Abstract
Heme oxygenase 1 (HO-1) uses molecular oxygen and electrons from NADPH cytochrome P450 reductase to convert heme to CO, ferrous iron, and biliverdin (BV). Enzymatic studies with the purified 30-kDa form of HO-1 routinely use a coupled assay containing biliverdin reductase (BVR), which converts BV to bilirubin (BR). BVR is believed to be required for optimal HO-1 activity. The goal of this study was to determine whether HO-1 activity could be monitored directly by following BV generation or iron release (using the ferrous iron chelator, ferrozine) in the absence of BVR. Using assays for each of the three end products, we found that HO-1 activity was stimulated in the presence of catalase and comparable rates were measured with each assay. Absorbance scans revealed characteristic spectra for BR, BV, and/or the ferrozine-iron complex. The optimal conditions were slightly different for the direct and coupled assays. BSA activated the coupled but inhibited the direct assays, and the assays had different pH optima. By measuring the activity of BVR directly using BV as a substrate, these differences were attributed to different enzymatic properties of BVR and HO-1. Thus, BVR is not needed to measure the activity of HO-1 when catalase is present. In fact, the factors affecting catalysis by HO-1 are better understood using the direct assays because the coupled assay can be influenced by properties of BVR.
Introduction
Heme oxygenase (HO) catalyzes the rate-limiting step of heme degradation. The reaction requires molecular oxygen and reducing equivalents that are provided through the interaction with the NADPH cytochrome P450 reductase (CPR). The reaction consumes seven electrons and three molecules of molecular oxygen and produces biliverdin (BV), carbon monoxide, and ferrous iron (Kikuchi et al., 2005). The next step of heme degradation involves the conversion of BV to bilirubin (BR) catalyzed by biliverdin reductase (BVR). Evidence suggests that both CPR and BVR bind competitively to HO-1 (Wang and Ortiz de Montellano, 2003; Higashimoto et al., 2008).
There are two isoforms of HO (HO-1 and HO-2). HO-1 is inducible by manifold sources of environmental stress including oxidative stress, heat shock, cytotoxic agents, and nutrient depletion (Ryter et al., 2002). The enzyme affords direct protection from reactive oxygen species by the removal of free heme, which has pro-oxidant properties, and by the formation of BV and subsequently BR, which have been shown to be potent antioxidants (Bauer and Bauer, 2002; Takahashi et al., 2004). HO-2 is expressed constitutively in brain and testes and is believed to primarily play a signaling role in these tissues, which is mediated by the CO formed by its enzymatic activity (Parfenova and Leffler, 2008).
The activity of HO-1 has been examined almost exclusively by using a coupled enzyme assay in which the BV formed by sHO-1 is converted to BR in the presence of excess BVR. The advantage of this coupled assay is that BR is a strong chromophore relative to BV. Furthermore, it is generally believed that HO-1 activity is stimulated by BVR because it has been proposed that the enzyme binds to HO-1 and causes the release of BV from the HO-1 active site in the process (Wilks, 2002; Kikuchi et al., 2005). This assumption has been largely derived from a study showing that the release of BV from the sHO-1 active site was the rate-limiting step of catalysis under anaerobic, single-turnover conditions. However, in the presence of BVR, the rate-limiting step became the conversion of verdoheme-bound sHO-1 to BV-bound ferric sHO-1 (Liu and Ortiz de Montellano, 2000). The putative binding site for BVR on sHO-1 has been modeled in a mutagenesis study (Wang and Ortiz de Montellano, 2003) and has been proposed to overlap with that for CPR. Thus, it is believed that CPR and BVR compete for binding to sHO-1.
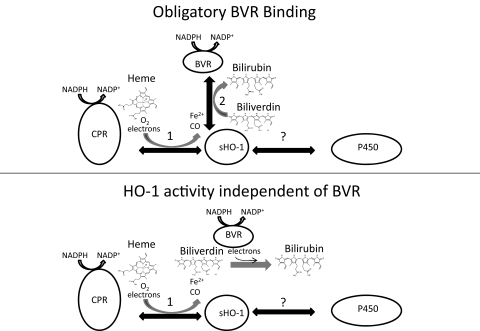
If both BVR and CPR need to interact with HO-1 to complete one catalytic cycle of the sHO-1 reaction, the protein-protein interactions must be coordinated in such a way that metabolism is not interrupted. Figure 1 demonstrates the potential complexity of sHO-1 catalysis. According to the accepted dogma (Fig. 1, top), the first part of the sHO-1 reaction involves an interaction with the CPR and the binding of O2 and heme to the sHO-1 active site. Catalysis by sHO-1 results in the production of CO, ferrous iron, and BV. However, before the BV can escape the sHO-1 active site, sHO-1 must first release CPR and bind to BVR, which induces the release of product. This scenario has important functional implications because it suggests that HO-1 catalysis is dependent on the relative concentrations of CPR, BVR, and cytochromes P450 (because the CPR also must interact with cytochromes P450) in the endoplasmic reticulum. In addition, studies have suggested that HO-1 also interacts with the cytochromes P450 (Bissell and Hammaker, 1976; Kutty et al., 1988). Thus, in addition to the likelihood that P450 competes with HO-1 for reducing equivalents from CPR, the putative binding between HO-1 and P450 may also interfere with the ability of CPR to deliver electrons to HO-1.
Fig. 1.
Schematic diagram describing the functional molecular interactions involved in sHO-1 catalysis. Top, sHO-1 reaction according to the widely accepted opinion that BVR binding causes the release of BV from the sHO-1 active site. The binding of proteins to sHO-1 are indicated by the black double-headed arrows. The binding of CPR (1) and BVR (2) must occur in the specified sequence to complete the catalytic cycle. The reaction arrows (curved, gray) indicate the products released/formed during each of the protein-binding events. This sequence of events implies that a complicated series of protein-protein interactions is required for HO-1 catalysis. Bottom, a reaction sequence (supported by the results in this study) in which BVR does not need to bind to the sHO-1 for optimal catalysis. As a result, the panel shows that only one binding event is required between the sHO-1 and the CPR to complete the sHO-1 catalytic cycle. Also shown in both panels is an arrow representing a putative binding event between P450 and sHO-1.
We showed previously that HO-1 catalysis was markedly inhibited by the hydrogen peroxide formed as a side product by the enzyme and that the addition of catalase dramatically improved the detection of activity (Huber et al., 2009). In the present study, we tested the possibility that the sHO-1 reaction could proceed efficiently in the absence of BVR (Fig. 1, bottom) upon addition of catalase. We found that the rates of sHO-1 metabolism in the presence and absence of BVR were equal. It was also demonstrated that the iron chelator, ferrozine, could be used in a direct assay at physiologic pH to trap the ferrous iron produced by sHO-1 (Liu and Ortiz de Montellano, 2000). Finally, by monitoring sHO-1 activity directly, the conditions influencing the activity of the enzyme could be assessed independently from those affecting the activity of BVR in the coupled HO-1 assay.
Materials and Methods
Materials.
Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). The BV used to directly measure the enzymatic properties of BVR was purchased from Frontier Scientific (Logan, UT).
Enzyme Sources.
The truncated human HO-1 expression system was provided by Dr. Paul Ortiz de Montellano (University of California, San Francisco, San Francisco, CA) and purified as reported previously (Wilks et al., 1995). Rabbit CPR was expressed from a recombinant plasmid, containing the wild-type cDNA insert in a vector using the T7 promoter, which was provided by Dr. Lucy Waskell (University of Michigan, Ann Arbor, MI) and has been described previously (Kelley et al., 2005). The recombinant human BVR was generously provided by Dr. Jawed Alam (Ochsner Medical Center, New Orleans, LA), and purity of the enzyme was determined by SDS-polyacrylamide gel electrophoresis.
Measurement of Enzyme Activity.
Reactions to measure the activity of the sHO-1 under the conditions described under Results were obtained by incubating 0.1 μM sHO-1 with 0.3 μM CPR in a solution containing 15 μM heme, 0.25 unit/μl catalase (unless indicated otherwise), and 0.1 M buffer salt at the pH indicated under Results. The heme was added to the assays from a 2.5 mM solution that was prepared by first dissolving the required amount of heme in a volume of 0.1 M NaOH that was one-fifth the final volume of the solution. After the heme was dissolved by vortexing, the solution was taken up to the final volume with the same buffer used in the enzymatic assays as indicated. All reactions with the sHO-1 were performed in a 0.1-ml volume at 37°C. All of the assays were performed in 96-well plates and measured using a plate reader (Molecular Devices, Sunnyvale, CA). Reactions were initiated with NADPH at a final concentration of 0.4 mM. When the activity of the sHO-1 was measured directly by the production of BV, the absorbance changes at 670 nm were monitored in real time, and the rates were taken from the linear portion of the profile. Duplicate blank samples containing all reaction components except NADPH were run simultaneously, and the averaged time-dependent absorbance changes in the blanks were subtracted from those of the assay incubations.
When the activity of the sHO-1 was measured by the formation of the ferrozine-ferrous iron complex, the reactions were performed as described above except that 250 μM ferrozine was also added to the incubations, and the absorbance changes at 564 nm were monitored in real time. In general, these assays also followed the formation of BV by the absorbance at 670 nm simultaneously with the formation of the ferrozine-iron complex. Duplicate blank samples containing all reaction components except NADPH were run simultaneously, and the averaged time-dependent absorbance changes in the blanks were subtracted from those of the assay incubations.
When the activity of the sHO-1 was measured by the production of BR from BV, the reactions were performed as described except that 0.2 μM BVR was added in addition to the reaction components described above. In general, these reactions also included 0.25 mg/ml bovine serum albumin (BSA), which has been shown to optimize the activity by the BVR (Noguchi et al., 1979). For these assays, the absorbance at 468 nm was measured in real time. Blanks for all of these reactions were derived from incubations of all of the reaction components except NADPH and subtracted from the scans obtained from the samples containing NADPH. After the assays described above were run for 5 min, the absorbance from 350 to 750 nm was measured to verify that the spectra were consistent with the intended reaction products. The averaged spectra of the “blank” samples described above were also subtracted from those of the sample wells to generate the spectra shown in Fig. 3.
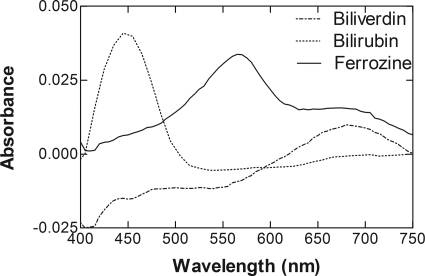
Fig. 3.
Absorbance spectra of the various complexes used for detection of sHO-1 activity. The spectra were taken 300 s after addition of NADPH for each of the reactions described in Fig. 1. The absorbances of “reference” solutions containing all of the reaction constituents except NADPH were subtracted from those of the reaction incubations. The curves represent BV (dotted line), ferrozine (solid line), and BR (dashed line).
Results
Effect of Catalase on HO-1 Activity.
In a previous study from this laboratory, it was shown that catalase had a dramatic effect on the magnitude and the time-dependent linearity of the activity by the full-length HO-1 enzyme (Huber et al., 2009). The previous study also showed that this effect was probably due to protection of HO-1 from hydrogen peroxide-generated damage. The tendency of HO-1 to produce hydrogen peroxide was shown to be related to its ability to couple with the CPR because sHO-1 produced proportionately high amounts of hydrogen peroxide, whereas the full-length enzyme only did so when the CPR concentration was higher than that of HO-1 (Huber et al., 2009). The effects of catalase in that study were so dramatic that we decided to determine whether the activity of sHO-1 could be measured directly if catalase was present.
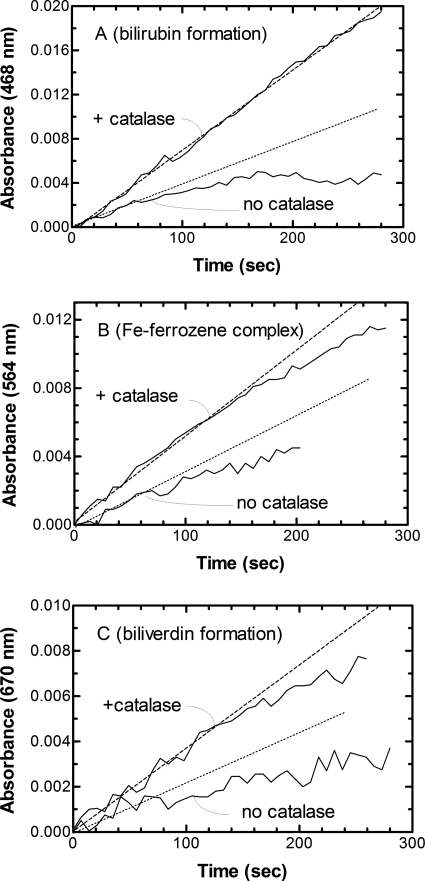
Figure 2 compares the kinetic traces resulting from the activity of sHO-1 in the presence and absence of catalase when measured directly (by the production of BV and the release of iron) and indirectly using the coupled assay (which follows the conversion of BV to BR by BVR). With each assay system, sHO-1 activity was more than doubled in the presence of catalase. In addition, the linearity of the reactions with time extended from less than 1 min in the absence of catalase to more than 2 min in its presence. The rates calculated using the extinction coefficients for the three products were roughly equivalent using the three methods (Fig. 6). Catalase seemed to benefit the coupled reaction even more than the direct assays for sHO-1 as the rate of BR formation using the coupled reaction was linear for the entire 5-min interval over which absorbance was monitored. Thus, the effects of catalase on BVR also were investigated by measuring the rate of BR formation when BV was used as a substrate. Although the averaged rate of BR formation by BVR in the presence of catalase (from triplicate determinations) was slightly higher, the difference was not statistically significant (data not shown). Thus, it appears that the effects of catalase are specific to sHO-1. Therefore, it is possible that the putative interaction of sHO-1 with BVR also protects sHO-1 from the inactivating effects of hydrogen peroxide formed during heme catabolism.
Fig. 2.
Comparison of sHO-1 activity by direct detection of BV and Fe-ferrozine and the coupled assay measuring BR formation: effect of catalase. All reactions contained 0.1 μM sHO-1, 0.3 μM CPR, and 15 μM heme in 100 mM MOPS buffer (pH 7.4), either in the absence or presence of 0.25 unit/μl catalase. All reactions were incubated at 37°C and were initiated by the addition of 0.4 mM NADPH. A, HO-1 activity was measured using a coupled assay containing 0.2 μM BVR as described under Materials and Methods. Formation of BR was monitored at 468 nm. B, when the Fe-ferrozine complex was used to measure HO-1 activity, the assay included 0.25 mM ferrozine in addition to the enzymes heme and NADPH. The ferrozine complex was monitored at 564 nm. C, BV formation was measured directly using its absorbance (670 nm). The dotted line represents an estimate of the initial rate of the reaction as determined by a fit through the linear portion of the data.
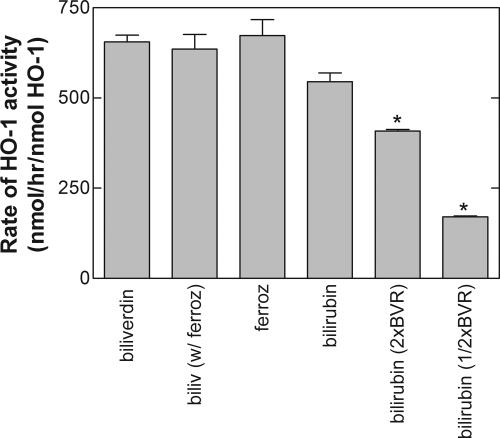
Fig. 6.
Direct comparison of the assays used to measure sHO-1 activity. Reactions containing sHO-1, CPR, heme, and catalase to measure the formation of BV, BR, and the iron-ferrozine complex were the same as those described under Materials and Methods except that 0.2, 0.4 (2× BVR), and 0.1 (1/2× BVR) μM BVR were added to the incubations labeled “bilirubin.” BV (biliv) formation was also measured in one group of samples in the presence of 0.25 mM ferrozine (ferroz), as indicated. The data represent the average ± S.E. of 12 determinations in four experiments. Statistical significance was determined using Dunnett's analysis of variance test, using the corresponding MOPS groups as a control (*, p < 0.05; **, p < 0.01).
Figure 3 demonstrates that the absorbance spectra observed in the reaction incubations of the three assays were consistent for those expected for the respective products because the absorbance maxima of BR, iron-ferrozine, and BV have been identified at 468, 564, and 670 nm, respectively (Yoshida and Kikuchi, 1978). In addition, the bilverdin peak was also apparent in the incubation containing ferrozine. Thus, these results demonstrate that sHO-1 activity could be accurately measured directly when catalase was added to the reactions.
Testing Impurities in Catalase for Effects on sHO-1 Metabolism.
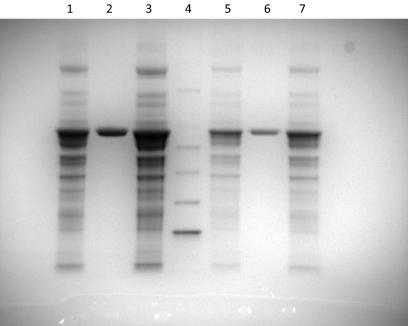
In many instances, the preparations of lyophilized bovine liver catalase that are commercially available are only partially purified. Figure 4 shows polyacrylamide gel electrophoresis images for three of these commercial preparations. To determine whether the effects of catalase on metabolism by sHO-1 were caused by an impurity in the Sigma-Aldrich preparation used for these studies, all three batches were tested in the HO-1 assay monitoring the formation of biliverdin as described under Materials and Methods. Because the preparation purchased from EMD Biosciences (San Diego, CA) was electrophoretically pure (12,000 units/mg powder), the effect of this batch of catalase should be less than those observed with the other commercial preparations if the effects were caused by a specific impurity in the samples. Alternatively, if the effects were attributable to the catalase in the preparations, all three batches of catalase should have the same stimulatory effect on metabolism by the sHO-1. The kinetic scans monitoring the formation of biliverdin with each batch of catalase were qualitatively similar (data not shown), and the rates of heme metabolism measured from the slopes of the traces were 425.8 ± 40.3, 485.6 ± 3.6, and 474.6 ± 14 nmol/h/nmol sHO-1 for the USB (Cleveland, OH), EMD Biosciences, and Sigma-Aldrich batches, respectively. Thus, there were no significant differences in the rates associated with the three commercial preparations, indicating that the effects on sHO-1 metabolism were caused by the catalase and not an impurity.
Fig. 4.
Purity of three preparations of commercially available catalase. Coomassie stain of polyacrylamide gel after electrophoresis of bovine liver catalase purchased from USB (lanes 1 and 5), EMD Biosciences (lanes 2 and 6), and Sigma-Aldrich (lanes 3 and 7). Fifteen microliters of each batch was loaded on the gel at 10 and 3.3 units/μl concentrations. Lane 4 shows 15 μl of a protein ladder (MagicMark from Invitrogen, Carlsbad, CA) molecular weight standard.
Effect of Buffer on the Activity of sHO-1.
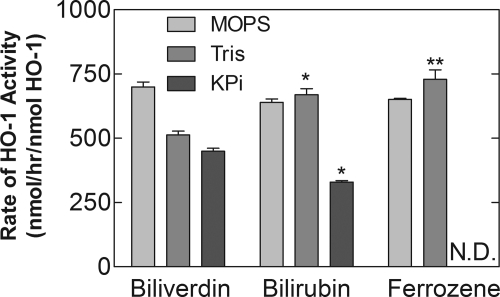
The activity of sHO-1 is routinely measured in 0.1 M potassium phosphate buffer (pH 7.4) (Maines et al., 1986). As reported previously in a single turnover study with HO-1, the release of ferrous iron by the enzyme during the metabolism of heme could be monitored by the formation of a complex with ferrozine (Liu and Ortiz de Montellano, 2000). The ferrozine-iron complex is a much stronger chromophore than BV and, thus, would serve as a better means to directly monitor HO-1 activity. However, it has been indicated that the binding of ferrous iron with ferrozine cannot be detected in potassium phosphate buffer (Liu and Ortiz de Montellano, 2000). We decided to test the activity of sHO-1 in a variety of buffers to find one in which the amount of iron release could be measured without a significant loss of activity relative to the commonly used BR assay. Figure 5 shows the activity of sHO-1 as measured using the three different assays in 0.1 M potassium phosphate, MOPS, and Tris buffers at pH 7.4. The relative activities of the enzyme in the different buffers varied depending on which assay was being assessed. Ironically, given its conventional use in the HO-1 assay, potassium phosphate appeared to be a poor buffer in which to measure optimal activity of the sHO-1 as the rates obtained by all three assays were lower in this buffer. Given the fact that the reaction is stimulated by catalase, the lower activity in phosphate buffer could be attributed to the fact that it has been shown to be a pro-oxidant under some conditions (Owen et al., 2000). Relative to the activity in MOPS, the production of BV was decreased in both Tris and potassium phosphate buffers (with a more significant effect occurring in the latter), whereas the activities of the enzyme in MOPS and Tris were approximately equal when the assays for BR formation and iron release were compared. The variance in rates using the BV and ferrozine assays in Tris buffer may reflect the ability of the chelator, ferrozine, to facilitate release of the ferrous iron from the enzyme (Liu and Ortiz de Montellano, 2000). From the comparison of the rates obtained with the different assays, it was found that the most consistent results were generated using MOPS buffer. Thus, this buffer was used to compare the direct and coupled assays in more detail.
Fig. 5.
Effect of buffer on sHO-1 activity. Activity of sHO-1 was measured directly by BV formation and iron release and indirectly by BR formation in 0.1 M MOPS, 0.1 M potassium phosphate (KPi), and 0.1 M Tris buffers at pH 7.4. Assays for the production of BV, BR, and the iron-ferrozine complex are described under Materials and Methods. The data represent the average ± S.E. of nine determinations in three experiments. Statistical significance was determined by Dunnett's analysis of variance test for MOPS and Tris data using the activity as determined by the direct detection of BV as a control and a Student's t test using the Welch correction for the potassium phosphate data (*, p < 0.05; **, p < 0.01). N.D., not detected.
Comparison of the Two Direct Assays for HO-1 with the Coupled Assay using BVR.
Figure 6 shows a direct comparison of the rates of metabolism by sHO-1 using the various methods of determination. The rates determined by measuring the production of BV and the iron-ferrozine complex were comparable. They were also similar to the rate of BV formation in the presence of ferrozine, indicating that ferrozine at this concentration (250 μM) did not interfere with BV production and provided a corroborating measure of HO-1 activity. The rate of HO-1 metabolism measured by BR production in the presence of the BVR was slightly lower than those determined by measuring HO-1 activity directly. Two other concentrations of the BVR were also tested in the assay to determine whether this was due to using BVR concentrations that were not optimal. The rate of BR formation was even lower at the other BVR concentrations. It seems likely that the lower rate of sHO-1 activity in the coupled assay with the BVR may have been caused by not having BSA in the assay buffer. When BSA was added to the MOPS buffer, the rate of BR formation was comparable to that of ferrozine-iron complex formation (see below).
Effects of Incubation Conditions on the Activities of sHO-1 and BVR.
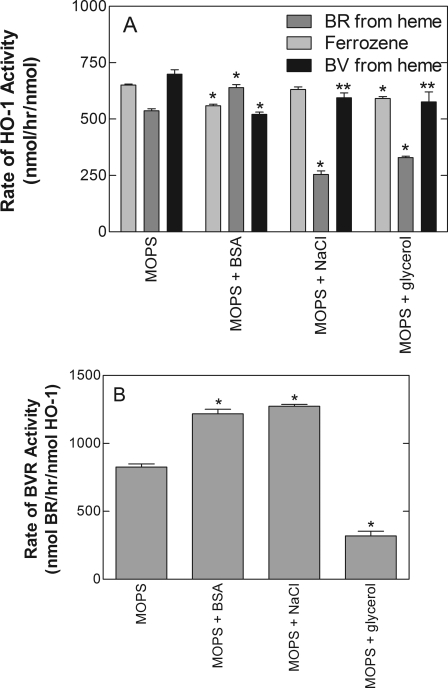
The effects of buffer constituents on sHO-1 activity were also compared using the direct and coupled assays. Figure 7A shows the effects of BSA, sodium chloride, and glycerol on the activities of sHO-1 and BVR. The buffer additives had disparate effects on the direct and coupled assays for HO-1. Sodium chloride (100 mM) and glycerol (20%) had only slight or no effects on the direct assays but dramatically inhibited the coupled assay. BSA clearly inhibited the direct sHO-1 assays (by approximately 15%) but significantly activated the coupled assay with BVR.
Fig. 7.
Effect of incubation conditions on the rates of metabolism by sHO-1 and BVR. A, activity of sHO-1 was measured by the formation of BV, BR, and the iron-ferrozine complex as described under Materials and Methods. All reactions contained 100 mM MOPS (pH 7.4) and 0.25 unit/μl catalase and the following reaction components as indicated: 0.125 mg/ml BSA, 100 mM NaCl, and 20% glycerol. B, BVR activity was measured by monitoring the generation of BR from BV. The assay contained 0.02 μM BVR and 20 μM BV in 100 mM MOPS and the components indicated in the figure. The data represent the average ± S.D. of six determinations from two experiments. Statistical significance was determined using Dunnett's test, using the corresponding MOPS groups as a control (*, p < 0.05; **, p < 0.01).
To better understand the varying effects of the buffer constituents on sHO-1 activity, we also examined their effects on the direct activity of BVR by using BV as a substrate instead of heme (Fig. 7B). The stimulation of the activity of BVR by BSA is well established (Tenhunen et al., 1970; Noguchi et al., 1979). Figure 7B corroborated the effect of BSA on this enzyme. However, the data in this study demonstrate that the activation of the coupled assay for sHO-1 activity by BSA was attributable to an effect solely on BVR. BSA actually had a weak inhibitory effect on sHO-1 activity as determined from the direct enzyme assays.
A similar relationship can explain the disparate effects of glycerol on the direct and coupled assays. The pronounced inhibition of sHO-1 activity by glycerol using the coupled assay was most likely related to inhibition of BVR (Fig. 7). The compound had little or no direct effect on sHO-1 activity as indicated by the assays for iron release and BV formation.
In contrast to its effect on the coupled assay for sHO-1 activity, 100 mM sodium chloride markedly stimulated the direct activity of BVR (Fig. 7B). Thus, the inhibitory effect of sodium chloride on the coupled sHO-1 assay may affect a putative binding event between sHO-1 and BVR and not be due to any direct effects on the activities of the two enzymes.
Effect of pH on sHO-1 Metabolism Measured Directly and in the Coupled Assay with BVR.
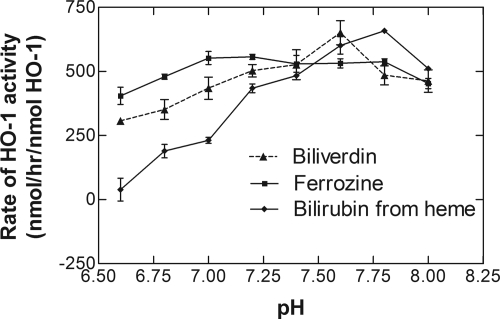
The pH optima of the metabolism of heme by sHO-1 was determined using the direct assays (ferrozine and BV formation) and the coupled assay with the BVR (Fig. 8). The direct assay measuring BV formation and the coupled assay with BVR showed optimal pH values of 7.6 and 7.8, respectively. The higher pH optimum for the coupled assay may have been the result of a higher pH optima (8.5) for BVR when NADPH was used as the source of electrons (Noguchi et al., 1979).
Fig. 8.
Effect of pH on sHO-1 activity. Reactions were performed as described under Materials and Methods in 100 mM MOPS buffer at the pH values indicated. The coupled assays, which included 0.2 μM BVR, were performed in the presence of 0.25 mg/ml BSA. The data represent the average ± S.D. of six determinations collected in two experiments.
The curve representing the activity as a function of pH using the ferrozine assay was relatively flat. Although it has been shown that there is rapid dissociation of the ferrous iron-BV-sHO-1 complex at pH 7.4 (Liu and Ortiz de Montellano, 2000), this may not have been the case at the lower pH values. Thus, under these conditions, the ferrozine may have facilitated release of ferrous iron from the sHO-1 active site. Such a mechanism is plausible because it was shown that desferroxamine facilitated release of ferric iron from the ferric iron-BV-sHO-1 complex (Liu and Ortiz de Montellano, 2000). This possibility would explain why the profile using ferrozine was relatively flat overall and the activities of sHO-1 at lower pH values were higher using the ferrozine assay than the rates determined by BV formation.
Discussion
In a previous study (Huber et al., 2009), we showed that catalase stabilized and stimulated the catalytic activity of HO-1 as measured by the coupled assay with BVR. Our findings were consistent with those of Noguchi et al. (1983), who showed that HO-1 formed H2O2 directly from the decay of a peroxo species and that the resulting H2O2 caused an inactivation of the enzyme. As shown in the present study, these protective effects of catalase made it possible to directly measure sHO-1 activity in the absence of BVR. We speculate that the ability of sHO-1 to function independently of BVR might not have been recognized previously because, in our hands, the activity of the enzyme was too low to be detected if catalase was not included in the reaction mixtures when 100 mM potassium phosphate (pH 7.4) was used as the buffer system (as was typically done in most studies in the literature).
By showing that heme catabolism by sHO-1 occurs at the same rate (or even higher under some conditions) in the absence of BVR, we have demonstrated that BVR is not essential for optimal sHO-1 activity. This finding dramatically changes the functional implications regarding the protein-protein interactions that are involved in catalysis by sHO-1. The most important implication is that the activity of the enzyme in vivo will not be dependent on the expression level of BVR.
Because sHO-1 is not dependent on BVR, the conditions affecting the activities of the two enzymes can be differentiated. As an initial attempt to demonstrate the different properties of BVR and sHO-1, we compared the effects of buffer additives on the activities of sHO-1 (using both the coupled and direct assays) and BVR (using biliverdin instead of heme as the substrate). BSA appeared to stimulate sHO-1 activity when measured using the coupled assay. The stimulating effects of BSA on BVR have been described extensively (Tenhunen et al., 1970). However, both of the direct assays show that BSA actually inhibited the sHO-1, and, thus, the stimulation in the coupled assay was probably due to effects on the BVR. Likewise, inhibition of the coupled HO-1 assay by glycerol seemed to be due to inhibition of the BVR and not a direct effect on the sHO-1. As indicated in our previous study (Huber et al., 2009), catalase prevented the inhibition of HO-1 by the hydrogen peroxide formed by uncoupled metabolism. In this study, we found that catalase had virtually no effect on BVR alone (data not shown). The present study also demonstrated that MOPS is a preferable buffer rather than potassium phosphate for measurement of sHO-1 activity because approximately 40% higher rates of heme metabolism were observed in MOPS. In this buffer, sHO-1 activity could be measured directly (albeit at lower rates) even in the absence of catalase. The results indicated that the coupled and direct assays for sHO-1 could lead to different conclusions about the effects of reaction conditions on the activity of sHO-1.
The most interesting effects of buffer additives were observed with 100 mM NaCl, which dramatically stimulated BVR. However, the NaCl had no effect on the direct assays for HO-1 and dramatically inhibited BR formation with the coupled sHO-1 assay. Because of the disparate effects on the direct assay for BVR and the coupled assay for HO-1, it seems that the salt may interfere with a putative interaction between sHO-1 and BVR that is necessary for BVR catalysis and seems to be driven in large part by ionic forces. An interaction between the enzymes may also be inferred from data showing that high concentrations of BVR inhibited sHO-1 activity (Fig. 6), suggesting that the putative interaction may inhibit interactions between CPR and sHO-1 at high BVR concentrations. Although an interaction between sHO-1 and BVR is inferred by the data, we have demonstrated that the interaction is not necessary for optimal sHO-1 catalysis as has been proposed by previous studies.
The three assays for HO-1 activity also led to different interpretations of the pH optimum of the reaction. The rates of HO-1 activity as measured by the production of BV and BR dropped off significantly at lower pH values (<7.2). However, the rates of metabolism measured using the ferrozine assay were relatively consistent over the range of pH values, suggesting that the chelator may facilitate the release of ferrous iron from the HO-1 active site at the lower pH values. Thus, the versatility of accurately measuring sHO-1 activity by the ferrozene assay may be limited to the physiologic range of pH values. Our findings also showed that the pH optimum of the coupled HO-1 assay was shifted to higher pH values, possibly because of the high pH optimum (8.5) of the BVR reaction and the low activity of BVR below pH 7.0 when NADPH is used as a cofactor (Noguchi et al., 1979).
As has been shown previously (Liu and Ortiz de Montellano, 2000), the complex of ferrous iron with the iron chelator, ferrozine, provided a useful chromophore to directly measure the rate of iron release during sHO-1 metabolism. The data in this study show that sHO-1 activity was not influenced by the chelator at 250 μM and neutral pH. Furthermore, the buffer components had similar affects on the activities measured by the ferrozene and the biliverdin assays. Thus, the ferrozine assay does appear to be a reliable alternative to measure the activity of the enzyme directly and may be suitable to extend the limit of detection for the direct measurement of sHO-1 activity.
Convincing evidence (Liu and Ortiz de Montellano, 2000) has been provided that BVR is necessary to increase the rate at which BV is released from the sHO-1 active site, which otherwise is the rate-limiting step of sHO-1 metabolism. However, the conditions that led to this conclusion included an anaerobic environment, single turnover concentrations of NADPH, and a ferric-BV-sHO-1 intermediate that was prepared by reaction of the enzyme with hydrogen peroxide and ascorbate. Thus, it is possible that BVR may play a different role under these unusual conditions. Our findings greatly simplify the interpretation of sHO-1 catalysis by decreasing the number of protein-protein interactions needed for a single sHO-1 turnover event (Fig. 1).
Because BVR is not required to increase the rate of sHO-1 metabolism, we have shown that it is better to assess the enzymatic properties of HO-1 by measuring its activity directly. Furthermore, it is not necessary to have BVR present to study the interactions of HO-1 with other membrane proteins such as CPR and cytochromes P450. Our laboratory is beginning to address these questions with full-length HO-1 to better understand the affects of these molecular interactions on enzymatic activities.
Acknowledgments.
We thank Dr. Jawed Alam at Ochsner Medical Center (New Orleans, LA) for providing purified human biliverdin reductase.
This work was supported by the U.S. Public Health Service National Institute of Environmental Health Sciences [Grant R01-ES004344].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.034777.
- HO
- heme oxygenase
- CPR
- NADPH cytochrome P450 reductase
- BR
- bilirubin
- BV
- biliverdin
- BVR
- biliverdin reductase
- sHO-1
- shortened form of heme oxygenase-1
- BSA
- bovine serum albumin
- MOPS
- 4-morpholinepropanesulfonic acid.
References
- Bauer M, Bauer I. (2002) Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal 4:749–758 [DOI] [PubMed] [Google Scholar]
- Bissell DM, Hammaker LE. (1976) Cytochrome P-450 heme and the regulation of hepatic heme oxygenase activity. Arch Biochem Biophys 176:91–102 [DOI] [PubMed] [Google Scholar]
- Higashimoto Y, Sugishima M, Sato H, Sakamoto H, Fukuyama K, Palmer G, Noguchi M. (2008) Mass spectrometric identification of lysine residues of heme oxygenase-1 that are involved in its interaction with NADPH-cytochrome P450 reductase. Biochem Biophys Res Commun 367:852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber WJ, 3rd, Marohnic CC, Peters M, Alam J, Reed JR, Masters BS, Backes WL. (2009) Measurement of membrane-bound human heme oxygenase-1 activity using a chemically defined assay system. Drug Metab Dispos 37:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RW, Reed JR, Backes WL. (2005) Effects of ionic strength on the functional interactions between CYP2B4 and CYP1A2. Biochemistry 44:2632–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G, Yoshida T, Noguchi M. (2005) Heme oxygenase and heme degradation. Biochem Biophys Res Commun 338:558–567 [DOI] [PubMed] [Google Scholar]
- Kutty RK, Daniel RF, Ryan DE, Levin W, Maines MD. (1988) Rat liver cytochrome P-450b, P-420b, and P-420c are degraded to biliverdin by heme oxygenase. Arch Biochem Biophys 260:638–644 [DOI] [PubMed] [Google Scholar]
- Liu Y, Ortiz de Montellano PR. (2000) Reaction intermediates and single turnover rate constants for the oxidation of heme by human heme oxygenase-1. J Biol Chem 275:5297–5307 [DOI] [PubMed] [Google Scholar]
- Maines MD, Trakshel GM, Kutty RK. (1986) Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem 261:411–419 [PubMed] [Google Scholar]
- Noguchi M, Yoshida T, Kikuchi G. (1979) Purification and properties of biliverdin reductases from pig spleen and rat liver. J Biochem 86:833–848 [DOI] [PubMed] [Google Scholar]
- Noguchi M, Yoshida T, Kikuchi G. (1983) A stoichiometric study of heme degradation catalyzed by the reconstituted heme oxygenase system with special consideration of the production of hydrogen peroxide during the reaction. J Biochem 93:1027–1036 [DOI] [PubMed] [Google Scholar]
- Owen RW, Spiegelhalder B, Bartsch H. (2000) Generation of reactive oxygen species by the faecal matrix. Gut 46:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, Leffler CW. (2008) Cerebroprotective functions of HO-2. Curr Pharm Des 14:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Otterbein LE, Morse D, Choi AM. (2002) Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234–235:249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Morita K, Akagi R, Sassa S. (2004) Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem 11:1545–1561 [DOI] [PubMed] [Google Scholar]
- Tenhunen R, Ross ME, Marver HS, Schmid R. (1970) Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry 9:298–303 [DOI] [PubMed] [Google Scholar]
- Wang J, de Montellano PR. (2003) The binding sites on human heme oxygenase-1 for cytochrome P450 reductase and biliverdin reductase. J Biol Chem 278:20069–20076 [DOI] [PubMed] [Google Scholar]
- Wilks A. (2002) Heme oxygenase: evolution, structure, and mechanism. Antioxid Redox Signal 4:603–614 [DOI] [PubMed] [Google Scholar]
- Wilks A, Black SM, Miller WL, Ortiz de Montellano PR. (1995) Expression and characterization of truncated human heme oxygenase (hHO-1) and a fusion protein of hHO-1 with human cytochrome P450 reductase. Biochemistry 34:4421–4427 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kikuchi G. (1978) Purification and properties of heme oxygenase from pig spleen microsomes. J Biol Chem 253:4224–4229 [PubMed] [Google Scholar]