Abstract
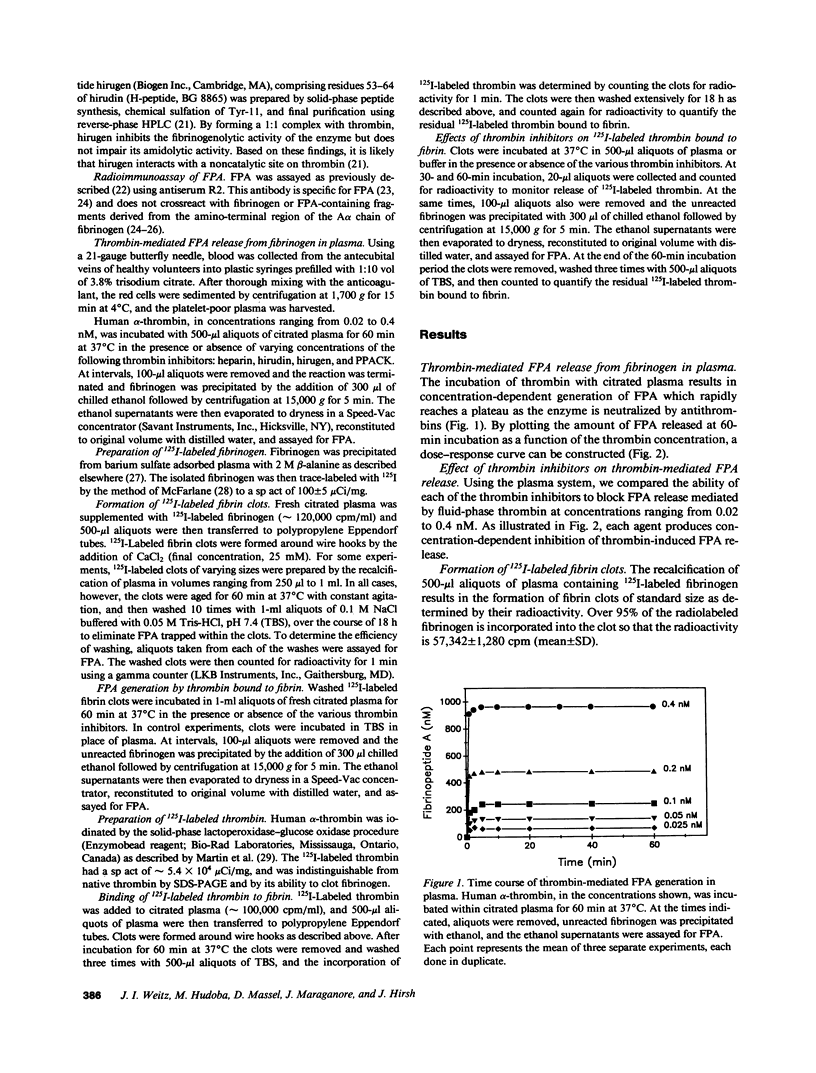
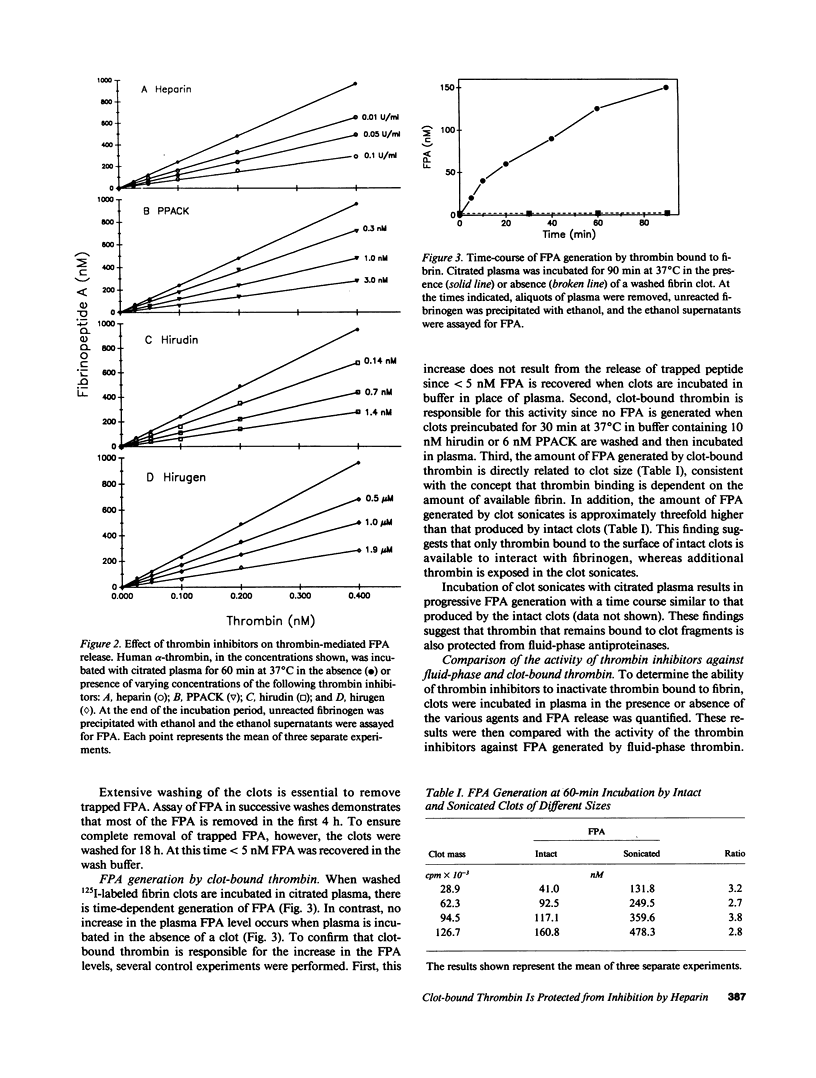
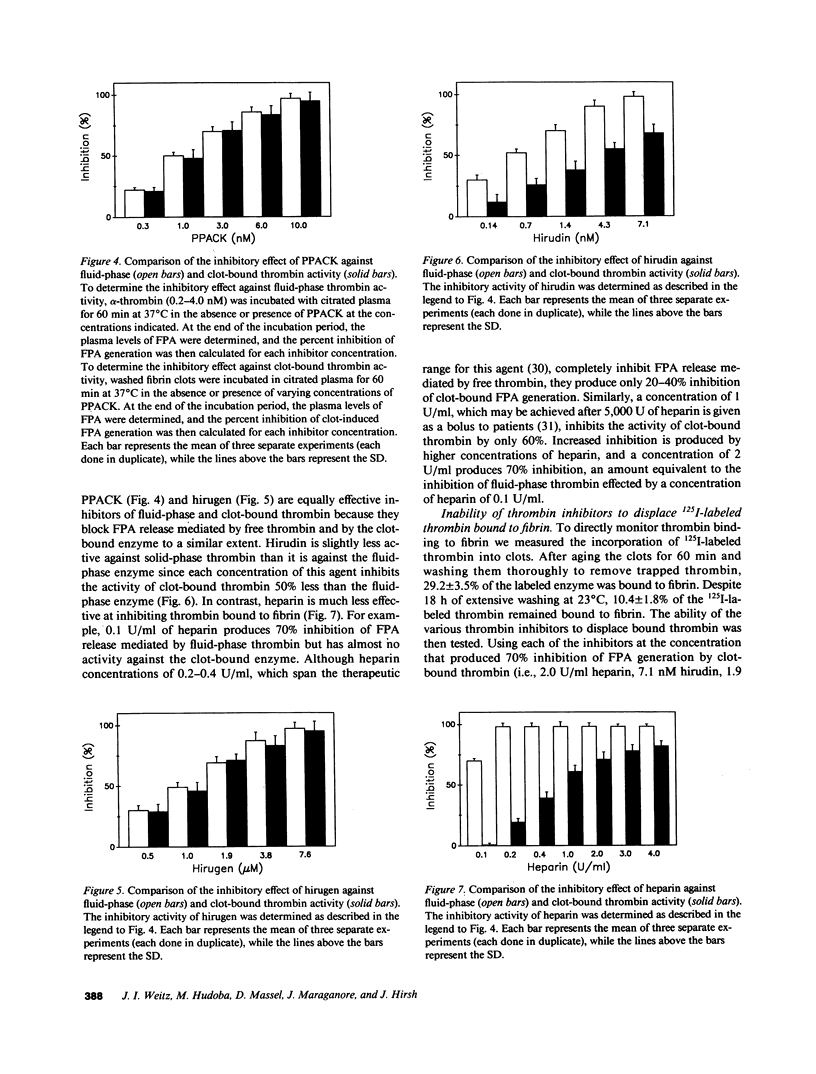
Propagation of venous thrombi or rethrombosis after coronary thrombolytic therapy can occur despite heparin administration. To explore potential mechanisms, we set out to determine whether clot-bound thrombin is relatively protected from inhibition by heparin-antithrombin III but susceptible to inactivation by antithrombin III-independent inhibitors. Using plasma fibrinopeptide A (FPA) levels as an index of thrombin activity, we compared the ability of thrombin inhibitors to block FPA release mediated by fluid-phase thrombin with their activity against the clot-bound enzyme. Incubation of thrombin with citrated plasma results in concentration-dependent FPA generation, which reaches a plateau within minutes. In contrast, there is progressive FPA generation when fibrin clots are incubated with citrated plasma. Heparin, hirudin, hirudin dodecapeptide (hirugen), and D-phenylalanyl-L-prolyl-L-arginyl chloromethyl ketone (PPACK) produce concentration-dependent inhibition of FPA release mediated by fluid-phase thrombin. However, heparin is much less effective at inhibiting thrombin bound to fibrin because a 20-fold higher concentration is necessary to block 70% of the activity of the clot-bound enzyme than is required for equivalent inhibition of fluid-phase thrombin (2.0 and 0.1 U/ml, respectively). In contrast, hirugen and PPACK are equally effective inhibitors of fluid- and solid-phase thrombin, while hirudin is only 50% as effective against the clot-bound enzyme. None of the inhibitors displace bound 125I-labeled thrombin from the clot. These studies indicate that (a) clot-bound thrombin is relatively protected from inhibition by heparin, possibly because the heparin binding site on thrombin is inaccessible when the enzyme is bound to fibrin, and (b) clot-bound thrombin is susceptible to inactivation by antithrombin III-independent inhibitors because the sites of their interaction are not masked by thrombin binding to fibrin. For these reasons, antithrombin III-independent inhibitors may be more effective than heparin in certain clinical settings.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berliner L. J., Sugawara Y., Fenton J. W., 2nd Human alpha-thrombin binding to nonpolymerized fibrin-Sepharose: evidence for an anionic binding region. Biochemistry. 1985 Nov 19;24(24):7005–7009. doi: 10.1021/bi00345a038. [DOI] [PubMed] [Google Scholar]
- Björk I., Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982 Oct 29;48(3):161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Boneu B., Buchanan M. R., Cade J. F., Van Ryn J., Fernandez F. F., Ofosu F. A., Hirsh J. Effects of heparin, its low molecular weight fractions and other glycosaminoglycans on thrombus growth in vivo. Thromb Res. 1985 Oct 1;40(1):81–89. doi: 10.1016/0049-3848(85)90352-4. [DOI] [PubMed] [Google Scholar]
- Bratt G., Törnebohm E., Granqvist S., Aberg W., Lockner D. A comparison between low molecular weight heparin (KABI 2165) and standard heparin in the intravenous treatment of deep venous thrombosis. Thromb Haemost. 1985 Dec 17;54(4):813–817. [PubMed] [Google Scholar]
- Canfield R. E., Dean J., Nossel H. L., Butler V. P., Jr, Wilner G. D. Reactivity of fibrinogen and fibrinopeptide A containing fibrinogen fragments with antisera to fibrinopeptide A. Biochemistry. 1976 Mar 23;15(6):1203–1209. doi: 10.1021/bi00651a004. [DOI] [PubMed] [Google Scholar]
- Danielsson A., Raub E., Lindahl U., Björk I. Role of ternary complexes, in which heparin binds both antithrombin and proteinase, in the acceleration of the reactions between antithrombin and thrombin or factor Xa. J Biol Chem. 1986 Nov 25;261(33):15467–15473. [PubMed] [Google Scholar]
- Hirsh J., van Aken W. G., Gallus A. S., Dollery C. T., Cade J. F., Yung W. L. Heparin kinetics in venous thrombosis and pulmonary embolism. Circulation. 1976 Apr;53(4):691–695. doi: 10.1161/01.cir.53.4.691. [DOI] [PubMed] [Google Scholar]
- Hogg P. J., Jackson C. M. Fibrin monomer protects thrombin from inactivation by heparin-antithrombin III: implications for heparin efficacy. Proc Natl Acad Sci U S A. 1989 May;86(10):3619–3623. doi: 10.1073/pnas.86.10.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm H. A., Ly B., Handeland G. F., Abildgaard U., Arnesen K. E., Gottschalk P., Høeg V., Aandahl M., Haugen K., Laerum F. Subcutaneous heparin treatment of deep venous thrombosis: a comparison of unfractionated and low molecular weight heparin. Haemostasis. 1986;16 (Suppl 2):30–37. doi: 10.1159/000215355. [DOI] [PubMed] [Google Scholar]
- Hull R. D., Raskob G. E., Hirsh J., Jay R. M., Leclerc J. R., Geerts W. H., Rosenbloom D., Sackett D. L., Anderson C., Harrison L. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986 Oct 30;315(18):1109–1114. doi: 10.1056/NEJM198610303151801. [DOI] [PubMed] [Google Scholar]
- Hull R., Delmore T., Genton E., Hirsh J., Gent M., Sackett D., McLoughlin D., Armstrong P. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979 Oct 18;301(16):855–858. doi: 10.1056/NEJM197910183011602. [DOI] [PubMed] [Google Scholar]
- Kaminski M., McDonagh J. Inhibited thrombins. Interactions with fibrinogen and fibrin. Biochem J. 1987 Mar 15;242(3):881–887. doi: 10.1042/bj2420881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner C., Shaw E. D-Phe-Pro-ArgCH2C1-A selective affinity label for thrombin. Thromb Res. 1979;14(6):969–973. doi: 10.1016/0049-3848(79)90014-8. [DOI] [PubMed] [Google Scholar]
- Lagerstedt C. I., Olsson C. G., Fagher B. O., Oqvist B. W., Albrechtsson U. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet. 1985 Sep 7;2(8454):515–518. doi: 10.1016/s0140-6736(85)90459-3. [DOI] [PubMed] [Google Scholar]
- Liu C. Y., Nossel H. L., Kaplan K. L. The binding of thrombin by fibrin. J Biol Chem. 1979 Oct 25;254(20):10421–10425. [PubMed] [Google Scholar]
- MCFARLANE A. S. Labelling of plasma proteins with radioactive iodine. Biochem J. 1956 Jan;62(1):135–143. doi: 10.1042/bj0620135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraganore J. M., Chao B., Joseph M. L., Jablonski J., Ramachandran K. L. Anticoagulant activity of synthetic hirudin peptides. J Biol Chem. 1989 May 25;264(15):8692–8698. [PubMed] [Google Scholar]
- Marder V. J., Soulen R. L., Atichartakarn V., Budzynski A. Z., Parulekar S., Kim J. R., Edward N., Zahavi J., Algazy K. M. Quantitative venographic assessment of deep vein thrombosis in the evaluation of streptokinase and heparin therapy. J Lab Clin Med. 1977 May;89(5):1018–1029. [PubMed] [Google Scholar]
- Martin B. M., Wasiewski W. W., Fenton J. W., 2nd, Detwiler T. C. Equilibrium binding of thrombin to platelets. Biochemistry. 1976 Nov 2;15(22):4886–4893. doi: 10.1021/bi00667a021. [DOI] [PubMed] [Google Scholar]
- Mirshahi M., Soria J., Soria C., Faivre R., Lu H., Courtney M., Roitsch C., Tripier D., Caen J. P. Evaluation of the inhibition by heparin and hirudin of coagulation activation during r-tPA-induced thrombolysis. Blood. 1989 Aug 15;74(3):1025–1030. [PubMed] [Google Scholar]
- Nossel H. L., Butler V. P., Jr, Wilner G. D., Canfield R. E., Harfenist E. J. Specificity of antisera to human fibrinopeptide A used in clinical fibrinopeptide A assays. Thromb Haemost. 1976 Feb 29;35(1):101–109. [PubMed] [Google Scholar]
- Nossel H. L., Yudelman I., Canfield R. E., Butler V. P., Jr, Spanondis K., Wilner G. D., Qureshi G. D. Measurement of fibrinopeptide A in human blood. J Clin Invest. 1974 Jul;54(1):43–53. doi: 10.1172/JCI107749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson S. T. Transient kinetics of heparin-catalyzed protease inactivation by antithrombin III. Linkage of protease-inhibitor-heparin interactions in the reaction with thrombin. J Biol Chem. 1988 Feb 5;263(4):1698–1708. [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Seegers W. H., Nieft M., Loomis E. C. NOTE ON THE ADSORPTION OF THROMBIN ON FIBRIN. Science. 1945 May 18;101(2629):520–521. doi: 10.1126/science.101.2629.520. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J. Kinetics of the inhibition of thrombin by hirudin. Biochemistry. 1986 Aug 12;25(16):4622–4628. doi: 10.1021/bi00364a025. [DOI] [PubMed] [Google Scholar]
- Talbot M. D., Ambler J., Butler K. D., Findlay V. S., Mitchell K. A., Peters R. F., Tweed M. F., Wallis R. B. Recombinant desulphatohirudin (CGP 39393) anticoagulant and antithrombotic properties in vivo. Thromb Haemost. 1989 Feb 28;61(1):77–80. [PubMed] [Google Scholar]
- Topol E. J., George B. S., Kereiakes D. J., Stump D. C., Candela R. J., Abbottsmith C. W., Aronson L., Pickel A., Boswick J. M., Lee K. L. A randomized controlled trial of intravenous tissue plasminogen activator and early intravenous heparin in acute myocardial infarction. Circulation. 1989 Feb;79(2):281–286. doi: 10.1161/01.cir.79.2.281. [DOI] [PubMed] [Google Scholar]
- Vali Z., Scheraga H. A. Localization of the binding site on fibrin for the secondary binding site of thrombin. Biochemistry. 1988 Mar 22;27(6):1956–1963. doi: 10.1021/bi00406a023. [DOI] [PubMed] [Google Scholar]
- Weitz J. I., Cruickshank M. K., Thong B., Leslie B., Levine M. N., Ginsberg J., Eckhardt T. Human tissue-type plasminogen activator releases fibrinopeptides A and B from fibrinogen. J Clin Invest. 1988 Nov;82(5):1700–1707. doi: 10.1172/JCI113783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz J. I., Landman S. L., Crowley K. A., Birken S., Morgan F. J. Development of an assay for in vivo human neutrophil elastase activity. Increased elastase activity in patients with alpha 1-proteinase inhibitor deficiency. J Clin Invest. 1986 Jul;78(1):155–162. doi: 10.1172/JCI112545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilner G. D., Danitz M. P., Mudd M. S., Hsieh K. H., Fenton J. W., 2nd Selective immobilization of alpha-thrombin by surface-bound fibrin. J Lab Clin Med. 1981 Mar;97(3):403–411. [PubMed] [Google Scholar]
- Yasuda T., Gold H. K., Fallon J. T., Leinbach R. C., Guerrero J. L., Scudder L. E., Kanke M., Shealy D., Ross M. J., Collen D. Monoclonal antibody against the platelet glycoprotein (GP) IIb/IIIa receptor prevents coronary artery reocclusion after reperfusion with recombinant tissue-type plasminogen activator in dogs. J Clin Invest. 1988 Apr;81(4):1284–1291. doi: 10.1172/JCI113446. [DOI] [PMC free article] [PubMed] [Google Scholar]



