additional homeostatic mechanisms for CO2/HCO3−/pH.
Bicarbonate sensing via sAC is not limited to systemic acid/base regulation. sAC activity was originally detected in mammalian testis [104, 105], specifically in male germ cells. A biochemically related but particulate activity was detected in spermatozoa [104], and its activity was postulated to be downstream of a NaHCO3 signal [106– 109]. Once the sAC gene was cloned [85], it was confirmed that sAC isoforms are highly expressed in testis and sperm [82]. Prior to ejaculation, sperm are stored in the lumen of the epididymis, where [HCO3−] is much lower than in plasma (≤5 mM) [110]. Sperm are immotile and incompetent to fertilize an egg while in the epididymis, but upon ejaculation, sperm are mixed with prostatic and seminal fluids containing 25 mM HCO3−, which enters sperm via Na+/HCO3− cotransporters [111]. The HCO3− influx induces two stages of sperm activation essential for fertilization, motility, and capacitation [112–114], which were pharmacologically [95] and genetically [93–95, 115] confirmed to be due to sAC. Thus, sAC functions as a physiological bicarbonate sensor in mammalian sperm essential for fertilization.
In recent years, HCO3− sensing via sAC has also been associated with the regulation of NaCl absorption in mammalian kidney [116] and fish intestine [117]; cystic fibrosis transmembrane conductance regulator (CFTR) in human airways [118, 119] and bovine cornea [120]; osteoclast formation [121], early embryo development [122], and K+ secretion in colon [123]; and fluid secretion in cholangiocytes [124] (Table 1).
Table 1.
Putative CO2/pH/HCO3− sensing via sAC
| Function | |
|---|---|
| pH and [HCO3−] sensing | |
| Acid/base and pH regulation | Regulation of V-ATPase recycling/translocation to the cell membrane in clear cells of the epididymis [59, 96], A-type intercalated cells of mammalian kidney [97, 98, 101], and base-secreting cells in shark gill [76, 77]. |
| Ion and fluid transport | NaCl absorption in renal collecting duct [116] and toadfish intestine [117], HCO3− transport in epithelial airways [118], Cl− secretion in cornea [120], K+ secretion in colon [123], and fluid and Cl− secretion in pancreas [124]. |
| Regulation of gene transcription | CREB phosphorylation [154]; CFTR gene transcription [119]. |
| Sperm function | Sperm motility, capacitation, and possibly acrosome reaction [93–95, 115, 155]. |
| Brain metabolic coupling | Glycogen breakdown and lactate release by astrocytes to be used by neurons [Choi HB, Gordon GRJ, Zhou N, Tai C, Ryu JK, McLarnon JG, Levin LR, Buck J, MacVicar BA (2010) Metabolic communication between astrocytes and neurons via bicarbonate responsive soluble adenylyl cyclase. unpublished]. |
| Development and cell differentiation | Osteoclastogenesis [121]; early embryo development [122]. |
| CO2 sensing | |
| Cilia movement | Cilliary beat frequency in airway epithelia [84]. |
| Metabolic regulation | Mitochondrial oxidative phosphorylation in response to CO2 from TCA cycle [78, 79]. |
Like sAC, GC-D is directly activated by HCO3−, but GC-D produces the second messenger cGMP instead of cAMP [74, 75]. The EC50 of GC-D for [HCO3−] is ~20 mM, both in intracellular cGMP accumulation experiments in cells overexpressing GC-D and in cGMP production assays with purified recombinant GC-D catalytic domain [74, 75]. In rodents, GC-D is present primarily, if not exclusively, in olfactory neurons [125], and it appears to be a pseudogene in primates [126].The proposed function for GC-D is as a putative sensor of atmospheric CO2 in rodents [75, 127]; however, it is difficult to rectify how an enzyme whose EC50 for [HCO3−] corresponds to ~4% CO2 could sense changes in atmospheric CO2 (~0.03%).
Carbon dioxide (CO2) sensors
In insects and nematodes, CO2 sensing induces either attraction or avoidance depending on the species and the particular neurons involved (recently reviewed in [128]). In CO2-sensing insects, like the malaria-carrying Anopheles mosquito and the fruit fly Drosophila, gaseous CO2 is sensed by chemosensing neurons of the olfactory system [129]. Drosophila also sense CO2 in solution, but this is mediated by different neurons of the gustatory system [130]. In fruit flies and the mosquito, two seven-transmembrane spanning members of the gustatory receptor family (Gr21A and Gr63A) are essential for sensing volatile CO2 [131]; however, the exact molecular mechanisms, including whether they sense CO2, HCO3−, or H+, and their downstream signaling cascade(s), remain unknown (reviewed in [128]).
In the nematode Caenorhabditis elegans, CO2 induces an avoidance behavior mediated via cGMP [132, 133]. The avoidance response is not induced by acidic pH or elevated [HCO3−] in media, leading the authors to suggest that the stimulus is a change in the concentration of H+, HCO3− and/or CO2 inside sensing cells [132]. The receptor guanylyl cyclase DAF-11 [133] as well as the cyclic nucleotide-gated channel subunits TAX-2 and TAX-4 [132, 133] are essential for C. elegans avoidance behavior and establish the link to cGMP. However, DAF-11 does not seem to be present in the same neurons as TAX-2 and TAX-4 [134], which possibly indicates a complex system with more than one chemoreceptor located in several sensing neurons.
In mammals, CO2 is sensed in kidney, airways, tongue, and peripheral and central chemoreceptors. Although some prokaryote adenylyl cyclases may be directly activated by CO2 [135], a similar regulation has not yet been unambiguously identified in mammals. In part, this may be due to the difficulties associated with differentiating between direct effects of CO2 from effects due to pH and HCO3−. The use of out-of-equilibrium CO2/HCO3− solutions [136] is one of the few ways in which this issue can be addressed. Using this approach, sensitivity to pharmacological inhibitors implicates tyrosine kinases in promoting acid secretion/bicarbonate reabsorption in response to basolateral (“blood”) CO2 in kidney proximal tubules [137, 138]. Interestingly, these experiments conclude that the inducing signal is CO2/HCO3− and not pHi, which suggest involvement of an as yet undefined CO2/HCO3− sensor instead of being mediated by Pyk2 and c-Src, which are proposed to function in the proximal tubules to activate apical H+ secretion by NHE3 in response to acidosis [46, 48, 49].
Because of the rapid equilibration of CO2, HCO3−, and H+ by carbonic anhydrases, in multiple physiological systems, CO2 is sensed by a pH sensor or a HCO3− sensor coupled with a carbonic anhydrase, for example, as mentioned above, the mechanism for extracellular CO2 sensing in sour taste receptor cells depends on extracellular carbonic anhydrase IV and PKD2L1 [139], the polycystickidney-disease-like acid-selective ion channel [21, 22]. Carbonic anhydrase IV hydrates CO2 into HCO3− and H+ almost instantaneously; the elevated [H+] opens PKD2L1 channels, which allow the entry of cations into the cell and elicit the neuronal response [139] (Fig. 1). Extracellular CO2 is also sensed via related variations in pHe by acid-sensing ion channels (ASICs)1 in the amygdala, and this pathway is important in fear-induced behaviors [44].
In vertebrates, peripheral chemoreceptors, specifically the aortic and carotid bodies, sense changes in arterial CO2 and pH while central chemoreceptors sense CO2 and pH of cerebral spinal fluid, and both types of chemoreceptors regulate breathing frequency and tidal volume. The chemosensing neurons may sense CO2, [HCO3−], pHe, pHi, transmembrane pH gradient, or oxidative stress, with the ultimate response possibly depending upon a combination of multiple sensors [140]. Although the potential direct effect of CO2/H+ on ion channels has been extensively studied ([141–144], see previous section on H+ sensors), no molecular chemosensor has been unambiguously identified. Bicarbonate-sensitive sAC represents an additional intriguing candidate which has not yet been directly tested. sAC is present in carotid body [145], and cAMP levels in carotid body are elevated during hypercapnia [145, 146], reviewed in [140]. sAC was hypothesized to sense intracellular increases in [HCO3−] derived from hypercapnia in type I (glomus) carotid body cells and to augment Ca2+ influx through L-type Ca2+ channels via PKA [146]. This stimulation is independent of changes in pHe or pHi [146] and, like the CO2 sensing mechanisms explained above, probably depends on a carbonic anhydrase (c.f.[147]). We have confirmed the presence of sAC in rat glomus cells by immunofluorescence (unpublished observations), which also express abundant intracellular carbonic anhydrase [148, 149]. Additionally, L-type Ca2+ channels are activated by elevated CO2, independently of pHi, in rat locus coeruleus neurons [143], suggesting sAC could also be important in central chemosensing; however, direct functional evidence for the role of sAC as a molecular sensor of hypercapnia in the carotid body or in other peripheral and central chemoreceptors, is lacking.
It has already been demonstrated that bicarbonate-regulated adenylyl cyclases, in conjunction with carbonic anhydrases, can function as CO2 sensors. In the pathological fungi Candida albicans and Cryptococcus neoformans, the adenylyl cyclases (Cyr1) are essential for pathological differentiation. In both cases, the ACs are directly regulated by HCO3− [150, 151], and deletion of either the cyclase or the carbonic anhydrase abrogates pathogenicity. These pathogenic fungi know when they are inside an infected host, and hence when they should undergo their pathogenic differentiation, by sensing the host’s internal [CO2] (5%), which is 150-fold higher than the ambient [CO2]e (0.03%).
In mammals, CO2 chemosensing via sAC has been demonstrated in the regulation of ciliary beat frequency (CBF) in lung epithelial cells. CO2 exposure increases CBF in cultured human lung epithelial cells differentiated at an air-liquid interface. This response is mediated by CO2/ HCO3−-dependent stimulation of sAC localized to the cilia’s axoneme [84]. Carbonic anhydrases are present in the apical area of ciliated cells of bronchiolar epithelium [152], but a direct link of carbonic anhydrases to CBF has not yet been established. Analogy with other CO2-sensing mechanisms suggests that carbonic anhydrases mediate the stimulation of sAC in cilia. Because CO2 levels are higher in exhaled breath compared to inhaled air, the regulation of CBF by sAC may ensure the proper clearance of mucus from airway epithelia, and impairments in this regulation may be associated with airway diseases such as asthma [84, 153].
Carbonic anhydrase and sAC also sense metabolically generated CO2 inside mitochondria. Mitochondria are the predominant source of CO2 in eukaryotic cells, and sAC is present inside mitochondria [83], where it coordinates the rate of ATP production via oxidative phosphorylation with nutritional availability [78, 79]. Mitochondrial sAC activity is stimulated by Krebs Cycle-generated CO2 in a carbonic anhydrase-dependent manner. CO2/HCO3− stimulation of sAC activates intramitochondrial PKA which phosphorylates complex IV of the electron transport chain, increasing its rate and capacity to handle electrons. Thus, a mitochondrial CO2–carbonic anhydrase–sAC pathway couples nutritional status to ATP production to ensure minimal generation of reactive oxygen species, and sAC senses variations of intracellular (metabolic) changes in CO2/HCO3− in addition to [HCO3−]i secondary to extracellular (blood, environment) CO2, HCO3− and pH changes.
Conclusions
Biological systems sense CO2, HCO3−, and pH via multiple different types of sensors, and while the specifics may vary, the fundamentals of sensing are evolutionarily conserved. HCO3 and pH are directly sensed by protein kinases and nucleotydyl cyclases from bacteria (e.g., CyaA, PhoQ) through vertebrates (e.g., sAC, Pyk2), and CO2 is indirectly sensed by HCO3− or pH sensors coupled to carbonic anhydrases. Thus, mechanisms for sensing pH, [HCO3−], and CO2 are present in all domains of life illustrating their importance for organismal function.
Acknowledgements
We thank Dr. Carsten Wagner (University of Zurich, Switzerland) for the insightful comments on the manuscript.
Footnotes
In this case, it is not known whether the mechanism relies on the presence of a carbonic anhydrase.
Contributor Information
Jochen Buck, Email: jobuck@med.cornell.edu.
Lonny R. Levin, Email: llevin@med.cornell.edu.
References
- 1.Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 2.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2101;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 4.Wang J-Q, Kon J, Mogi C, Tobo M, Damirin A, Sato K, Komachi M, Malchinkhuu E, Murata N, Kimura T, Kuwabara A, Wakamatsu K, Koizumi H, Uede T, Tsujimoto G, Kurose H, Sato T, Harada A, Misawa N, Tomura H, Okajima F. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J Biol Chem. 2004;279:45626–45633. doi: 10.1074/jbc.M406966200. [DOI] [PubMed] [Google Scholar]
- 5.Komarova SV, Pereverzev A, Shum JW, Sims SM, Dixon SJ. Convergent signaling by acidosis and receptor activator of NF-κB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc Natl Acad Sci USA. 2005;102:2643–2648. doi: 10.1073/pnas.0406874102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomura H, Mogi C, Sato K, Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal. 2005;17:1466–1476. doi: 10.1016/j.cellsig.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Tomura H, Wang J-Q, Komachi M, Damirin A, Mogi C, Tobo M, Kon J, Misawa N, Sato K, Okajima F. Prostaglandin I2 production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J Biol Chem. 2005;280:34458–34464. doi: 10.1074/jbc.M505287200. [DOI] [PubMed] [Google Scholar]
- 8.Seuwen K, Ludwig M-G, Wolf RM. Receptors for protons or lipid messengers or both? J Recept Signal Transduct Res. 2006;26:599–610. doi: 10.1080/10799890600932220. [DOI] [PubMed] [Google Scholar]
- 9.An S, Tsai C, Goetzl EJ. Cloning, sequencing and tissue distribution of two related G protein-coupled receptor candidates expressed prominently in human lung tissue. FEBS Lett. 1995;375:121–124. doi: 10.1016/0014-5793(95)01196-l. [DOI] [PubMed] [Google Scholar]
- 10.Yang LV, Radu CG, Roy M, Lee S, McLaughlin J, Teitell MA, Iruela-Arispe ML, Witte ON. Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor. Mol Cell Biol. 2007;27:1334–1347. doi: 10.1128/MCB.01909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi J-W, Lee SY, Choi Y. Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol. 1996;168:78–84. doi: 10.1006/cimm.1996.0051. [DOI] [PubMed] [Google Scholar]
- 12.Ishii S, Kihara Y, Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem. 2005;280:9083–9087. doi: 10.1074/jbc.M407832200. [DOI] [PubMed] [Google Scholar]
- 13.Radu CG, Nijagal A, McLaughlin J, Wang L, Witte ON. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc Natl Acad Sci USA. 2005;102:1632–1637. doi: 10.1073/pnas.0409415102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosa N, Murakami M, Jia WY, Yokoyama M, Masunaga T, Iwabuchi C, Inobe M, Iwabuchi K, Miyazaki T, Onoe K, Iwata M, Uede T. Critical function of T cell death-associated gene 8 in glucocorticoid-induced thymocyte apoptosis. Int Immunol. 2003;15:741–749. doi: 10.1093/intimm/dxg070. [DOI] [PubMed] [Google Scholar]
- 15.Radu CG, Cheng D, Nijagal A, Riedinger M, McLaughlin J, Yang LV, Johnson J, Witte ON. Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Mol Cell Biol. 2006;26:668–677. doi: 10.1128/MCB.26.2.668-677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 17.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 18.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitzmann D, Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev. 2008;88:1119–1182. doi: 10.1152/physrev.00020.2007. [DOI] [PubMed] [Google Scholar]
- 21.Huang AL, Chen XK, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJP, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 24.Delmas P. Polycystins: polymodal receptor/ion-channel cellular sensors. Pflügers Arch Eur J Physiol. 2005;451:264–276. doi: 10.1007/s00424-005-1431-5. [DOI] [PubMed] [Google Scholar]
- 25.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 26.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflüg Archiv Europ J Physiol. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 27.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 28.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 29.Geppetti P, Trevisani M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141:1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJP, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 32.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 33.Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 34.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 35.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9Å resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 36.Gründer S, Chen X. Sructure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94. [PMC free article] [PubMed] [Google Scholar]
- 37.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan Z-Y, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ Res. 2007;101:1009–1019. doi: 10.1161/CIRCRESAHA.107.154377. [DOI] [PubMed] [Google Scholar]
- 40.Gründer S, Geissler H-S, Bässler E-L, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. NeuroReport. 2000;11:1607–1611. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- 41.Jahr H, van Driel M, van Osch GJVM, Weinans H, van Leeuwen JPTM. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun. 2005;337:349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 42.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res. 2008;75:202–210. doi: 10.1016/j.mvr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension. 2008;51:1265–1271. doi: 10.1161/HYPERTENSIONAHA.107.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard Iii MA, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aronson PS, Nee J, Suhm MA. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982;299:161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- 46.Gluck SL. Acid sensing in renal epithelial cells. J Clin Invest. 2004;114:1696–1699. doi: 10.1172/JCI23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein-tyrosine kinase Pyk2 involved in Ca2+-induced regulation of ion-channel and map kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 48.Li SY, Sato S, Yang XJ, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest. 2004;114:1782–1789. doi: 10.1172/JCI18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preisig PA. The acid-activated signaling pathway: starting with Pyk2 and ending with increased NHE3 activity. Kidney Int. 2007;72:1324–1329. doi: 10.1038/sj.ki.5002543. [DOI] [PubMed] [Google Scholar]
- 50.Yamaji Y, Tsuganezawa H, Moe OW, Alpern RJ. Intracellular acidosis activates c-Src. Am J Physiol Cell Physiol. 1997;272:C886–C893. doi: 10.1152/ajpcell.1997.272.3.C886. [DOI] [PubMed] [Google Scholar]
- 51.Ambuhl PM, Amemiya M, Danczkay M, Lotscher M, Kaissling B, Moe OW, Preisig PA, Alpern RJ. Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am J Physiol Renal Physiol. 1996;271:F917–F925. doi: 10.1152/ajprenal.1996.271.4.F917. [DOI] [PubMed] [Google Scholar]
- 52.Yang XJ, Amemiya M, Peng Y, Moe OW, Preisig PA, Alpern RJ. Acid incubation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Cell Physiol. 2000;279:C410–C419. doi: 10.1152/ajpcell.2000.279.2.C410. [DOI] [PubMed] [Google Scholar]
- 53.Espiritu DJD, Bernardo AA, Robey RB, Arruda JAL. A central role for Pyk2-Src interaction in coupling diverse stimuli to increased epithelial NBC activity. Am J Physiol Renal Physiol. 2002;283:F663–F670. doi: 10.1152/ajprenal.00338.2001. [DOI] [PubMed] [Google Scholar]
- 54.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the C-Src protooncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 55.Orr AW, Murhpy-Ulrich JE. Regulation of endothelial cell function by FAK and Pyk2. Front Biosci. 2004;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- 56.Kodama H, Fukuda K, Takahashi E, Tahara S, Tomita Y, Ieda M, Kimura K, Owada KM, Vuori K, Ogawa S. Selective involvement of p130Cas/Crk/Pyk2/c-Src in endothelin-1-induced JNK activation. Hypertension. 2003;41:1372–1379. doi: 10.1161/01.HYP.0000069698.11814.F4. [DOI] [PubMed] [Google Scholar]
- 57.Tsuganezawa H, Sato S, Yamaji Y, Preisig PA, Moe OW, Alpern RJ. Role of c-SRC and ERK in acid-induced activation of NHE3. Kidney Int. 2002;62:41–50. doi: 10.1046/j.1523-1755.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 58.Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol. 2008;132:329–338. doi: 10.1085/jgp.200810044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Yu X-J, McGourty K, Liu M, Unsworth KE, Holden DW. pH sensing by intracellular Salmonella induces effector translocation. Science. 2010;328:1040–1043. doi: 10.1126/science.1189000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tews I, Findeisen F, Sinning I, Schultz A, Schultz JE, Linder JU. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science. 2005;308:1020–1023. doi: 10.1126/science.1107642. [DOI] [PubMed] [Google Scholar]
- 63.Dittrich D, Keller C, Ehlers S, Schultz JE, Sander P. Characterization of a Mycobacterium tuberculosis mutant deficient in pH-sensing adenylate cyclase Rv1264. Int J Med Microbiol. 2006;296:563–566. doi: 10.1016/j.ijmm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Tao M, Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci USA. 1969;63:86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castanie-Cornet M-P, Penfound TA, Smith D, Elliott JF, Foster JW. Control of acid resistance in Escherichia coli. J Bacteriol. 1999;181:3525–3535. doi: 10.1128/jb.181.11.3525-3535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Z, Richard H, Foster JW. pH-dependent modulation of cyclic AMP levels and GadW-dependent repression of RpoS affect synthesis of the GadX regulator and Escherichia coli acid resistance. J Bacteriol. 2003;185:6852–6859. doi: 10.1128/JB.185.23.6852-6859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava J, Barber DL, Jacobson MP. Intracellular pH sensors: design principles and functional significance. Physiology. 2007;22:30–39. doi: 10.1152/physiol.00035.2006. [DOI] [PubMed] [Google Scholar]
- 69.Brown D, Paunescu TG, Breton S, Marshansky V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol. 2009;212:1762–1772. doi: 10.1242/jeb.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hassel B. Carboxylation and anaplerosis in neurons and glia. Mol Neurobiol. 2000;22:21–40. doi: 10.1385/MN:22:1-3:021. [DOI] [PubMed] [Google Scholar]
- 71.Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008;78:131–156. doi: 10.1016/S0083-6729(07)00007-6. [DOI] [PubMed] [Google Scholar]
- 72.Sadowski JA, Esmon CT, Suttie JW. Vitamin K-dependent carboxylase. Requirements of the rat liver microsomal enzyme system. J Biol Chem. 1976;251:2770–2776. [PubMed] [Google Scholar]
- 73.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 74.Guo D, Zhang JJ, Huang XY. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry. 2009;48:4417–4422. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tresguerres M, Parks SK, Salazar E, Levin LR, Goss GG, Buck J. Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc Natl Acad Sci USA. 2010;107:442–447. doi: 10.1073/pnas.0911790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tresguerres M, Parks SK, Wood CM, Goss GG. V-H+-ATPase translocation during blood alkalosis in dogfish gills: interaction with carbonic anhydrase and involvement in the postfeeding alkaline tide. Am J Physiol Reg Int Comp Physiol. 2007;292:R2012–R2019. doi: 10.1152/ajpregu.00814.2006. [DOI] [PubMed] [Google Scholar]
- 78.Acin-Perez R, Salazar E, Brosel S, Yang H, Schon EA, Manfredi G. Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol Med. 2009;1:392–406. doi: 10.1002/emmm.200900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farrell J, Ramos L, Tresguerres M, Kamenetsky M, Levin LR, Buck J. Somatic "soluble" adenylyl cyclase isoforms are unaffected in Sacytm1Lex/Sacytm1Lex “knockout” mice. PLoS ONE. 2008;3:e3251. doi: 10.1371/journal.pone.0003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geng W, Wang Z, Zhang J, Reed BY, Pak CY, Moe OW. Cloning and characterization of the human soluble adenylyl cyclase. Am J Physiol Cell Physiol. 2005;288:C1305–C1316. doi: 10.1152/ajpcell.00584.2004. [DOI] [PubMed] [Google Scholar]
- 82.Sinclair ML, Wang XY, Mattia M, Conti M, Buck J, Wolgemuth DJ, Levin LR. Specific expression of soluble adenylyl cyclase in male germ cells. Mol Reprod Dev. 2000;56:6–11. doi: 10.1002/(SICI)1098-2795(200005)56:1<6::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 83.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 84.Schmid A, Sutto Z, Nlend MC, Horvath G, Schmid N, Buck J, Levin LR, Conner GE, Fregien N, Salathe M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J Gen Physiol. 2007;130:99–109. doi: 10.1085/jgp.200709784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steegborn C, Litvin TN, Hess KC, Capper AB, Taussig R, Buck J, Levin LR, Wu H. A novel mechanism for adenylyl cyclase inhibition from the crystal structure of its complex with catechol estrogen. J Biol Chem. 2005;280:31754–31759. doi: 10.1074/jbc.M507144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steegborn C, Litvin TN, Levin LR, Buck J, Wu H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol. 2005;12:32–37. doi: 10.1038/nsmb880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Linder J. Class III adenylyl cyclases: molecular mechanisms of catalysis and regulation. Cell Mol Life Sci. 2006;63:1736–1751. doi: 10.1007/s00018-006-6072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Linder JU. Structure-function relationships in Escherichia coli adenylate cyclase. Biochem J. 2008;415:449–454. doi: 10.1042/BJ20080350. [DOI] [PubMed] [Google Scholar]
- 90.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of "soluble" adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 91.Jaiswal BS, Conti M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J Biol Chem. 2001;276:31698–31708. doi: 10.1074/jbc.M011698200. [DOI] [PubMed] [Google Scholar]
- 92.Chaloupka JA, Bullock SA, Iourgenko V, Levin LR, Buck J. Autoinhibitory regulation of soluble adenylyl cyclase. Mol Reprod Dev. 2006;73:361–368. doi: 10.1002/mrd.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–362. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 94.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB. The "soluble" adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol. 2008;294:C488–C494. doi: 10.1152/ajpcell.00537.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HA, Breton S, Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol. 2008;294:F130–F138. doi: 10.1152/ajprenal.00406.2007. [DOI] [PubMed] [Google Scholar]
- 98.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol-Renal Physiol. 2010;298:F1162–F1169. doi: 10.1152/ajprenal.00645.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology. 2005;20:417–428. doi: 10.1152/physiol.00036.2005. [DOI] [PubMed] [Google Scholar]
- 100.Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol. 2006;17:2368–2382. doi: 10.1681/ASN.2006060620. [DOI] [PubMed] [Google Scholar]
- 101.Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol-Renal Physiol. 2010;298:F643–F654. doi: 10.1152/ajprenal.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tresguerres M, Parks SK, Katoh F, Goss GG. Microtubule-dependent relocation of branchial V-H+-ATPase to the basolateral membrane in the Pacific spiny dogfish (Squalus acanthias): a role in base secretion. J Exp Biol. 2006;209:599–609. doi: 10.1242/jeb.02059. [DOI] [PubMed] [Google Scholar]
- 103.Tresguerres M, Katoh F, Fenton H, Jasinska E, Goss GG. Regulation of branchial V-H+-ATPase Na+/K+-ATPase and NHE2 in response to acid and base infusions in the Pacific spiny dogfish (Squalus acanthias) J Exp Biol. 2005;208:345–354. doi: 10.1242/jeb.01382. [DOI] [PubMed] [Google Scholar]
- 104.Braun T. The effect of divalent cations on bovine spermatozoal adenylate cyclase activity. J Cyclic Nucleotide Res. 1975;1:271–281. [PubMed] [Google Scholar]
- 105.Neer EJ. Multiple forms of adenylate cyclase. Adv Cyclic Nucleotide Res. 1978;9:69–83. [PubMed] [Google Scholar]
- 106.Garbers DL, Tubb DJ, Hyne RV. A requirement of bicarbonate for Ca2+−induced elevations of cyclic AMP in guinea pig spermatozoa. J Biol Chem. 1982;257:8980–8984. [PubMed] [Google Scholar]
- 107.Garty NB, Salomon Y. Stimulation of partially purified adenylate cyclase from bull sperm by bicarbonate. FEBS Lett. 1987;218:148–152. doi: 10.1016/0014-5793(87)81036-0. [DOI] [PubMed] [Google Scholar]
- 108.Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 1985;260:9699–9705. [PubMed] [Google Scholar]
- 109.Visconti PE, Muschietti JP, Flawia MM, Tezon JG. Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim Biophys Acta. 1990;1054:231–236. doi: 10.1016/0167-4889(90)90246-a. [DOI] [PubMed] [Google Scholar]
- 110.Levine N, Marsh DJ. Micropuncture studies of electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, epididymis and vas deferens in rats. J Physiol. 1971;213:557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demarco IA, Espinosa F, Edwards J, Sosnik J, de la Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE. Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J Biol Chem. 2003;278:7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- 112.Boatman DF, Robbins RS. Bicarbonate: carbon-dioxide regulation of sperm capacitation, hyperactivated motility, and acrosome reactions. Biol Reprod. 1991;44:806–813. doi: 10.1095/biolreprod44.5.806. [DOI] [PubMed] [Google Scholar]
- 113.Lee MA, Storey BT. Bicarbonate is essential for ferilization of mouse eggs: mouse sperm require it to undergo the acrosome reaction. Biol Reprod. 1986;34:349–356. doi: 10.1095/biolreprod34.2.349. [DOI] [PubMed] [Google Scholar]
- 114.Visconti PE, Galantino-Homer H, Moore GD, Bailey JL, Ning X, Fornes M, Kopf GS. The molecular basis of sperm capacitation. J Androl. 1998;19:242–248. [PubMed] [Google Scholar]
- 115.Schuh SM, Carlson AE, McKnight GS, Conti M, Hille B, Babcock DF. Signaling pathways for modulation of mouse sperm motility by adenosine and catecholamine agonists. Biol Reprod. 2006;74:492–500. doi: 10.1095/biolreprod.105.047837. [DOI] [PubMed] [Google Scholar]
- 116.Hallows KR, Wang HM, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem. 2009;284:5774–5783. doi: 10.1074/jbc.M805501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tresguerres M, Levin LR, Buck J, Grosell M. Modulation of NaCl absorption by [HCO3−] in the marine teleost intestine is mediated by soluble adenylyl cyclase. Am J Physiol Regul Integr Comp Physiol. 2010;299:62–71. doi: 10.1152/ajpregu.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y, Lam CS, Wu F, Wang W, Duan Y, Huang P. Regulation of CFTR channels by HCO3−sensitive soluble adenylyl cyclase in human airway epithelial cells. Am J Physiol Cell Physiol. 2005;289:C1145–C1151. doi: 10.1152/ajpcell.00627.2004. [DOI] [PubMed] [Google Scholar]
- 119.Baudouin-Legros M, Hamdaoui N, Borot F, Fritsch J, Ollero M, Planelles G, Edelman A. Control of basal CFTR gene expression by bicarbonate-sensitive adenylyl cyclase in human pulmonary cells. Cell Physiol Biochem. 2008;21:075–086. doi: 10.1159/000113749. [DOI] [PubMed] [Google Scholar]
- 120.Sun XC, Zhai CB, Cui M, Chen Y, Levin LR, Buck J, Bonanno JA. HCO3dependent soluble adenylyl cyclase activates cystic fibrosis transmembrane conductance regulator in corneal endothelium. Am J Physiol Cell Physiol. 2003;284:C1114–C1122. doi: 10.1152/ajpcell.00400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Geng W, Hill K, Zerwekh JE, Kohler T, Müller R, Moe OW. Inhibition of osteoclast formation and function by bicarbonate: role of soluble adenylyl cyclase. J Cell Physiol. 2009;220:332–340. doi: 10.1002/jcp.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen MH, Chen H, Zhou Z, Ruan YC, Wong HY, Lu YC, Guo JH, Chung YW, Huang PB, Huang HF, Zhou WL, Chan HC. Involvement of CFTR in oviductal HCO3− secretion and its effect on soluble adenylate cyclase-dependent early embryo development. Hum Reprod. 25:1744–1754. doi: 10.1093/humrep/deq094. (210) [DOI] [PubMed] [Google Scholar]
- 123.Halm ST, Zhang J, Halm DR. β-adrenergic activation of electrogenic K+ and Cl− secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2010;299:81–95. doi: 10.1152/ajpgi.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Strazzabosco M, Fiorotto R, Melero S, Glaser S, Francis H, Spirli C, Alpini G. Diferentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology. 2009;50:244–252. doi: 10.1002/hep.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fülle HJ, Vassar R, Foster DC, Yang RB, Axel R, Garbers DL. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci USA. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Young JM, Waters H, Dong C, Fülle H-J, Liman ER. Degeneration of the olfactory guanylyl cyclase D gene during primate evolution. PLoS ONE. 2007;2:e884. doi: 10.1371/journal.pone.0000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 128.Sharabi K, Lecuona E, Helenius IT, Beitel GJ, Sznajder JI, Gruenbaum Y. Sensing, physiological effects and molecular response to elevated CO2 levels in eukaryotes. J Cell Mol Med. 2009;13:4304–4318. doi: 10.1111/j.1582-4934.2009.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 130.Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- 131.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 132.Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hammer A, Hodgson DRW, Cann MJ. Regulation of prokaryotic adenylyl cyclases by CO2. Biochem J. 2006;396:215–218. doi: 10.1042/BJ20060372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao J, Hogan EM, Bevensee MO, Boron WF. Out-of-equilibrium CO2/HCO3− solutions and their use in characterizing a new K/HCO3 cotransporter. Nature. 1995;374:636–639. doi: 10.1038/374636a0. [DOI] [PubMed] [Google Scholar]
- 137.Zhou Y, Bouyer P, Boron WF. Role of a tyrosine kinase in the CO2-induced stimulation of HCO3− reabsorption by rabbit S2 proximal tubules. Am J Physiol Renal Physiol. 2006;291:F358–F367. doi: 10.1152/ajprenal.00520.2005. [DOI] [PubMed] [Google Scholar]
- 138.Zhou Y, Zhao J, Bouyer P, Boron WF. Evidence from renal proximal tubules that HCO3− and solute reabsorption are acutely regulated not by pH but by basolateral HCO3− and CO2. Proc Natl Acad Sci USA. 2005;102:3875–3880. doi: 10.1073/pnas.0500423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJP, Zuker CS. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- 141.Wellner-Kienitz M-C, Shams H, Scheid P. Contribution of Ca2+-activated K+ channels to central chemosensitivity in cultivated neurons of fetal rat medulla. J Neurophysiol. 1998;79:2885–2894. doi: 10.1152/jn.1998.79.6.2885. [DOI] [PubMed] [Google Scholar]
- 142.Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton-and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- 143.Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol (Lond) 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nunes AR, Monteiro EC, Johnson SM, Gauda EB. Bicarbonate-regulated soluble adenylyl cyclase (sAC) mRNA expression and activity in peripheral chemoreceptors. Adv Exp Med Biol. 2009;648:235–241. doi: 10.1007/978-90-481-2259-2_27. [DOI] [PubMed] [Google Scholar]
- 146.Summers BA, Overholt JL, Prabhakar NR. CO2 and pH independently modulate L-type Ca2+ current in rabbit carotid body glomus cells. J Neurophysiol. 2002;88:604–612. doi: 10.1152/jn.2002.88.2.604. [DOI] [PubMed] [Google Scholar]
- 147.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid-body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 148.Nurse CA. Carbonic-anhydrase and neuronal enzymes in cultured glomus cells of the carotid-body of the rat. Cell Tissue Res. 1990;261:65–71. doi: 10.1007/BF00329439. [DOI] [PubMed] [Google Scholar]
- 149.Rigual R, Iniguez C, Carreres J, Gonzalez C. Carbonicanhydrase in the carotid-body and the carotid-sinus nerve. Histochemistry. 1985;82:577–580. doi: 10.1007/BF00489979. [DOI] [PubMed] [Google Scholar]
- 150.Mogensen EG, Janbon G, Chaloupka J, Steegborn C, Fu MS, Moyrand F, Klengel T, Pearson DS, Geeves MA, Buck J, Levin LR, Muhlschlegel FA. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot Cell. 2006;5:103–111. doi: 10.1128/EC.5.1.103-111.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schroppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, Levin LR, Buck J, Muhlschlegel FA. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Spicer SS, Ge Z-H, Tashian RE, Hazen-Martin DJ, Schulte BA. Comparative distribution of carbonic anhydrase isozymes III and II in rodent tissues. Am J Anat. 1990;187:55–64. doi: 10.1002/aja.1001870107. [DOI] [PubMed] [Google Scholar]
- 153.Sutto Z, Conner GE, Salathe M. Regulation of human airway ciliary beat frequency by intracellular pH. J Physiol. 2004;560:519–532. doi: 10.1113/jphysiol.2004.068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, Levin LR, Buck J. Bicarbonate-responsive "soluble" adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164:527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beltran C, Vacquier VD, Moy G, Chen Y, Buck J, Levin LR, Darszon A. Particulate and soluble adenylyl cyclases participate in the sperm acrosome reaction. Biochem Biophys Res Commun. 2007;358:1128–1135. doi: 10.1016/j.bbrc.2007.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]