Abstract
Neuroventilation is highly plastic and exposure to either of two distinct teratogens, nicotine or ethanol, during development results in a similar loss of the neuroventilatory response to hypercapnia in bullfrog tadpoles. Whether this functional deficit is permanent or transient following nicotine or ethanol exposure was unknown. Here we tested the persistence of hypercapnic neuroventilatory response impairments in tadpoles exposed to either 30 µg/L nicotine or 0.12 - 0.06 g/dL ethanol for 10 wk. Brainstem breathing-related neural activity was assessed in tadpoles allowed to develop teratogen-free after either nicotine or ethanol exposure. Nicotine-exposed animals responded normally to hypercapnia after a 3-wk teratogen-free period but the hypercapnic response in ethanol-exposed tadpoles remained impaired. Tadpoles allowed to develop for only 1 wk nicotine free after chronic exposure were unable to respond to hypercapnia. The hypercapnic response of ethanol-exposed tadpoles returned by 6 wk following chronic ethanol exposure. These findings suggest that some nicotine- and ethanol-induced impairments can be resolved during early development. Understanding both the disruptive effects of nicotine and ethanol exposure and how impaired responses return when teratogen exposure stops may offer insight on the function and plasticity of respiratory control.
Keywords: control of breathing, development, alcohol, nicotine
Introduction
Neuroventilation is considerably plastic, (Carroll, 2003; Mitchell and Johnson, 2003) and experiences generate short- to long-term changes in the function and morphology of the breathing control network (Bavis and Mitchell, 2008). The varying effects of these changes have a widespread impact on CO2 homeostasis, because compensating for shifts in pH by offloading CO2 is a critical homeostatic function of breathing (Milsom, 1995; Nattie, 1999; Putnam et al., 2004). Early exposure to either nicotine or ethanol during development results in impairment of the ventilatory response that normally counteracts elevated CO2 (hypercapnia; Eugenin et al., 2008; Taylor et al., 2008). This may explain why prenatal exposure to both nicotine and ethanol are risk factors for Sudden Infant Death Syndrome (SIDS; Iyasu et al., 2002; Kinney, 2009). An inability to adequately respond to hypercapnia has been hypothesized to contribute to SIDS (Shannon et al., 1977; Dunne et al., 1992; Richerson et al., 2001). The neuroplastic changes to the breathing control network that are evoked by nicotine and ethanol exposure result in impaired responses to hypercapnia in mice and bullfrog tadpoles (Eugenin et al., 2008; Taylor et al., 2008; Brundage and Taylor, 2009). Characterizing the neuroplastic changes induced by nicotine and ethanol could improve the understanding of the respiratory control network and improve how clinical medicine addresses this vulnerability.
The timeline of when impairments in hypercapnic responses occur offers insight on the development and lability of respiratory control. Response to hypercapnia is lost faster in early tadpole development during nicotine exposure compared to ethanol exposure (Brundage and Taylor, 2009; Brundage and Taylor, 2010). Despite the disparate actions of nicotine and ethanol in the brain, the breathing-related functional deficit following chronic exposure is the same. The duration of impairment of the hypercapnic response may also differ following nicotine and ethanol exposure. The nicotine-induced impairment of the hypercapnic response is transient in mice. Mice prenatally exposed to nicotine are vulnerable to hypercapnia for 0 – 3 postnatal days; normal responses to hypercapnia return by postnatal day 8 (Eugenin et al., 2008). Persistence in the loss in hypercapnic responses following ethanol exposure, to our knowledge, has not been evaluated. It would be interesting if the timeline for recovery from nicotine and ethanol exposure were similar and would suggest that the neuroplastic changes evoked by nicotine and ethanol exposure may be comparable. Dissecting the mechanisms involved in nicotine and ethanol impairment of central hypercapnic drive can be aided significantly by first characterizing the developmental timeline for these impairments and their recovery.
The developing bullfrog tadpole has been used as a model to investigate the effects of nicotine and ethanol exposure on control of breathing (Taylor et al., 2008; Brundage and Taylor, 2009). The neuroventilation of bullfrogs can be quantified using an isolated brainstem preparation at all free-living developmental stages, and the exposure conditions of the tadpoles to nicotine and ethanol can be highly controlled due to their aquatic environment (Brundage and Taylor, 2009). Ten wk of chronic nicotine or ethanol exposure results in the loss of the neuroventilatory response to hypercapnia in early metamorphic tadpoles (Taylor et al., 2008; Brundage and Taylor, 2009). We hypothesized that similar to mice, normal tadpole responses to hypercapnia would return if animals were allowed to live in a nicotine-free environment after their chronic nicotine exposure. Vulnerability to SIDS is resolved by the first year of life (Filiano and Kinney, 1994; Kinney, 2009), and we have found no reports of higher incidence among infants with fetal alcohol spectrum disorders. Thus, we also hypothesized that the effects of ethanol would also be resolved following an ethanol-free period. This is the first study to look at the persistence of any teratogen-induced impairment of neuroventilatory responses to hypercapnia in the tadpole, and the first to make such a consideration for any animal following ethanol exposure.
Methods
Animals
Studies were performed on bullfrogs Lithobates catesbeianus tadpoles (n = 34) purchased from a commercial supplier (Sullivan Co. Inc., www.researchamphibians.com). Tadpoles were maintained at room temperature and were fed goldfish food daily. Tadpoles were housed for 10 wk in aquaria with either dechlorinated water only, dechlorinated water containing nicotine (30 µg/L (−)-nicotine hydrogen tartrate salt; Sigma, www.sigmaaldrich.com) or dechlorinated water containing ethanol (0.12 - 0.06 g/dL). We chose 10-wk exposure because that duration of exposure impairs ventilatory responses to hypercapnia in both early- and late-stage tadpoles (Taylor et al., 2008; Brundage and Taylor, 2009; Brundage and Taylor 2010). The concentration of nicotine was similar to that found in the body fluids of an average smoker (Moyer et al., 2002). The ethanol concentration varied due to the volatilization of ethanol from the tank and was equivalent to 0.75–1.5 times the 0.08 g/dL blood alcohol content that serves as the legal limit in many western countries (Caldeira et al., 2004). Following 10 wk of nicotine exposure, tadpoles were allowed to recover for either 1 wk (n = 6) or 3 wk (n = 8) in nicotine-free dechlorinated water. Ethanol-exposed tadpoles recovered for either 3 wk (n = 5) or 6 wk (n = 7).
Tadpole developmental stages were determined at the start of treatment and at the end of the 10-wk exposure to ensure developmental homogeneity. At the time of dissection each tadpole was at an early stage of metamorphosis corresponding to developmental stages 7–15 in the classification scheme of Taylor and Köllros (1946). The teratogen-exposed animals appeared to develop and grow similarly and at a similar pace compared with unexposed animals, although development and growth were not specifically quantified for individual animals. All animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Alaska Fairbanks and complied with all state and federal ethical guidelines.
Surgical preparation
Each tadpole was anesthetized by immersion for 1–2 min in cold (4 °C) 0.2 mM tricaine methanesulfonate (MS222; Sigma, www.sigmaaldrich.com) in dechlorinated water buffered to pH 7.8 with NaHCO3. The front of the head rostral to the nares and the back of the body (hind limbs and tail, if present) were removed. The dorsal cranium and forebrain rostral to the diencephalon were resected and the fourth ventricle opened by removing the choroid plexus. The remaining brainstem and spinal cord were removed en bloc and further trimmed rostrally to the optic tectum and caudally at the brachial nerve. During dissection, exposed tissues were superfused with cold artificial cerebral spinal fluid (aCSF) composed of (in mM) 104 NaCl, 4 KCl, 1.4 MgCl2, 10 D-glucose, 25 NaHCO3, and 2.4 CaCl2 equilibrated with 100 % O2. These methods have been used in our previous tadpole studies (Taylor et al., 2008; Davies et al., 2009).
The isolated brainstem was transferred to a 2.5-ml, Plexiglas, flow-through recording chamber and was supported, ventral side up, between coarse nylon mesh such that all surfaces were bathed with aCSF flowing from rostral to caudal at a rate of 5 ml/min. A supply of aCSF, equilibrated with O2-CO2 mixtures that produced the desired pH, flowed through plastic tubing to the recording chamber and bathed the isolated brainstem. The pH of the aCSF was maintained at either pH 7.8 (1.5 % CO2: 98.5 % O2; normocapnia) or pH 7.4 (5.0 % CO2: 95.0 % O2; hypercapnia) by adjusting the fractional concentrations of O2 and CO2 in the equilibration gas. CO2 was monitored with a CO2 analyzer (Capstar 100; CWE, www.cwe-inc.com). After isolation the brainstem was allowed to stabilize for 1 h while superfused at 23 °C, with aCSF of pH 7.8 (~9 torr PCO2).
Neurogram recording
Roots of the facial and hypoglossal nerves were drawn into glass suction electrodes pulled from 1-mm-diameter capillary glass to tip diameters that fit the nerve roots. Whole-nerve discharge was amplified (X100 by DAM 50 amplifiers, World Precision Instruments, www.wpiinc.com; X1000 by a four-channel model 1700 amplifier, A-M Systems, www.a-msystems.com) and filtered (100 Hz high pass to 1 kHz low pass). The amplified and filtered nerve output was sent to a data acquisition system (Powerlab, AD Instruments, www.adinstruments.com), which sampled at 1 kHz. The data were archived as whole-nerve discharge, and duplicate integrated (full-wave rectified and averaged over 200 ms) neurograms were acquired simultaneously. Such recordings were made during the initial 1 h post-isolation stabilization period and recorded continuously throughout the duration of each treatment protocol.
Data analyses and statistics
Neurograms recorded from the isolated tadpole brainstems were quantified for 30 min of normocapnia, 30 min of hypercapnia, and a subsequent 30 min return to normocapnia. Burst activity patterns were designated as either putative gill or putative lung breaths on the basis of the amplitude of the integrated nerve activity and the presence or absence of coincident firing in both the facial and hypoglossal nerves as previously described (Torgerson et al., 1998). Putative gill breaths had lower integrated burst amplitude on the facial nerve than putative lung breaths and little or no coincident burst activity in the hypoglossal nerve. Putative lung breaths had higher integrated burst amplitude in the facial nerve and coincident burst activity in the hypoglossal nerve.
The frequency of lung and gill ventilation was quantified as the number of bursts per minute for the last 3 min of normocapnia and hypercapnia. Taylor et al. (2003a) demonstrated that an increase in lung burst frequency is the primary manifestation of the bullfrog hypercapnic response at all stages of their development (Fig. 1). Gill burst frequency during normocapnia, but not hypercapnia was decreased after 10-wk exposure to nicotine but not ethanol (Brundage et al., 2010b). Therefore, gill neuroventilation during normocapnia in post-ethanol exposed tadpoles was not considered in this study. Percent changes in lung burst frequency during the last 3 min of hypercapnia were determined relative to the last 3 min of normocapnia. The mean values for each of the quantified burst frequencies were compared using repeated-measures analysis of variance (RM-ANOVA; SigmaStat, www.systat.com). When a RM-ANOVA indicated that significant differences existed, multiple comparisons were made using the Holm-Sidak multiple comparison test. Comparisons between control and treatment groups were conducted using t-test comparisons (SigmaStat, www.systat.com). Values reported in the text are always mean ± SE.
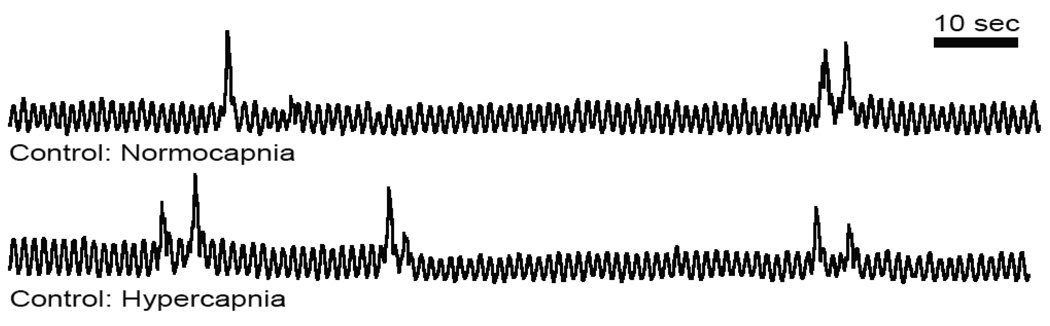
Fig 1. Lung burst frequency increased in response to hypercapnia.
Representative integrated neurograms recorded over 2 min from the facial nerve root of a control bullfrog tadpole during normocapnia and hypercapnia. Control tadpoles increased the number of lung bursts per minute in response to hypercapnia.
Results
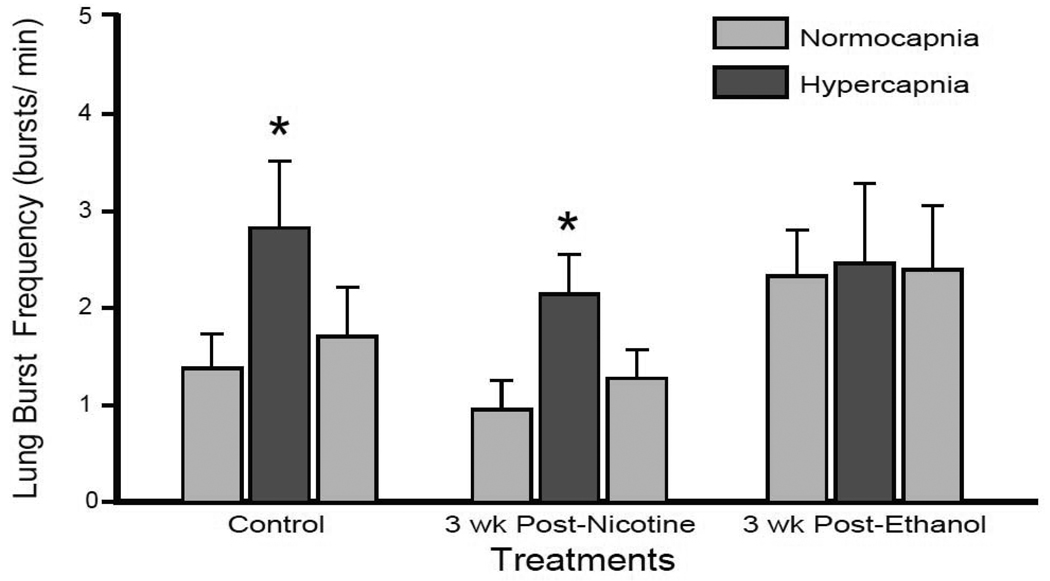
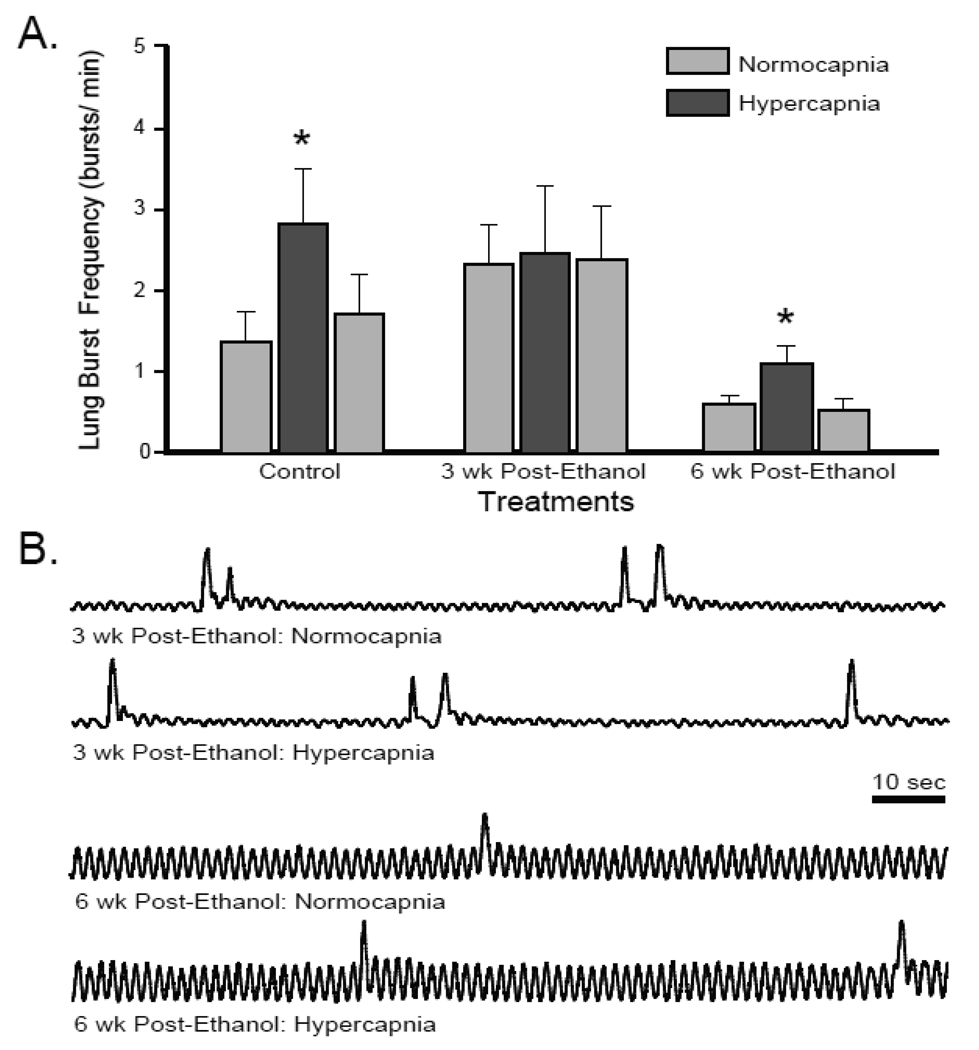
Lung burst frequency after a 3-wk teratogen-free period following 10 wk of either nicotine or ethanol exposures are presented in Fig. 2. Control tadpoles increased lung burst frequency from 1.38 ± 0.36 bursts/min during normocapnia to 1.71 ± 0.51 bursts/min during hypercapnia (P = 0.029). 3 wk of recovery following 10 wk of either nicotine or ethanol exposure did not significantly alter tadpole normocapnic lung burst frequencies (0.95 ± 0.30 bursts/min P = 0.393; 2.33 ± 0.48 bursts/min P = 0.138 for post-nicotine and post-ethanol exposure, respectively). The limited size of the treatment group for 3-wk post-ethanol exposed animals may have prejudiced this observation, as the latter test was below the desired power (0.198). Tadpoles previously exposed to nicotine for 10 wk responded similarly to control animals after 3 wk nicotine-free recovery by increasing lung burst frequency to 2.14 ± 0.41 bursts/min (P = 0.023); a 232. 6 ± 0.5 % increase compared to the 166.7 ± 85.9 % response to hypercapnia in controls. In contrast, 3-wk post-ethanol exposed tadpoles failed to increase lung burst frequency significantly during hypercapnia (2.46 ± 0.53 bursts/min; P = 0.987) demonstrating only a 2.8 ± 21.9 % change in lung burst frequency during hypercapnia. Thus, responses to hypercapnia were still impaired following 3-wk recovery from chronic ethanol but not nicotine exposure.
Fig 2. Central hypercapnic response returned following 3-wk recovery in nicotine- but not ethanol-exposed tadpoles.
Mean lung bursts per min over the last 3 min of normocapnia, hypercapnia, and return to normocapnia. Control and 3-wk post-nicotine exposed tadpoles responded significantly to hypercapnia (* = P < 0.05). 3-wk post-ethanol exposed tadpoles did not respond to hypercapnia (P > 0.05). Data presented are mean ± SE for 6 – 8 tadpoles.
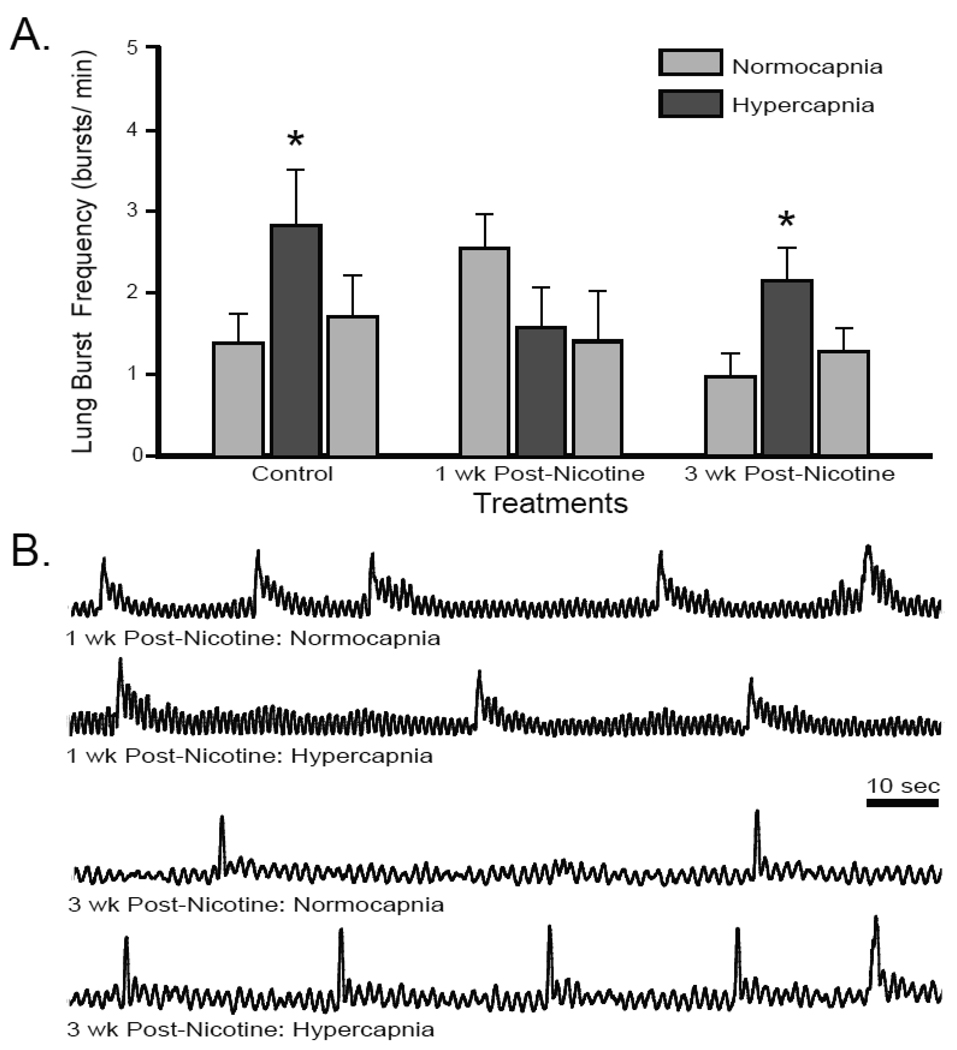
The disparate responses following 3-wk post-exposure periods prompted interest to determine if any persistent impairment of the neuroventilatory hypercapnic response occurred in chronic nicotine-exposed tadpoles, because the previous studies (Taylor et al., 2008; Brundage and Taylor, 2009) had quantified this central hypercapnic response immediately after chronic exposure, without any nicotine-free period. 10-wk nicotine-exposed tadpoles were, therefore, allowed to recover for 1 wk in nicotine-free water before experimentation. The normocapnic lung burst frequency of 1-wk post-nicotine exposed tadpoles was slightly, although not significantly, elevated compared to controls, (2.53 ± 0.41 bursts/min P = 0.059; Fig. 3), but was significantly higher than the 3-wk post-nicotine exposed tadpoles (P = 0.009). Responses to hypercapnia, however, were blocked, in fact there was a modest reduction in lung burst frequency during hypercapnia (1.56 ± 0.49 bursts/min; P = 0.287) a −43.2 ± 9.5 % change in lung burst frequency. This reduction in lung burst frequency was not significant nor was there any significant change in lung burst frequency after 30-min return to normocapnia (1.39 ± 0.62 bursts/min). Therefore, the hypercapnia-induced increase in lung burst frequency that was impaired following 10-wk nicotine exposure remained impaired 1 wk following nicotine exposure, but was fully returned after 3 wk in a nicotine-free environment.
Fig 3. Central hypercapnic response was impaired 1-wk post-nicotine exposure but returned by 3-wk post-nicotine exposure.
(A) Mean lung bursts per min over the last 3 min of normocapnia, hypercapnia, and return to normocapnia. 1-wk post-nicotine exposed tadpoles did not respond to hypercapnia. 3-wk post-nicotine exposed tadpoles increased lung burst frequency during hypercapnia (*= P < 0.05). Data presented are mean ± SE for 6 – 8 tadpoles. (B) Representative integrated neurograms recorded over 2 min from the facial nerve root during normocapnia and hypercapnia.
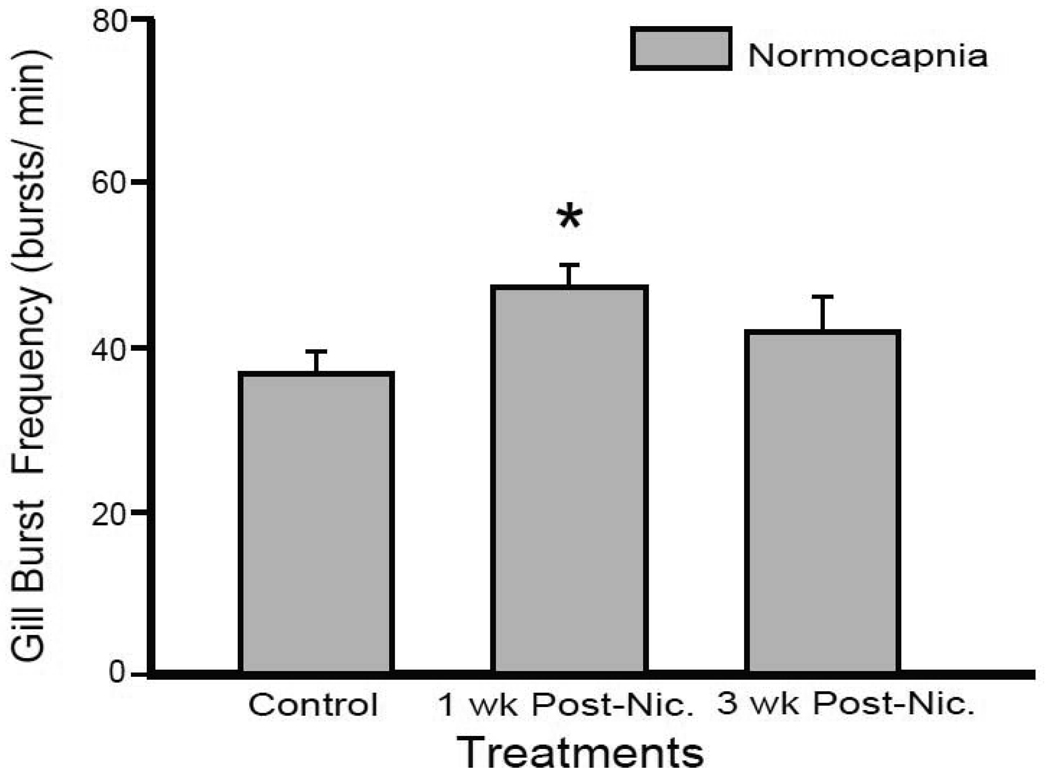
Our previous work identified a diminished normocapnic gill burst frequency in tadpoles immediately following 10-wk nicotine exposure (Brundage et al., 2010b). Here gill burst frequency was quantified during normocapnia in both the 1-wk and 3-wk post-nicotine exposed tadpoles (Fig. 4). 1 wk after exposure the gill burst frequency was significantly elevated compared to control tadpoles (36.2 ± 2.8 and 46.9 ± 2.7 bursts/min for control and 1-wk post-nicotine tadpoles, respectively; P = 0.02). Gill burst frequency of 3-wk post-nicotine exposed tadpoles was not significantly different from controls (41.3 ± 4.4 bursts/min; P = 0.343), nor was it significantly different from 1-wk post-nicotine exposed tadpoles (P = 0.342). Thus, although lung burst responses to hypercapnia continued to be impaired after a 1-wk nicotine free-period, the decreased gill burst frequency induced by chronic nicotine exposure was replaced with a full recovery and facilitation by 1-wk post-nicotine exposure.
Fig 4. Gill burst frequency was elevated 1-wk post-nicotine but not 3-wk post-nicotine exposure.
Mean gill bursts per min over the last 3 min of normocapnia for control, 1-wk, and 3-wk post-nicotine exposed tadpoles. 1-wk post-nicotine exposed tadpoles had an elevated gill burst frequency compared to controls (*= P < 0.05). The gill burst frequency of 3-wk post-nicotine exposed tadpoles was similar to control preparations. Data presented are mean ± SE for 6 – 8 tadpoles.
The persistent impairment of the hypercapnic neuroventilatory response in 3-wk post-ethanol exposed tadpoles prompted interest to determine if the reduction in hypercapnic ventilatory drive was permanent following chronic ethanol exposure. Tadpoles allowed to recover for 6 wk after 10-wk ethanol exposure had a normocapnic lung burst frequency that was not significantly different than controls (0.57 ± 0.10 bursts/min; P = 0.065; Fig. 5) but was significantly lower than the 3-wk post-ethanol exposed tadpoles (P = 0.002). 6-wk post-ethanol exposed tadpoles did increase lung burst frequency in response to hypercapnia (1.05 ± 0.22 bursts/min; P = 0.036). This response to hypercapnia was reduced, but was not significantly different, from that of controls (86.9 ± 38.9 %; P = 0.497). Lung burst frequency fully returned to pre-hypercapnic levels by the end of the normocapnic recovery period (0.49 ± 0.14 bursts/min). Thus, impairments in the hypercapnic response of chronic ethanol-exposed tadpoles persisted for 3 wk but not 6 wk after ethanol exposure was stopped.
Fig 5. Central hypercapnic response was lost 3-wk post-ethanol but returned by 6-wk post-ethanol exposure.
(A) Mean lung bursts per min over the last 3 min of normocapnia, hypercapnia, and return to normocapnia. 3-wk post-ethanol exposed tadpoles did not respond to hypercapnia. Control and 6-wk post-ethanol exposed tadpoles increased lung burst frequency during hypercapnia (*= P < 0.05). Data presented are mean ± SE for 6 – 8 tadpoles. (B) Representative integrated neurograms recorded over 2 min from the facial nerve root during normocapnia and hypercapnia
Discussion
It took between 1 and 3 wk for the tadpole hypercapnic neuroventilatory response to return following 10 wk of chronic nicotine exposure. This is considerably more rapid than the 3- to 6-wk recovery period required following 10 wk of chronic ethanol exposure. Thus, we have demonstrated that, like mice in which the central hypercapnic response is transiently impaired by developmental nicotine exposure (Eugenin et al 2008), nicotine- and ethanol-induced impairments of the tadpole central hypercapnic response are transient. It is noteworthy that, just as there is a difference in the duration of chronic exposure required to induce an impairment (Brundage and Taylor 2009; Brundage and Taylor 2010), nicotine and ethanol differ in the duration that their impairments persist after teratogen exposure is stopped. The mechanisms that underlie the differences in these two teratogens, regarding both the time to impairment and time to recovery are of particular interest.
Normocapnic lung burst frequency was not significantly different between control and post-nicotine or post-ethanol treatment groups. This may have been confounded at times due to individual variation and limited sample size. Normocapnic lung ventilation previously has not been affected by either nicotine or ethanol treatment. Lung burst frequencies were, however, significantly higher in the early post-treatment groups relative to those later (1-wk post-nicotine and 3-wk post-ethanol relative to 3-wk post-nicotine and 6-wk post-ethanol). Lung burst frequencies of all groups were consistent with those previously reported for the metamorphic stage. Nonetheless, normocapnic lung activity may have been augmented following ethanol exposure, and that may have influenced subsequent responses to hypercapnia. These allegations, however, warrant additional experimentation.
Persistent impairment of the tadpole central hypercapnic response that results from chronic nicotine or ethanol exposure may be an example of developmental neuroplasticity. Developmental neuroplasticity has been defined as a long-term change induced by experiences during a critical period of development and has recently been recognized as an important phenomenon in respiratory control (Carroll, 2003; Bavis and Mitchell, 2008). The term developmental neuroplasticity may be more appropriately applied to the effect of chronic nicotine exposure, because early metamorphic tadpoles have a increased vulnerability to nicotine exposure compared to later developmental stages (Brundage and Taylor, 2009; Brundage et al., 2010a). Chronic ethanol exposure, although not evaluated in adult bullfrogs, causes similar impairment of the central hypercapnic responses during both early and late periods of tadpole metamorphosis (Brundage and Taylor 2010). Nonetheless, chronic exposure to nicotine or ethanol during tadpole development resulted in a deficit that lasted for 1–6 wk after exposure, and in the case of nicotine, similar exposure after metamorphosis did not induce a similar impairment in the central hypercapnic response (Brundage et al., 2010a). Thus, we have identified two paradigms of persistent change in the respiratory control network induced by environmental factors experienced during tadpole development. We believe these research paradigms can now be applied to investigation of the mechanisms underlying nicotine- and ethanol-induced developmental neuroplasticity of the breathing control network, a developmental neuroplasticity that is deleterious and may be shared broadly among vertebrates.
The mechanisms of neural change that underlie this plasticity, which manifest as a loss of central hypercapnic ventilatory responses, may result from persistent nicotinic acetylcholine receptor stimulation by nicotine or the potentiation of GABAergic signaling in the case of ethanol (Aguayo et al., 2002; Slotkin et al., 2002; Breese et al., 2006). Chronic stimulation may result in desensitization to subsequent acetylcholine/nicotine, GABA, or other neurotransmitter systems reportedly altered by chronic nicotine and ethanol exposure (Allan et al., 1998; Covernton and Lester, 2002; Fregosi and Pilarski, 2008; Dopico and Lovinger, 2009; Duncan et al., 2009). Nicotinic and GABA receptors are involved in neurogenesis (Zahalka et al., 1992; Represa and Ben-Ari, 2005; Dwyer et al., 2008). The persistent loss of hypercapnic responses may not be limited to cell signaling but also reflect altered neurodevelopment, a change in the types or numbers of cells participating in breathing-related signaling. Investigations of the mechanisms underlying nicotine- and ethanol-induced developmental neuroplasticity of the breathing control network are warranted and should be pursued on both the cellular and receptor level to identify the specific mode of impairment in this animal model.
When nicotine or ethanol was removed from the tadpole environment, there was a subsequent change in neural control of breathing, a central ventilatory response to high CO2 returned. Thus, we have identified another type of neuroplasticity in breathing control, one that is beneficial but not necessarily developmental in nature. Identifying the mechanisms contributing to the return of the central hypercapnic response will provide important information about neuroplasticity in the breathing control network. Although the response to hypercapnia returned, it is unclear if the original mechanisms providing that response recovered or if a new mechanism, one that accommodated the deleterious effects of nicotine and ethanol, arose and facilitated a functional central hypercapnic response. Whether through recovery an original mechanism or emergence of a new mechanism, a change in the neural network that controls breathing was induced after nicotine and ethanol exposure were stopped. This change in signaling of the central hypercapnic response, as another instance of neuroplasticity, is expected to have resulted from neurogenesis, synaptogenesis, or changes in synaptic composition, and any or all of these have the potential to contribute to the renewed manifestation of the central hypercapnic response (Bavis and Mitchell, 2008; Dwyer et al., 2008).
Immediately following 10 wk of chronic nicotine exposure the normocapnic gill burst frequency in isolated tadpole brainstems was decreased (Brundage et al., 2010b). After 1 wk post-nicotine exposure gill burst frequency during normocapnia was greater in brainstems isolated from post-exposure tadpoles than controls although not significantly greater than the frequencies reported in the previous study. Here gill burst facilitation returned to control levels by 3-wk post-nicotine exposure. Thus, with respect to gill burst activity during normocapnia, chronic nicotine exposure induced an initial decrease and recovery from nicotine exposure induced an over-corrective increase followed by a return to baseline levels. This pattern of change in response to changes in nicotine exposure may be consistent with lung ventilation and could be the result of a nicotine-induced desensitization of nicotinic acetylcholine receptors that is compensated by an increase in receptor density and a further change in receptors when the desensitization is rectified. Such alteration in receptor density have been reported for cortical neurons and is termed homeostatic neuroplasticity (Turrigiano, 1999; Desai, 2003; Wierenga et al., 2006; Aoki et al., 2009), which is an adaptation to maintain signal strength in the face of receptor desensitization. There is no direct evidence to support that a change in receptors occurred, rather we raise this issue to emphasize that the mechanism underlying this phenomenon is potentially of interest. The return in functionality of the central hypercapnic response was not the only neuroplastic change resulting from the cessation of chronic nicotine exposure, the baseline frequency of gill neuroventilation was potentially affected as well.
Developmental consistency exists in the hypercapnic response; it is evident even early in metamorphosis and is maintained across ontogeny (Brundage and Taylor, 2010) Although the actual frequency of hypercapnic ventilation increases with development, the hypercapnia-induced percent change in ventilation is relatively constant (Taylor et al., 2003; Brundage and Taylor, 2010). Thus, although the normocapnic ventilation varied between treatment groups the response to hypercapnia should have been consistent. The major reductions in the percent change in ventilation during in hypercapnia in the 1-wk post-nicotine and 3-wk post-ethanol groups suggest these responses were impaired and not the result of intra-group variability.
In this study we used a tadpole model to investigate development of neural control of breathing, and we saw that the SIDS risk factors of chronic developmental exposure to nicotine (Milerad and Sundell, 1993; Adgent, 2006) and ethanol (Iyasu et al., 2002; Kinney, 2009) impaired central response to a ventilatory stressor, hypercapnia. The impairments persisted after exposure to the teratogens had stopped, but the central hypercapnic response was eventually recovered, as it is in mice (Eugenin et al., 2008) after prenatal nicotine exposure. Characterizing the mechanistic consequences of chronic nicotine and ethanol exposure, the persistence of these consequences, as well as the mechanisms that support the return of functionality, may offer significant insight into neuroplasticity of the breathing control network and its hypercapnic response, which may extend the understanding of the pathogenesis of SIDS.
Acknowledgments
This work was supported by NIH-NINDS grant #U54 NS041069-06A1.
References
- Adgent MA. Environmental tobacco smoke and sudden infant death syndrome: a review. Birth Defects Res B Dev Reprod Toxicol. 2006;77:69–85. doi: 10.1002/bdrb.20068. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acid A1 receptor-gated chloride ion channel in adult rat offspring. J Pharmacol Exp Ther. 1998;284:250–257. [PubMed] [Google Scholar]
- Aoki C, Kojima N, Sabaliauskas N, Shah L, Ahmed TH, Oakford J, Ahmed T, Yamazaki H, Hanamura K, Shirao T. Drebrin a knockout eliminates the rapid form of homeostatic synaptic plasticity at excitatory synapses of intact adult cerebral cortex. J Comp Neurol. 2009;517:105–121. doi: 10.1002/cne.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Long-term effects of the perinatal environment on respiratory control. J Appl Physiol. 2008;104:1220–1229. doi: 10.1152/japplphysiol.01086.2007. [DOI] [PubMed] [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, Otis TS, Parsons LH, Penland SN, Roberto M, Siggins GR, Valenzuela CF, Wallner M. Basis of the gabamimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage CM, Taylor BE. Timing and duration of developmental nicotine exposure contribute to attenuation of the tadpole hypercapnic neuroventilatory response. Dev Neurobiol. 2009;69:451–461. doi: 10.1002/dneu.20720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage CM, Taylor BE. Chronic ethanol exposure during development impairs central hypercapnic ventilatory drive. J Appl Physiol. 2010 doi: 10.1016/j.resp.2012.11.006. submitted to. [DOI] [PubMed] [Google Scholar]
- Brundage CM, Nelson CA, Taylor BE. Cholinergic sensitivity of the developing bullfrog (Rana catesbeiana) does not explain vulnerability to chronic nicotine exposure. Adv Exp Med Biol. 2010a;669:103–107. doi: 10.1007/978-1-4419-5692-7_21. [DOI] [PubMed] [Google Scholar]
- Brundage CM, McLane LH, Taylor BE. Chronic nicotine and ethanol exposure both disrupt central ventilatory responses to hypoxia in bullfrog tadpoles. J Physiol London. 2010b doi: 10.1016/j.resp.2013.04.004. submitted to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira JC, Wu Y, Mameli M, Purdy RH, Li PK, Akwa Y, Savage DD, Engen JR, Valenzuela CF. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J Neurochem. 2004;90:1530–1539. doi: 10.1111/j.1471-4159.2004.02686.x. [DOI] [PubMed] [Google Scholar]
- Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Covernton POJ, Lester RAJ. Prolonged stimulation of presynaptic nicotinic acetylcholine receptors in the rat interpeduncular nucleus has differential effects on transmitter release. Intl J Dev Neurosci. 2002;20:247–258. doi: 10.1016/s0736-5748(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Davies BL, Brundage CM, Harris MB, Taylor BE. Lung respiratory rhythm and pattern generation in the bullfrog: role of neurokinin-1 and mu-opioid receptors. J Comp Physiol B. 2009;179:579–592. doi: 10.1007/s00360-009-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS. Homeostatic plasticity in the CNS: synaptic and intrinsic forms. J Physiol Paris. 2003;97:391–402. doi: 10.1016/j.jphysparis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Garland M, Myers MM, Fifer WP, Yang M, Kinney HC, Stark RI. Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: implications for sudden infant death syndrome. J Appl Physiol. 2009;107:1579–1590. doi: 10.1152/japplphysiol.91629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne KP, Fox GP, O'Regan M, Matthews TG. Arousal responses in babies at risk of sudden infant death syndrome at different postnatal ages. Ir Med J. 1992;85:19–22. [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Eugenin J, Otarola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von Bernhardi R. Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J Neurosci. 2008;28:13907–13917. doi: 10.1523/JNEUROSCI.4441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir Physiol Neurobiol. 2008;164:80–86. doi: 10.1016/j.resp.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyasu S, Randall LL, Welty TK, Hsia J, Kinney HC, Mandell F, McClain M, Randall B, Habbe D, Wilson H, Willinger M. Risk factors for sudden infant death syndrome among northern plains Indians. J Am Med Assoc. 2002;288:2717–2723. doi: 10.1001/jama.288.21.2717. [DOI] [PubMed] [Google Scholar]
- Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol. 2009;51:223–233. doi: 10.1002/dev.20367. [DOI] [PubMed] [Google Scholar]
- Milerad J, Sundell H. Nicotine exposure and the risk of SIDS. Acta Paediatr Suppl 82 Suppl. 1993;389:70–72. doi: 10.1111/j.1651-2227.1993.tb12882.x. [DOI] [PubMed] [Google Scholar]
- Milsom WK. The role of CO2/pH chemoreceptors in ventilatory control. Braz J Med Biol Res. 1995;28:1147–1160. [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, Hurt RD. Simultaneous analysis of nicotine, nicotine metabolites, and tobacco alkaloids in serum or urine by tandem mass spectrometry, with clinically relevant metabolic profiles. Clin Chem. 2002;48:1460–1471. [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Shannon DC, Kelly DH, O'Connell K. Abnormal regulation of ventilation in infants at risk for sudden-infant-death syndrome. N Engl J Med. 1977;297:747–750. doi: 10.1056/NEJM197710062971403. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain Res Dev Brain Res. 2002;133:175–179. doi: 10.1016/s0165-3806(02)00281-x. [DOI] [PubMed] [Google Scholar]
- Taylor AC, Kollros JJ. Stages in the normal development of Rana pipiens larvae. Anat. Rec. 1946;94:7–24. doi: 10.1002/ar.1090940103. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Croll AE, Drucker ML, Wilson AL. Developmental exposure to ethanol or nicotine inhibits the hypercapnic ventilatory response in tadpoles. Respir Physiol Neurobiol. 2008;160:83–90. doi: 10.1016/j.resp.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Harris MB, Coates EL, Gdovin MJ, Leiter JC. Central CO2 chemoreception in developing bullfrogs: anomalous response to acetazolamide. J Appl Physiol. 2003;94:1204–1212. doi: 10.1152/japplphysiol.00558.2002. [DOI] [PubMed] [Google Scholar]
- Torgerson CS, Gdovin MJ, Remmers JE. Fictive gill and lung ventilation in the pre- and postmetamorphic tadpole brain stem. J Neurophysiol. 1998;80:2015–2022. doi: 10.1152/jn.1998.80.4.2015. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Walsh MF, Turrigiano GG. Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol. 2006;96:2127–2133. doi: 10.1152/jn.00107.2006. [DOI] [PubMed] [Google Scholar]
- Zahalka EA, Seidler FJ, Lappi SE, McCook EC, Yanai J, Slotkin TA. Deficits in development of central cholinergic pathways caused by fetal nicotine exposure: differential effects on choline acetyltransferase activity and [3H]hemicholinium-3 binding. Neurotoxicol Teratol. 1992;14:375–382. doi: 10.1016/0892-0362(92)90047-e. [DOI] [PubMed] [Google Scholar]