Abstract
The goal of this study was to evaluate the pharmacokinetics, mass balance, metabolism, routes and extent of elimination, and safety of a single oral dose of 14C-labeled brivanib alaninate and the safety and tolerability of brivanib after multiple doses in patients with advanced or metastatic solid tumors. This was a two-part, single-center, open-label, single oral-dose (part A) followed by multiple-dose (part B) study in patients with advanced or metastatic solid tumors. In part A, patients received a single dose of [14C]brivanib alaninate and in part B patients received 800 mg of nonradiolabeled brivanib alaninate every day. Four patients (two white, two black: two with non–small-cell lung cancer, one with ovarian cancer, and one with renal cell carcinoma) were treated in both parts. The median time to reach the maximal plasma concentration of brivanib was 1 h, geometric mean maximal plasma concentration was 6146 ng/ml, mean terminal half-life was 13.8 h, and geometric mean apparent oral clearance was 14.7 l/h. After a single oral dose of [14C]brivanib alaninate, 12.2 and 81.5% of administered radioactivity was recovered in urine and feces, respectively. Brivanib alaninate was completely converted to the active moiety, brivanib, and the predominant route of elimination was fecal. Renal excretion of unchanged brivanib was minimal. Brivanib was well tolerated; fatigue was the most frequent adverse event occurring in all patients and the most frequent treatment-related adverse event in three (75%). The best clinical response in one patient was stable disease; the other three had progressive disease. Brivanib alaninate was rapidly absorbed and extensively metabolized after a single 800-mg oral dose; the majority of drug-related radioactivity was excreted in feces.
Introduction
Angiogenesis, or neovascularization, is strictly required for tumor expansion, with respect to both growth and metastasis (Carmeliet and Jain, 2000). Tumor-induced angiogenesis is mediated by proangiogenic factors, including vascular endothelial growth factor A (VEGF-A), platelet-derived growth factor, and fibroblast growth factor-1 (FGF-1) and -2 (FGF-2) (Nyberg et al., 2005).
Several agents targeting the VEGF/VEGF receptor pathway have demonstrated a survival advantage for patients with advanced cancer, either as monotherapy [sunitinib or sorafenib in renal cell carcinoma (Escudier et al., 2007; Motzer et al., 2007) and sorafenib in hepatocellular carcinoma (HCC) (Llovet et al., 2008b)] or as part of a combination therapy regimen [bevacizumab in colorectal cancer (Hurwitz et al., 2008) and lung cancer (Sandler et al., 2006; Hurwitz et al., 2008)]. However, responses to these therapies are modest and transient, and significant toxicities may occur at therapeutic concentrations (Je et al., 2009).
The tyrosine kinase inhibitors (TKIs) comprise a relatively new group of anticancer drugs that has been developed as oral formulations and administered on a daily basis. The mechanism of action of TKIs includes modulation of key pathways and mechanisms of angiogenesis and tumorigenesis such as VEGF receptor, ErB, and platelet-derived growth factor receptor (Arora and Scholar, 2005). TKIs compete with the ATP binding site of the catalytic domain of several oncogenic tyrosine kinases to inhibit transduction of extracellular signals to the cytoplasm (Arora and Scholar 2005). However, tyrosine kinase involvement and activity may vary from tumor to tumor, resulting in differing responses to different TKIs. To date, most of the available pharmacokinetics (PK) information available is based on information obtained from in vitro experiments, animal studies, drug-drug interactions, and mass-balance studies in healthy volunteers with a single dose of the TKI (van Erp et al., 2009). Absorption, distribution, metabolism, and excretion studies are a crucial part of a drug's development program, particularly an anticancer drug, because plasma levels of anticancer agents are often associated with both activity and toxicity, creating a narrow therapeutic window (Beumer et al., 2006). In particular, data are limited on the effects of mild, moderate, or severe renal or hepatic impairment on the PK of TKIs (van Erp et al., 2009). Absorption, distribution, metabolism, and excretion studies can help determine the likely impact of renal or hepatic impairment on the PK of a new agent.
Brivanib, a small-molecule TKI, is the first selective dual inhibitor of FGF and VEGF signaling (Bhide et al., 2006) and is formulated as an orally administered l-alanine ester prodrug, brivanib alaninate (Cai et al., 2008; Marathe et al., 2009) (Fig. 1). Preclinical studies have demonstrated that dual inhibition of FGF and VEGF signaling by brivanib has strong antiangiogenic effects (Huynh et al., 2008; Bhide et al., 2010) and potent direct effects on tumor cells across a range of tumor types, including breast (Bhide et al., 2010), liver (Huynh et al., 2008; Bhide et al., 2010), colon, and lung (Cai et al., 2008; Bhide et al., 2010). Promising clinical activity has been seen with brivanib in a phase II study in patients with advanced HCC in the first- and second-line settings (Raoul et al., 2009). Brivanib is being investigated in a phase III development program in HCC and in combination with other anticancer agents for the treatment of several tumor types, including colorectal cancer.
Fig. 1.
Structures of brivanib and brivanib alaninate. *, site labeled with 14C.
Preclinical in vitro and in vivo PK studies in four animal species (mouse, rat, dog, and monkey) have demonstrated that brivanib alaninate is rapidly and efficiently converted to its active moiety, brivanib, and the clearance of brivanib in humans is expected to be low to intermediate, whereas its volume of distribution is expected to be high (Marathe et al., 2009). The goal of the current study was to report on the PK, mass balance, metabolism, routes and extent of elimination, and safety of a single oral dose of 14C-labeled brivanib alaninate and the safety and tolerability of brivanib after multiple doses in patients with advanced or metastatic solid tumors.
Materials and Methods
Patients.
Patients aged 18 or older with histologically or cytologically confirmed metastatic or locally advanced solid tumors for which no other systemic, surgical, or local therapeutic options exist were enrolled. Patients had to have a life expectancy of at least 3 months, an Eastern Cooperative Oncology Group performance status of 0 to 2, an ability to comply with visits/procedures, and adequate hematologic, hepatic, and renal function. Prior anticancer treatments were permitted if they had achieved adequate recovery from recent therapy. At least 1 week must have elapsed since minor surgery, 12 weeks since major surgery or last dose of radiation therapy or immunotherapy, or 4 weeks since the last dose of chemotherapy (6 weeks from last dose of nitrosoureas, mitomycin C, or liposomal doxorubicin). Patients were excluded from the study if they were women who were pregnant or breast-feeding, women of child-bearing potential who are unwilling or unable to use an acceptable method of contraception to avoid pregnancy for the entire study period and for at least 3 months after the study, or sexually active fertile men who were unwilling or unable to use a barrier contraception or whose partners were women of child-bearing potential not using an adequate method of birth control from the time of enrollment and for 3 months after participation in the study. Patients were also excluded if they had brain metastasis or signs and symptoms suggestive of brain metastasis; concomitant secondary malignancies (except nonmelanomatous skin cancers or early stage prostate or cervical cancers), unless a complete remission was achieved at least 5 years before study entry and no additional therapy was required or anticipated to be required during the study period; uncontrolled or significant cardiovascular disease; a serious uncontrolled medical disorder or active infection that would impair their ability to receive protocol therapy or whose control might have been jeopardized by the complications of this therapy; thromboembolic disease requiring full anticoagulation within the previous 6 months; an inability to swallow capsules or tolerate venipuncture; a known history of gastrointestinal disease that could potentially affect the absorption of the study drug; a history of allergy to brivanib or related compounds; exposure to any investigational drug within 4 weeks of enrollment; or other concurrent chemotherapy, hormonal therapy (except hormone replacement therapy and luteinizing hormone-releasing hormone agonist therapy for prostate cancer), immunotherapy, or radiotherapy.
This study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization-Good Clinical Practice and in accordance with the ethical principles underlying European Union Directive 2001/20/EC and the U.S. Code of Federal Regulations, Title 21, Part 50. Informed consent was obtained from each patient. The protocol, amendments, and subject informed consent received appropriate approval by the local institutional review board or independent ethics committee before the initiation of study at each site.
Study Design.
This was a two-part, single-center, open-label, single oral dose followed by multiple dose study conducted in patients with advanced or metastatic solid tumors. Part A denotes the period of assessment of PK, mass balance, metabolism, and elimination of a single dose of [14C]brivanib alaninate (Fig. 2). The position of 14C labeling in brivanib alaninate is shown in Fig. 1. Patients were admitted to the clinical facility on the morning of day −1, at which time they underwent baseline assessments, including physical examination and measurements, echocardiogram, electrocardiogram, brain natriuretic peptide levels, troponin I levels, urinalysis, and clinical laboratory tests.
Fig. 2.
Study design.
Based on dosimetry calculations performed using data from a single-dose [14C]brivanib alaninate limited tissue distribution study of total radioactivity (TRA) in male Long-Evans rats, it was estimated that the administration of a 100-μCi oral dose of [14C]brivanib alaninate would expose human patients to a total committed effective dose equivalent of 1.32 mrem, which was within the range of natural background radiation (up to 50 mrem/year), below the effective dose equivalent for a routine diagnostic chest X-ray (5–20 mrem), and well below the exposure limit for a single dose in human isotope studies (3000 mrem; data on file).
On day 1, patients received a single oral in tablet form dose of 800 mg of [14C]brivanib alaninate as an oral solution containing 100 μCi of TRA (0.125 μCi/mg). In part B, patients received brivanib alaninate tablets at a dose of 800 mg once daily.
Serial blood samples were collected for analyses of PK and TRA over a 10-day period at predose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 18, 24, 36, 48, 60, 72, 96, 120, 144, 168, 192, 216, and 240 h. All blood samples were collected into 3-ml tubes containing EDTA as the anticoagulant and 0.45 ml of 3.14 M glycine buffer (to prevent the ex vivo conversion of brivanib alaninate to brivanib). Plasma was prepared from the blood samples by centrifuging at room temperature for 15 min at 1300g. The plasma samples were then stored at −20°C until analysis. Complete urinary and fecal output was collected at 24-h intervals over the 10-day period or until discharge and analyzed for TRA. A single oral dose (30 ml) of magnesium hydroxide was administered on the evening of day 7 to ensure defecation before release from the clinical facility. Patients were discharged from the clinic on day 10, after ensuring that at least 80% of the total dose of radioactivity had been collected or that the day 8 measurement of radioactivity in the urine and feces combined (for the 24-h period) was ≤1% of the total administered radioactivity. Patients whose TRA on day 8 exceeded 1% remained in the clinical facility until the level of a 24-h period was ≤1% or until day 17, whichever was earlier. Part B denotes the period of safety analysis and began after patients had completed part A. Patients received nonradiolabeled brivanib alaninate administered orally at a dose of 800 mg once daily starting on day 15, 16, or 17. Patients continued on this study until disease progression or unacceptable toxicity. No dose escalation was permitted, and a maximum of two dose reductions, by 25 and 50% (to 600 or 400 mg q.d., respectively) for the first and second dose reductions, respectively, was allowed for patients experiencing toxicity.
Analysis of Brivanib Concentration in Plasma and Urine Samples.
The brivanib concentration in plasma and urine was measured after solid-phase extraction or acetonitrile treatment, respectively, followed by a liquid chromatography/tandem mass spectrometry method using a stable-labeled internal standard of brivanib ([13C3,15N2]BMS-540215). The internal standard (50 μl at 100 ng/ml for plasma and 10 μl at 250 ng/ml for urine) was added to 0.1 and 0.05 ml of plasma and urine samples, respectively. The plasma samples were buffered with ammonium formate before loading onto a solid-phase extraction plate obtained from Waters (Milford, MA). Solid-phase extraction plates were conditioned with methanol and an ammonium formate solution. After addition of the plasma sample, the cartridges were washed with an ammonium formate solution and acetonitrile-water. Samples were eluted from the plate with a formic acid solution and evaporated to dryness under nitrogen and then were reconstituted with an acetonitrile-water-ammonium formate-formic acid solution. The urine samples were buffered with ammonium formate followed by acetonitrile, vortexed, and then centrifuged; these extracts were loaded onto the high-performance liquid chromatography (HPLC) system. The HPLC system consisted of a Shimadzu HPLC pump (Shimadzu Corporation, Columbia, MD) and a PerkinElmer Autosampler (PerkinElmer Life Sciences, Inc., Boston, MA). The column used was a Gemini C6-phenyl column (5 μm, 2.0 × 50 mm; Phenomenex, Torrance, CA), and the mobile phase flow rate was 0.3 ml/min. A gradient of two solvent systems, A and B, was used for HPLC profiling. Solvent A consisted of 0.01 M ammonium formate solution. Solvent B consisted of 0.01 M ammonium formate with 0.12% formic acid in acetonitrile-water. The HPLC system was interfaced to a Sciex API 4000 mass spectrometer (Applied Biosystems, Foster City, CA) that was operated in the positive ion electrospray ionization mode. The analysis time was ∼2.3 min for both brivanib and the internal standard in plasma sample analysis and ∼1.6 min for both brivanib and the internal standard in urine sample analysis. The limits of quantitation in plasma and urine were 2 to 2000 and 1 to 1000 ng/ml, respectively.
Analysis of Radioactivity Concentration in Blood, Plasma, Feces, and Urine Samples.
TRA of [14C]brivanib was measured in blood, plasma, feces homogenates, and urine by liquid scintillation counting. Twenty-five to 50 μl of each plasma sample were transferred to an accelerator mass spectrometry quartz tube containing 100 μl of carrier carbon tributyrin solution (42.2 mg C/ml), with the exception of predose samples, which were transferred to an empty accelerator mass spectrometry tube. 14C-labeled standards were combusted to 14CO2 together with the study samples to determine combustion efficacy. Resultant graphite samples were analyzed for total 14C radioactivity using a Shimadzu V-series Total Organic Carbon Analyzer. Carbon concentrations (grams per milliliter) were reported and used in the calculation of 14C radioactivity derived from [14C]brivanib.
Pharmacokinetic Analysis.
The following single-dose PK parameters were derived: maximum plasma concentration (Cmax), time to reach maximum observed plasma concentration (Tmax), area under the concentration-time curve from time 0 extrapolated to infinity (AUC0–inf), AUC from time 0 to the last quantifiable concentration (AUC0–T), terminal elimination half-life (t1/2), renal clearance (CLR), and apparent total body clearance (CL/F).
Safety and Adverse Events.
Patients were monitored for adverse events (AEs) throughout the study. Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3). Safety was assessed by monitoring vital signs, physical examinations, electrocardiograms, echocardiograms, brain natriuretic peptide levels, troponin I levels, and clinical laboratory tests.
Objectives.
The primary objective was to assess the PK, mass balance, metabolism, and routes and extent of elimination of a single oral dose of [14C]brivanib alaninate containing 100 μCi of TRA (0.125 μCi/mg) in patients with advanced or metastatic solid tumors. Secondary objectives were 1) to assess the safety of a single oral dose administration of 800 mg of [14C]brivanib alaninate, 2) to evaluate the safety and tolerability of brivanib after multiple oral dose administration in patients with advanced and metastatic solid tumors who participated in part B, and 3) to assess preliminary evidence of efficacy as deemed appropriate by the institutional or investigator's standard of care.
Statistical Analyses.
Individual subject PK parameter values were derived by a noncompartmental method using a validated pharmacokinetic program (Kinetica 4.4.1 within the eToolbox, version 2.6.1; Thermo Fisher Scientific, Waltham, MA) with the PK values for each parameter calculated for each patient and then averaged for the whole study population. Summary statistics were calculated for brivanib and plasma TRA PK parameters: Cmax, Tmax, AUC0–inf, AUC0–T, t1/2, CL/F, CLR, AUC(brivanib)/AUC(TRA) ratio, and TRA blood AUC/plasma AUC ratio. Geometric means and coefficients of variation are presented for Cmax, AUC0–T, AUC0–inf, CLR, and AUC(brivanib)/AUC(TRA) ratio. Medians and ranges are presented for Tmax. Mean and SD are presented for t1/2. Summary statistics are calculated for percentage of brivanib and radioactivity excreted in urine, percentage of radioactivity excreted in feces, and percentage of total administered radioactivity recovered for each collection interval and cumulative over the entire period of collection. Means and SD are presented for all other parameters.
Results
Patient Population.
Seven patients were enrolled and provided written informed consent; two patients later withdrew consent and one no longer met the study inclusion criteria. Of the remaining four patients, two with non–small-cell lung cancer, one with ovarian cancer, and one with renal cell carcinoma were treated in part A and part B of this study (Table 1). Three of the treated patients were male and one was female; the mean age was 65 years (range 51–79 years).
TABLE 1.
Patient baseline characteristics and demographics
n = 4.
| Characteristic | Value |
|---|---|
| Mean age (years) | |
| Mean ± S.D. | 65 ± 13 |
| Minimum, maximum | 51, 79 |
| Gender, n (%) | |
| Male | 3 (75) |
| Female | 1 (25) |
| Race, n (%) | |
| White | 2 (50) |
| Black | 2 (50) |
| Body mass index (kg/m2) | |
| Mean ± S.D. | 24.1 ± 4.1 |
| Minimum, maximum | 18.4, 28.1 |
Pharmacokinetics.
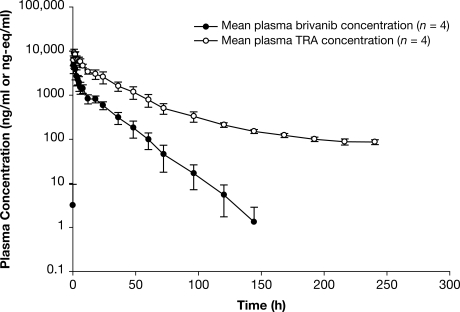
The mean plasma concentration-time profile of TRA and brivanib in plasma is shown in Fig. 3. There were minimal differences in the plasma concentration-time profile among patients. The median Tmax of brivanib was 1 h and the geometric mean Cmax was 6146 ng/ml. The mean t1/2 of brivanib was 13.8 h, and the geometric mean CL/F was 14.7 l/h (Table 2). TRA readily appeared in plasma, with a median Tmax value of 1 h after a single oral dose of 800 mg of [14C]brivanib alaninate, reflecting a composite of plasma concentrations of brivanib and other metabolites. The mean t1/2 of TRA in plasma declined slowly, with a mean of 105 h, and the geometric mean Cmax of TRA in plasma was approximately one-third higher than that of brivanib in plasma. With use of the geometric mean plasma AUC0–inf ratios of unchanged brivanib to TRA, the percentage of TRA in the form of brivanib was approximately 23%, suggesting the presence of metabolites.
Fig. 3.
Mean ± S.D. plasma concentration-time profile of brivanib and total radioactivity after a single oral 800-mg dose of [14C]brivanib alaninate.
TABLE 2.
Summary of brivanib and total radioactivity plasma PK parameters in patients
n = 4. Statistics are presented as follows: geometric mean (coefficient of variation percent) for Cmax, AUC, AUC ratios, CL/F, and CLR; median (minimum, maximum) for Tmax; and mean ± SD for t1/2.
| Parameter | Brivanib | Plasma TRA |
|---|---|---|
| Cmax (ng/ml) | 6146 (15) | 9350 (19) |
| Tmax (h) | 1 (0.5, 1) | 1 (0.5, 1.9) |
| AUC0–inf (ng · h/ml) | 45,892 (9) | 203,823 (20) |
| t1/2 (h) | 13.8 ± 2.8 | 105 ± 27 |
| CL/F (ml/min) | 14.7 (10) | 4 (21) |
| CLR (l/h) | 0.001 (42) | 0.496 (44) |
| Plasma AUC(brivanib)/AUC(TRA) | 0.23 (13) | |
| TRA blood AUC/plasma AUC ratio | 0.52 (32) | |
Mass Balance.
After a single oral dose of [14C]brivanib alaninate, 12.2 and 81.5% of the administered radioactivity was recovered in urine and feces, respectively, for a total recovery of 93.7% (Table 3). After oral administration, brivanib alaninate was completely converted to the active moiety, brivanib. Brivanib alaninate was not detected in plasma. The predominant route of elimination of drug-related material was fecal. Thus, the percentage of brivanib absorbed when administered as brivanib alaninate was at least 86% (93.7%; total recovery − 7.5%). Renal excretion of unchanged brivanib was minimal.
TABLE 3.
Recovery of brivanib and total radioactivity in urine and feces up to day 10
| Patient | Urine (Brivanib) | Urine TRA | Feces TRA | Total TRA |
|---|---|---|---|---|
| % | ||||
| 1 | 0.010 | 12.56 | 80.85 | 93.41 |
| 2 | 0.009 | 12.06 | 80.27 | 92.33 |
| 3 | 0.004 | 6.71 | 87.64 | 94.35 |
| 4 | 0.006 | 17.37 | 77.40 | 94.77 |
| Mean | 0.007 | 12.18 | 81.5 | 93.72 |
| S.D. | 0.003 | 4.36 | 4.34 | 1.08 |
Safety.
Brivanib was well tolerated. Fatigue was the most frequent AE that occurred in all four patients and the most frequent treatment-related AE occurring in three patients (75%). All four patients experienced gastrointestinal events, the most common being nausea, diarrhea, and constipation. There were no deaths, serious AEs, or discontinuations due to AEs in part A. In part B, one patient discontinued treatment because of a serious AE of grade 3 dysphagia, which was not related to treatment. All AEs in part A and the majority of events in part B were mild (grade 1 or 2) in severity. Grade 2 AEs included single events of fatigue, abdominal pain, dysphagia, back pain, cognitive disorder, and cachexia. No grade 4 events were reported, and one patient died of disease progression, which was reported as a grade 5 serious AE.
Efficacy.
During treatment in the study, which ranged from approximately 4 to 9 weeks, the best clinical response in one patient with non–small-cell lung cancer was stable disease and the other three patients with non–small-cell lung cancer, ovarian cancer, and renal cell carcinoma had progressive disease.
Discussion
This study was performed in tandem with a study to provide information on the routes of elimination of brivanib alaninate and its metabolites to anticipate any likely effects of renal or hepatic impairment on the disposition of brivanib alaninate. Four patients with advanced/metastatic solid tumors received single oral doses of [14C]brivanib alaninate in part A and then continued to receive treatment with 800 mg daily of brivanib alaninate in part B for approximately 4 to 9 weeks. Brivanib alaninate, administered as a single radioactive-labeled dose, was extensively metabolized in patients with advanced cancer, and elimination occurred primarily via feces. Recovery of radioactivity was good, with minimal differences among patients. Toxicities associated with once-daily oral dosing of brivanib alaninate were manageable in this patient population. After a single oral 800-mg dose of [14C]brivanib alaninate, the drug was rapidly absorbed, as indicated by the early Tmax of 1 h for both brivanib and TRA. The prodrug was not detected in plasma, demonstrating that the prodrug was efficiently converted to its active moiety. As seen in the study mentioned above that was completed in tandem, brivanib was metabolized by multiple metabolic routes and excreted primarily as metabolites in feces, with 81.54% of total radioactivity recovered in feces and 7.5% as unchanged brivanib. Brivanib absorption was high (>86%) in patients with advanced cancer when administered orally as brivanib alaninate. Renal excretion of brivanib was minimal; therefore, renal impairment is unlikely to affect brivanib exposure. Recovery of radioactivity was high (93.7%) and minimally variable among patients, suggesting similar elimination of this drug by the diverse population of patients with advanced cancer in this study. Biotransformation and metabolite identification analyses were conducted on plasma, urine, and fecal samples in a separate study using patient samples from the study reported here. Brivanib was found to be the major active circulating component in plasma samples collected before 12 h. Minor metabolites were detected in plasma but none are expected to be biologically active.
Results from the current study and demonstrate that brivanib is extensively metabolized, with metabolites accounting for more than 90% of the excreted radioactivity. In addition, another study (data not shown) has shown that brivanib was primarily metabolized by multiple liver enzymes, including CYP1A2, CYP3A4, and multiple sulfotransferases. It is anticipated that the involvement of multiple enzymes in the metabolism of brivanib would make brivanib less susceptible to drug-drug interactions compared with other agents such as sunitinib and sorafenib, which are metabolized primarily by CYP3A4 with additional glucuronidation by UDP glucuronyl transferase A19 in the case of sorafenib (Faivre et al., 2006; Kane et al., 2006; Miller et al., 2009). As a result, concomitant treatment with CYP3A4 inhibitors and inducers must be avoided with these two agents. Because brivanib is primarily metabolized in the liver, the effect of hepatic impairment on brivanib is currently being investigated in an ongoing PK study (clinicaltrials.gov identifier NCT00437424). The t1/2 of brivanib was 13.8 h in this study, whereas the reported t1/2 of sorafenib ranges from 41 to 86 h (Faivre et al., 2006) and that of sunitinib from 40 to 60 h (Hartmann et al., 2009). The shorter t1/2 of brivanib compared with sorafenib and sunitinib therefore allows for once-daily dosing with limited accumulation in plasma upon multiple daily dosing.
Although this was not a trial to establish efficacy and the duration of treatment was short (4–9 weeks), the response to treatment was encouraging, with one of four patients achieving stable disease. These data correlate with and support the preliminary clinical activity observed with brivanib in an ongoing phase II study of first-line brivanib treatment in patients with HCC in which the disease control rate was 51% (4 partial responses, 2 unconfirmed partial responses, and 22 stable disease) when assessed with modified World Health Organization criteria, and 78% (7 complete responses, 12 partial responses, and 24 stable disease) when assessed with the new modified Response Evaluation Criteria in Solid Tumors for HCC (Llovet et al., 2008a; Raoul et al., 2009).
The safety profile of brivanib was manageable in this population with advanced cancer. The most frequent AEs were fatigue [4 (100%) patients] and gastrointestinal events [nausea, diarrhea, and constipation, each reported in 2 (50%) patients]. Although hypertension has been reported with other agents in this class (Morabito et al., 2006; Hartmann et al., 2009), there were no reported AEs of increased blood pressure among the 4 patients treated in this study, and no reported cases of hand-foot syndrome.
Brivanib alaninate represents a potential therapeutic advance over current VEGF-directed therapies through dual targeting of the FGF signaling pathway, which appears to play a role in the development of resistance to VEGF-targeted therapies and the VEGF signaling pathway. Thus, dual targeting of FGF and VEGF signaling pathways may provide prolonged clinical benefit to patients. In this study, brivanib was rapidly absorbed and extensively metabolized in humans after a single 800-mg oral dose, with the majority of drug-related radioactivity excreted in feces. Consistent with the results from other studies, brivanib was well tolerated and has a manageable safety profile in this group of patients with advanced cancer.
Acknowledgments.
Editorial support was provided by Dr. Mark English (PAREXEL, Stamford, CT) and was funded by Bristol-Myers Squibb.
This work was supported by the National Institutes of Health National Cancer Institute [Grant 5P30-CA043703] (to R.G.).
Parts of this work were previously presented at the following conference: Ganapathi R, Mekhail T, Wu C-Y, Fischer B, Gong J, Iyer R, Gan J, Pursley J, Patricia D, and Masson E (2009) Mass balance, pharmacokinetics and metabolism of 14C of brivanib in subjects with advanced or metastatic solid tumors. 2009 Annual Meeting of the American Society of Clinical Oncology; 2009 May 29–June 2; Orlando, FL. American Society of Clinical Oncology, Alexandria, VA.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.033951.
- VEGF
- vascular endothelial growth factor
- FGF
- fibroblast growth factor
- HCC
- hepatocellular carcinoma
- TKI
- tyrosine kinase inhibitor
- PK
- pharmacokinetics
- BMS-540215
- (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1f][1,2,4]triazin-6-yloxy)propan-2-ol
- HPLC
- high-performance liquid chromatography
- AE
- adverse event
- TRA
- total radioactivity
- Cmax
- maximum plasma concentration
- Tmax
- time to reach maximum observed plasma concentration
- AUC0-inf
- area under the concentration-time curve from time 0 extrapolated to infinity
- AUC0-T
- AUC from time 0 to the last quantifiable concentration
- t1/2
- terminal elimination half-life
- CLR
- renal clearance
- CL/F
- apparent total body clearance.
References
- Arora A, Scholar EM. (2005) Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 315:971–979 [DOI] [PubMed] [Google Scholar]
- Beumer JH, Beijnen JH, Schellens JH. (2006) Mass balance studies, with a focus on anticancer drugs. Clin Pharmacokinet 45:33–58 [DOI] [PubMed] [Google Scholar]
- Bhide RS, Cai ZW, Zhang YZ, Qian L, Wei D, Barbosa S, Lombardo LJ, Borzilleri RM, Zheng X, Wu LI, et al. (2006) Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem 49:2143–2146 [DOI] [PubMed] [Google Scholar]
- Bhide RS, Lombardo LJ, Hunt JT, Cai ZW, Barrish JC, Galbraith S, Jeyaseelan R, Sr, Mortillo S, Wautlet BS, Krishnan B, et al. (2010) The antiangiogenic activity in xenograft models of brivanib, a dual inhibitor of vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinases. Mol Cancer Ther 9:369–378 [DOI] [PubMed] [Google Scholar]
- Cai ZW, Zhang Y, Borzilleri RM, Qian L, Barbosa S, Wei D, Zheng X, Wu L, Fan J, Shi Z, et al. (2008) Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215). J Med Chem 51:1976–1980 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. (2000) Angiogenesis in cancer and other diseases. Nature 407:249–257 [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134 [DOI] [PubMed] [Google Scholar]
- Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, et al. (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35 [DOI] [PubMed] [Google Scholar]
- Hartmann JT, Haap M, Kopp HG, Lipp HP. (2009) Tyrosine kinase inhibitors—a review on pharmacology, metabolism and side effects. Curr Drug Metab 10:470–481 [DOI] [PubMed] [Google Scholar]
- Hurwitz H, LoRusso P, Shapiro I, Wolanski A, Chemidlin J, Masson E, Syed S, Kollia G, Fischer B, Conlon K. (2008) Phase 1 study of food effects on pharmacokinetics of brivanib alaninate in patients with advanced or metastatic solid tumors, in Proceedings of the 20th EORTC-NCI-AACR Symposium on “Molecular Targets and Cancer Therapeutics”; 2008 October 21–24; Geneva, Switzerland Abstr 429, European CanCer Organisation, Brussels, Belgium [Google Scholar]
- Huynh H, Ngo VC, Fargnoli J, Ayers M, Soo KC, Koong HN, Thng CH, Ong HS, Chung A, Chow P, et al. (2008) Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res 14:6146–6153 [DOI] [PubMed] [Google Scholar]
- Je Y, Schutz FA, Choueiri TK. (2009) Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol 10:967–974 [DOI] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, et al. (2006) Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res 12:7271–7278 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, et al. (2008a) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100:698–711 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. (2008b) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390 [DOI] [PubMed] [Google Scholar]
- Marathe PH, Kamath AV, Zhang Y, D'Arienzo C, Bhide R, Fargnoli J. (2009) Preclinical pharmacokinetics and in vitro metabolism of brivanib (BMS-540215), a potent VEGFR2 inhibitor and its alanine ester prodrug brivanib alaninate. Cancer Chemother Pharmacol 65:55–66 [DOI] [PubMed] [Google Scholar]
- Miller AA, Murry DJ, Owzar K, Hollis DR, Kennedy EB, Abou-Alfa G, Desai A, Hwang J, Villalona-Calero MA, Dees EC, et al. (2009) Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 27:1800–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. (2006) Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist 11:753–764 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124 [DOI] [PubMed] [Google Scholar]
- Nyberg P, Xie L, Kalluri R. (2005) Endogenous inhibitors of angiogenesis. Cancer Res 65:3967–3979 [DOI] [PubMed] [Google Scholar]
- Raoul J-L, Finn R, Kang YK, Park JW, Harris R, Coric V, Donica M, Walters I. (2009) An open-label phase II study of first- and second-line treatment with brivanib in patients with hepatocellular carcinoma (HCC) (abstract). J Clin Oncol 27(15s):abstr 4577 [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550 [DOI] [PubMed] [Google Scholar]
- van Erp NP, Gelderblom H, Guchelaar HJ. (2009) Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 35:692–706 [DOI] [PubMed] [Google Scholar]